Abstract
Objectives
To compare the outcomes of patients after interlaminar computed tomography (CT)-guided epidural injections of the lumbar spine with particulate vs. non-particulate steroids.
Methods
531 consecutive patients were treated with CT-guided lumbar interlaminar epidural injections with steroids and local anaesthetics. 411 patients received a particulate steroid and 120 patients received a non-particulate steroid. Pain levels were assessed using the 11-point numerical rating scale (NRS) and overall reported ‘improvement’ was assessed using the Patients Global Impression of Change (PGIC) at 1 day, 1 week and 1 month post-injection.
Descriptive and inferential statistics were applied.
Results
Patients receiving particulate steroids had statistically significantly higher NRS change scores (p = 0.0001 at 1 week; p = 0.0001 at 1 month).
A significantly higher proportion of patients receiving particulate steroids reported relevant improvement (PGIC) at both 1 week and 1 month post injection (p = 0.0001) and they were significantly less likely to report worsening at 1 week (p = 0.0001) and 1 month (p = 0.017).
Conclusion
Patients treated with particulate steroids had significantly greater pain relief and were much more likely to report clinically relevant overall ‘improvement’ at 1 week and 1 month compared to the patients treated with non-particulate steroids.
Key Points
• CT-guided epidural injections of the lumbar spine with particulate vs. non-particulate steroids.
• Good outcomes with particulate steroids.
• Less pain relief in patients with non-particulate steroids.
• Less improvement in patients with non-particulate steroids.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The number of epidural steroid injections has increased significantly over the last two decades [1], fuelled by the growth of the population of patients with low back pain [2]. Epidural steroid injection is the most frequently administered therapy for patients with disc herniation and spinal canal stenosis [3–6] and can be administered by three different approaches, namely the transforaminal periradicular epidural injection, the caudal injection and the interlaminar injection with varying long-term outcomes for the different techniques and overall strong short-term evidence [5, 7–10].
The most commonly used drugs for lumbar epidural injections are an injectable particulate or a non-particulate steroid, which is usually administered in a combination with a local anaesthetic. For many years, triamcinolone acetonide, a particulate depot steroid, was used ‘off label’ for epidural steroid injections with what appeared to be good clinical outcomes [11]. Triamcinolone acetonide is considered to be more effective because of greater particle size and therefore prolonged ability to remain in the epidural space compared to soluble preparations [12]. The particle size is also discussed to be the problem related to adverse events such as embolization because triamcinolone acetonide has variable particle sizes and some particles may be larger than red blood cells [13].
Epidural steroid injections are considered an ‘off label’ procedure because steroids have never been authorized for epidural use by the US Food and Drug Administration (FDA). Furthermore, the FDA issued a warning in 2011 that triamcinolone acetonide “is not for epidural use” [14] and in 2014 the FDA published a safety announcement that “injections into the epidural space may result in rare but serious adverse events, including loss of vision, stroke, paralysis, and death” [15]. This announcement was based on 15 references of the medical literature and a review of cases from the FDA Adverse Event Reporting System (FAERS) [15]. The majority of these cases were cervical transforaminal epidural injections. As a result of this FDA warning, many physicians changed from using the particulate corticosteroid preparations, such as triamcinolone acetonide, to using non-particulate corticosteroid preparations for epidural steroid injections. This change also occurred at our university orthopaedic hospital in May 2014.
Because the radiology department of our specialized orthopaedic/rheumatology university hospital has developed and continues to expand a large database to monitor outcomes from imaging-guided therapeutic musculoskeletal injections, up-to-date prospective information is available on outcomes from patients receiving epidural injections before and after the change from particulate to non-particulate corticