Abstract
Objectives
Intrauterine growth restriction (IUGR) is a pathologic fetal condition known to affect the fetal brain regionally and associated with future neurodevelopmental abnormalities. This study employed MRI to assess in utero regional brain volume changes in IUGR fetuses compared to controls.
Methods
Retrospectively, using MRI images of fetuses at 30–34 weeks gestational age, a total of 8 brain regions—supratentorial brain and cavity, cerebral hemispheres, temporal lobes and cerebellum—were measured for volume in 13 fetuses with IUGR due to placental insufficiency and in 21 controls. Volumes and their ratios were assessed for difference using regression models. Reliability was assessed by intraclass correlation coefficients (ICC) between two observers.
Results
In both groups, all structures increase in absolute volume during that gestation period, and the rate of cerebellar growth is higher compared to that of supratentorial structures. All structures’ absolute volumes were significantly smaller for the IUGR group. Cerebellar to supratentorial ratios were found to be significantly smaller (P < 0.05) for IUGR compared to controls. No other significant ratio differences were found. ICC showed excellent agreement.
Conclusions
The cerebellar to supratentorial volume ratio is affected in IUGR fetuses. Additional research is needed to assess this as a radiologic marker in relation to long-term outcome.
Key Points
• IUGR is a pathologic fetal condition affecting the brain
• IUGR is associated with long-term neurodevelopmental abnormalities; fetal characterization is needed
• This study aimed to evaluate regional brain volume differences in IUGR
• Cerebellar to supratentorial volume ratios were smaller in IUGR fetuses
• This finding may play a role in long-term development of IUGR fetuses
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Intrauterine growth restriction (IUGR) is a pathologic slowing of fetal growth, resulting in a fetus that is unable to reach its growth potential [1]. Placental insufficiency is a common aetiology for IUGR that affects 5–10 % of all pregnancies [2]. It has been associated with metabolic [3], structural [4–7] and microstructural [3, 8] effects on the fetal and postnatal brain, and may be a source of long-term future neurodevelopment abnormalities that these fetuses have been shown to have at increased rates [9].
Additional fetal studies are needed to further characterize neurostructural differences in the IUGR brain and to target imaging biomarkers.
Studies in IUGR neonates and infants showed several brain areas to have aberrant volume [10–13]. As for IUGR fetuses, the volumetric brain evaluation approach was done by Benavides-Serralde et al., who utilized 3D ultrasound imaging to measure the frontal, thalamic and cerebellar regions of 24–34 weeks gestational age IUGR and control fetuses, with a pulsatility index in the umbilical artery above the 95th centile. They showed that the IUGR fetuses have significantly smaller net volumes for all structures except the thalamus. Significant intergroup differences in the ratios between structures were found only for those involving the frontal region [14]. In another volumetric study by Sanz-Cortes et al. using MRI, the cerebellar volume was shown to be increased in small for gestational age (normal umbilical artery pulsatility index below the 95th percentile) compared to appropriate for gestational age fetuses [15].
These results highlight the need for in utero MRI volumetric evaluation of the IUGR brain. Volumetric MRI studies of the fetal brain have been published in recent years, showing volume growth trajectories during gestation [16, 17], and associating certain fetal pathologies with aberrant volume values [15, 18–21].
The fetal brain is prone to blood flow redistribution in the IUGR state [22, 23], which may affect the brain structures regionally. Therefore, such an in utero multi-structural volumetric study approach can provide important information on relative volume differences between brain regions, revealing radiological biomarkers useful for characterization of these fetuses in the future.
In this retrospective study we aimed to evaluate volume and growth of several brain regions in fetuses suffering from early onset IUGR due to placental insufficiency. A total of eight brain regions and 10 of their ratios were assessed.
Materials and methods
Subjects
The study subjects comprised singleton pregnant women who underwent MRI scans for fetal evaluation at the Chaim Sheba Medical Center between 2011 and 2014. We focused on 30–34 weeks gestational age fetuses. In our institution, the majority of fetal MRI examinations are performed during the third trimester, a critical period during which significant brain volume growth occurs [24].
As MRI is not a routine examination in antenatal diagnosis [25], 21 fetuses were recruited as controls according to the following criteria:
-
(a)
No brain pathology was found on MRI scan according to expert neuroradiologist opinion.
-
(b)
Normal biometric brain values match gestational age according to biometric population studies.
-
(c)
No evidence of IUGR.
Among the fetuses who had an MRI brain examination in our institution during these years, 13 were found to be suitable for inclusion in the IUGR group. The group was defined according to the following criteria in order to create a homogeneous group and take into account that IUGR is a heterogeneous condition:
-
(a)
Referred to MRI for indication of IUGR.
-
(b)
Fetal IUGR weight estimation was confirmed as less than the 10th percentile adjusted to gestational age in accordance with Dollberg curves [26].
-
(c)
IUGR explained by placental insufficiency, demonstrated by abnormal flow in umbilical artery (systolic to diastolic ratio larger than 3 or absent end diastolic flow or reverse flow), or abnormal uterine artery flow, and/or thickened placenta on ultrasound, or low amniotic fluid index.
-
(d)
Absence of any other reason for IUGR after full investigation was completed, in particular infections and chromosomal and other genetic abnormalities.
For each fetus, information about presentation, sex, gestational age and maternal age was obtained. Pregnancies were dated by crown–rump length measurement during the first trimester.
MRI scans
This study was based on the routine fetal MRI procedure carried out in our institution. Fetal brain MRI was performed using a 1.5-Tesla system (Optima scanner, GE Healthcare Technologies, Milwaukee, Wisconsin). Single-shot fast spin echo T2-weighted sequences in three orthogonal planes were performed using half Fourier technique (NEX = 0.53) with the following parameters: section thickness of 3 or 4 mm, no gap, flexible coil (8-channel cardiac coil), FOV was determined by the size of the fetal head with a range of 24 cm × 24 cm to 30 cm × 30 cm, acquisition time between 40 and 45 s, matrix 320/224, TE 90 ms, TR 1298 ms, pixel bandwidth 122 Hz/pixel, SAR values were between 1.1 and 1.7 W/kg.
Target variables
All measurements were taken on the coronal plane. For each fetus a total of eight structures were evaluated for volume: supratentorial brain, supratentorial cavity, cerebellum, right and left cerebral hemispheres, and temporal lobes—total, right and left. In addition, 10 ratios between these volumes were assessed.
Volume measurement method
The best coronal sequence in terms of fetal motion was chosen for all measurements in each fetus to get a clear detailed view of each structure and its boundaries.
Image data was transformed from DICOM to TIFF format and analysed with ImageJ (NIH) software.
Volumetric values were obtained by Cavalieri’s principle, a calculation method used in similar studies [5, 18, 20]: delineation was drawn manually through cursor-guided free-hand traces on individual images. The region of interest (ROI) traced on each image created an area that was multiplied by the slice thickness to calculate the volume. ROI volumes from successive slices were then summed to yield the full volume of the desired region. Values are expressed in millilitres.
Anatomic boundaries
Coronal identification of structures was aided by reference to MRI and histological atlases of the human fetal brain [27, 28].
Supratentorial brain
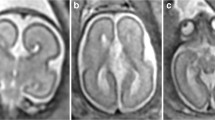
Anterior, posterior, superior and lateral boundaries were delineated by the cerebral cortex (outer edge). The inferior border matched the cortex and an imaginary line crossing the brainstem between the two ends of tentorium cerebelli (Fig. 1a).
Supratentorial cavity
The supratentorial brain as described above in addition to cerebrospinal fluid (CSF). Infratentorial structures—cerebellum and cisterna magna—were excluded (Fig. 1b).
Hemispheres
Elucidated by midline cut of supratentorial brain delineation. Left and right sidedness was discerned in accord with the stomach and cardiac apex.
Temporal lobes
Anterior, inferior and lateral borders were demarcated by cortex; superiorly the Sylvian fissure and a straight line connecting it with the uncus. The posterior boundary was marked by the slice where the frontal and temporal horns of lateral ventricles merge into the atria. Both lobes were drawn separately (Fig. 1c, d).
Cerebellum
Cerebellar hemispheres were drawn with the cerebellar peduncles and vermis. Brainstem and the 4th ventricle were excluded (Fig. 1e).
Statistical analysis
Variables are the brain structures mentioned in the previous section and their ratios. Gestational age was represented as a decimal number.
To test whether IUGR fetuses have differences in volumes and ratios compared to controls, a multiple regression model was constructed, in which the dependent variable was regional brain volume or ratio and the independent variables were a dichotomized IUGR variable and gestational age. Thus, the model coefficient for the IUGR variable could be interpreted as the difference in regional brain volumes between cases and controls, adjusted for gestational age. P < 0.05 was considered statistically significant.
The associations between gestational age and volumes and ratios were assessed in linear regression models separately for cases and controls. Figures and parameters estimated (slope and Y intercept at 30 weeks) with its confidence interval (CI, defined as 95 %) are presented. Linear dependence was assumed on the basis of other fetal brain volumetric studies showing linear fit to be appropriate during this gestational period [17, 19].
Analyses were done using IBM SPSS version 22.
Interobserver reliability
All results shown in this study were obtained by measurements taken by the same observer. To validate consistency of measurements and reliability of results, all regions in the normal group were delineated independently by another observer. Interobserver variability was assessed by the intraclass correlation coefficient (ICC) and its 95 % confidence intervals (CI). We considered an ICC value of more than 0.7 as excellent agreement [4].
Results
Clinical characteristics of study population
The groups’ mean gestational age (SD, range) at MRI was 32.3 weeks (0.9, 30.6–34.3) and 31.6 weeks (1.1, 30–33.3) for control and IUGR, respectively.
Eleven out of 21 (52.4 %) fetuses in the control group were male, while six out of 13 (46 %) in IUGR group were male. All fetuses except two (belonging to the IUGR group) were imaged while on cephalic presentation.
Controls were referred due to suspicious ultrasound findings (n = 10), a family history of developmental delay, inborn malformations or genetic background (n = 6), extracranial malformation (n = 3) and insignificant genetic finding (n = 2). No overt pathologic MRI findings were found, as determined by a neuroradiologist.
All IUGR fetuses were below the 10th percentile in weight according to Dollberg curves [26]; five of them were less than the 3rd percentile and 10 were less than the 5th percentile. Eight of the fetuses were documented to have abnormal flow in the umbilical artery (systolic to diastolic ratio larger than 3 or absent end diastolic flow or reverse flow) or uterine artery. Five were documented with thickened placenta by ultrasound imaging and low amniotic fluid index, which pointed to placental insufficiency aetiology. Other reasons for IUGR, in particular genetics or infections, were disproved for each of the fetuses.
Interobserver variability assessment results
Overall volumetric estimations show excellent interobserver reliability for all regions: the lowest ICC for volume was 0.91 (Table 1).
Multiple regression model
As shown in Table 2, all volumes tested were significantly smaller for the IUGR group (P < 0.05). Significant differences were also found for ratios involving the cerebellum in relation to the supratentorial brain and cavity. However, most ratios are similar between groups. No lobe asymmetry was found.
Linear regression model
All structures evaluated showed a tendency for positive volume growth during the relevant gestational period (Table 3, Fig. 2). Rates of growth were similar for both groups. While volume ratios (Fig. 3) remain steady for both groups, those involving the cerebellum did not. The cerebellum grows progressively more in both groups compared to temporal lobes, supratentorial brain and supratentorial cavity (Fig. 3g, i, j).
Simple linear regression models of the target variable as a function of time are shown for each volume. A plot was not drawn for some because of negligible differences. For each variable, a linear model, Y = b + a × X, was calculated for both groups, where Y is the variable tested (absolute volume) and X is gestational week time. Abbreviations are defined in Table 1
Simple linear regression models of the target variable as a function of time are shown for each volume. A plot was not drawn for some because of negligible differences. For each variable, a linear model, Y = b + a × X, was calculated for both groups, where Y is the variable tested (volume ratio) and X is gestational week time. Abbreviations are defined in Table 1
Discussion
As the IUGR state manifests with neural [3–5] and blood distribution [22, 23, 29] changes in the brain, our interest was to look for relative regional brain volume changes. The results evidence relative cerebellar decrease compared with other brain structures in IUGR fetuses, in addition to absolute reduction in all net volumes.
Volume values in our study are comparable to those that were shown by other MRI volumetric studies.
The supratentorial brain measurements yield volumes comparable to cerebrum values shown by Clouchoux et al. [17].
A temporal lobe effect in the IUGR brain was suggested by studies demonstrating reduced hippocampal volume on premature IUGR infants at term [30], and temporal lobe grey and white matter volumetric changes in premature IUGR infants at 12 months of age [12]. In our study temporal lobes to supratentorial brain and cavity ratios were not significantly different compared to control (Table 2; Fig. 3c, d).
Our results showed that all structures increased during the 30–34 weeks gestation period and that the cerebellum enlarges progressively more compared to the supratentorial structures (with positive CI except for the IUGR temporal lobe and cerebellar ratios; see Fig. 3g, i, j). This observation is in agreement with data from Clouchoux et al. [17], who showed that the cerebellum has a greater maturation rate in volume compared to other brain regions.
Average cerebellar volumes in our study were 9.1 ± 1.6 mL for control and 6.3 ± 1.6 mL for IUGR. Grossman et al. showed that the cerebellar volume is in the range of roughly 5–15 mL for this gestational age [20]. Clouchoux et al. presented figures of around 10–15 mL [17]. Our results for control and IUGR fit the 95th and 50th percentiles, respectively, of cerebellar volume data shown by Hatab et al. [16]. Discrepancies may be explained by variations of boundary definitions, distinct planes of measurements or different slice thickness.
Studies have already demonstrated the cerebellar volume to be altered in the IUGR or small for gestational age (SGA) states. Sanz-Cortes et al. performed MRI volumetric assessment of the cerebellum in 37 weeks gestational age fetuses and showed it to be increased in small for SGA fetuses compared to appropriate for gestational age (AGA) fetuses [15]. Their SGA cohort contained fetuses with normal umbilical artery pulsatility index below the 95th percentile, i.e. placental insufficiency was not an inclusion criterion. Another study, using 2D ultrasound, presented increased transverse cerebral diameter to abdominal circumference ratio in growth-restricted fetuses [31]. It is noteworthy that the 3D ultrasonography evaluation of Benavides-Serralde et al., mentioned in the “Introduction”, did not indicate significant cerebellar volume difference in IUGR of fetuses 28–34 weeks gestational age [14], and Padilla et al. actually showed preterm IUGR infants to have reduced volume of cerebellar white matter at 12 months of age [12]. The results of these studies, in addition to ours, demonstrate that the cerebellum is prone to volume changes in the IUGR state. The fluctuations between results may reflect the fact that the IUGR population is heterogeneous. In this regard we remark that 10 out of 13 IUGR fetuses in our study were under the 5th percentile, five of them being less than the 3rd. Additional research is needed to further characterize these differences in the IUGR population, to determine whether these changes persist into later life and their relation to neurobehavioral outcome [9, 32].
A pathophysiologic explanation for cerebellar volume changes in IUGR may reside in the hypoxic nature of this condition. Studies in sheep showed that a prolonged period of placental insufficiency, resulting in fetal hypoxemia, can affect growth of the cerebellum, with a reduction in cell number and cell size [33, 34]. An additional proposed mechanism is haemodynamic brain redistribution [22, 23] in IUGR that can induce regional reorganization of the brain reflected as regional volume changes.
Intrauterine growth retardation children were shown to have lower school achievement and intelligence scores [9]. As the role of the cerebellum in high cognitive functioning is well recognized [35], an interesting question arises as to whether abnormal cerebellar growth during pregnancy is the key for this observation.
We acknowledge some limitations of this study. Follow-up after birth to assess if findings are associated with neurodevelopmental outcome is absent from our research. Another limitation is the relatively small sample size. However, considering other fetal brain volumetric studies [14, 19, 20], we focused on a shorter period of gestation—the number of fetuses evaluated per unit of time is not equivalently smaller. As this study’s approach is multi-structural with the aim of assessing of several structures, manual measurements of each fetus were very time consuming. Nevertheless, this study was able to produce statistically significant statements. Finally, in comparison to our MRI protocol, a 3D MRI protocol enables more accurate volume measurement, although this protocol is still not in common use worldwide. Our method is technologically simple, easy to learn and reproducible.
As for the strengths, our assessment was done using MRI, which provides good visibility of the whole brain independent of the fetal presentation, produces slices easily in three planes and makes it possible to measure the brain itself in the supratentorial space [36]. Volumetric MRI research of the fetal brain is gaining popularity and showing some interesting results [19, 21, 37]. Automated segmentation methods have been developed to make this approach a less time-consuming and more effective tool in the clinical field [17]. Considering that IUGR is a heterogeneous condition with several aetiologies, we examined a homogenous IUGR group, which is an additional strength of our study.
To conclude, the study shows that in IUGR fetuses of 30–34 weeks gestational age the cerebellum to supratentorial volume ratio is smaller. This is added to results of other studies showing this structure’s volume to be affected in the IUGR brain. Future research is needed to characterize these changes in light of IUGR pathophysiological processes and aetiologies, and to provide information about its effect on neurodevelopmental outcome.
Abbreviations
- CER:
-
Cerebellum
- LH:
-
Left cerebral hemisphere
- LT:
-
Left temporal lobe
- RH:
-
Right cerebral hemisphere
- RT:
-
Right temporal lobe
- STB:
-
Supratentorial brain
- STC:
-
Supratentorial cavity
- TT:
-
Total temporal lobes
References
Romo A, Carceller RTJ (2006) Intrauterine growth retardation (IUGR): epidemiology and etiology. Pediatr Endocrinol Rev 6:332–336
Batalle D, Eixarch E, Figueras F et al (2012) Altered small-world topology of structural brain networks in infants with intrauterine growth restriction and its association with later neurodevelopmental outcome. Neuroimage 60:1352–1366
Sanz-Cortés M, Figueras F, Bargalló N, Padilla N, Amat-Roldan I, Gratacós E (2010) Abnormal brain microstructure and metabolism in small-for-gestational-age term fetuses with normal umbilical artery Doppler. Ultrasound Obstet Gynecol 36:159–165
Egaña-Ugrinovic G, Sanz-Cortés M, Couve-Pérez C, Figueras F, Gratacós E (2014) Corpus callosum differences assessed by fetal MRI in late-onset intrauterine growth restriction and its association with neurobehavior. Prenat Diagn 34:843–849
Egaña-Ugrinovic G, Sanz-Cortes M, Figueras F, Bargalló N, Gratacós E (2013) Differences in cortical development assessed by fetal MRI in late-onset intrauterine growth restriction. Am J Obstet Gynecol 209:126.e1–126.e8
Egaña-Ugrinovic G, Sanz-Cortes M, Figueras F, Couve-Perez C, Gratacós E (2014) Fetal MRI insular cortical morphometry and its association with neurobehavior in late-onset small-for-gestational-age fetuses. Ultrasound Obstet Gynecol 44:322–329
Dubois J, Benders M, Borradori-Tolsa C et al (2008) Primary cortical folding in the human newborn: An early marker of later functional development. Brain 131:2028–2041
Sanz-Cortés M, Figueras F, Bonet-Carne E et al (2013) Fetal brain MRI texture analysis identifies different microstructural patterns in adequate and small for gestational age fetuses at term. Fetal Diagn Ther 33:122–129
Leitner Y, Fattal-Valevski A, Geva R et al (2007) Neurodevelopmental outcome of children with intrauterine growth retardation: a longitudinal, 10-year prospective study. J Child Neurol 22:580–587
Thompson DK, Warfield SK, Carlin JB et al (2007) Perinatal risk factors altering regional brain structure in the preterm infant. Brain 130:667–677
Taft PB, Lethab H, Ring PB, Peitmmb B, Lou HC, Henriksena O (1995) Volumetric analysis of the normal infant brain and in intrauterine growth retardation. Early Hum Dev 43:15–29
Padilla N, Falcón C, Sanz-Cortés M et al (2011) Differential effects of intrauterine growth restriction on brain structure and development in preterm infants: a magnetic resonance imaging study. Brain Res 1382:98–108
Tolsa CB, Zimine S, Warfield SK et al (2004) Early alteration of structural and functional brain development in premature infants born with intrauterine growth restriction. Pediatr Res 56:132–138
Benavides-Serralde A, Hernández-Andrade E, Fernández-Delgado J (2009) Three-dimensional sonographic calculation of the volume of intracranial structures in growth-restricted and appropriate-for-gestational age fetuses. Ultrasound Obstet Gynecol 33:530–537
Sanz-Cortes M, Egaña-Ugrinovic G, Zupan R, Figueras F, Gratacos E (2014) Brainstem and cerebellar differences and their association with neurobehavior in term small-for-gestational-age fetuses assessed by fetal MRI. Am J Obstet Gynecol 210:452.e1–452.e8
Hatab MR, Kamourieh SW, Twickler DM (2008) MR volume of the fetal cerebellum in relation to growth. J Magn Reson Imaging 27:840–845
Clouchoux C, Guizard N, Evans AC, du Plessis AJ, Limperopoulos C (2012) Normative fetal brain growth by quantitative in vivo magnetic resonance imaging. Am J Obstet Gynecol 206:173.e1–173.e8
Hoffmann C, Grossman R, Bokov I, Lipitz S, Biegon A (2010) Effect of cytomegalovirus infection on temporal lobe development in utero: quantitative MRI studies. Eur Neuropsychopharmacol 20:848–854
Limperopoulos C, Tworetzky W, McElhinney DB et al (2010) Brain volume and metabolism in fetuses with congenital heart disease: evaluation with quantitative magnetic resonance imaging and spectroscopy. Circulation 121:26–33
Grossman R, Hoffman C, Mardor Y, Biegon A (2006) Quantitative MRI measurements of human fetal brain development in utero. Neuroimage 33:463–470
Andescavage N, Yarish A, Donofrio M et al (2015) 3-D volumetric MRI evaluation of the placenta in fetuses with complex congenital heart disease. Placenta 36:1024–1030
Hernandez-Andrade E, Figueroa-Diesel H, Jansson T, Rangel-Nava H, Gratacos E (2008) Changes in regional fetal cerebral blood flow perfusion in relation to hemodynamic deterioration in severely growth-restricted fetuses. Ultrasound Obstet Gynecol 32:71–76
Figueroa-Diesel H, Hernandez-Andrade E, Acosta-Rojas R, Cabero L, Gratacos E (2007) Doppler changes in the main fetal brain arteries at different stages of hemodynamic adaptation in severe intrauterine growth restriction. Ultrasound Obstet Gynecol 30:297–302
Lodygensky GA, Vasung L, Sizonenko SV (2010) Neuroimaging of cortical development and brain connectivity in human newborns and animal models. J Anat 217:418–428
Garel C (2005) Fetal cerebral biometry: normal parenchymal findings and ventricular size. Eur Radiol 15:809–813
Dollberg S, Haklai Z, Mimouni FB, Gorfein IGE (2005) Birth weight standards in the live-born population in Israel. Isr Med Assoc J 7:311–314
Bayer SA, Altman J (2003) Atlas of human central nervous system development. Vol 22: the human brain during the third trimester. CRC, Boca Raton
Garel C (2004) MRI of the fetal brain normal development and cerebral pathologies. Springer, Berlin Heidelberg New York
Benavides-Serralde A, Scheier M, Cruz-Martinez R et al (2011) Changes in central and peripheral circulation in intrauterine growth-restricted fetuses at different stages of umbilical artery flow deterioration: new fetal cardiac and brain parameters. Gynecol Obstet Investig 71:274–280
Lodygensky GA, Seghier ML, Warfield SK et al (2008) Intrauterine growth restriction affects the preterm infant’s hippocampus. Pediatr Res 63:438–443
Vinkesteijn AS, Mulder PG, Wladimiroff JW (2000) Fetal transverse cerebellar diameter measurements in normal and reduced fetal growth. Ultrasound Obstet Gynecol 15:47–51
Courchesne E, Karns CM, Davis HR et al (2001) Unusual brain growth patterns in early life in patients with autistic disorder: an MRI study. Neurology 57:245–254
Mallard C, Rees S, Stringer S (1998) Effects of chronic placental insufficiency on brain development in fetal sheep. Pediatr Res 43:262–270
Rees S, Breen S, Loeliger M, McCrabb G, Harding R (1999) Hypoxemia near mid-gestation has long-term effects on fetal brain development. J Neuropathol Exp Neurol 58:932–945
Baillieux H, De Smet HJ, Paquier PF, De Deyn PP, Mari P (2008) Cerebellar neurocognition: insights into the bottom of the brain. Clin Neurol Neurosurg 110:763–773
Blondiaux E, Garel C (2013) Fetal cerebral imaging - ultrasound vs. MRI: an update. Acta Radiol 54:1046–1054
Schellen C, Ernst S, Gruber GM et al (2015) Fetal MRI detects early alterations of brain development in Tetralogy of Fallot. Am J Obstet Gynecol 213:392.e1–392.e7
Acknowledgments
The scientific guarantor of this publication is Dr. Eldad Katorza, Sheba Medical Center, Israel. The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article. The authors state that this work has not received any funding. Estela Derazne kindly provided statistical advice for this manuscript. Institutional review board approval was obtained. Written informed consent was waived by the institutional review board. Methodology: retrospective, case-control study, performed at one institution.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Polat, A., Barlow, S., Ber, R. et al. Volumetric MRI study of the intrauterine growth restriction fetal brain. Eur Radiol 27, 2110–2118 (2017). https://doi.org/10.1007/s00330-016-4502-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-016-4502-4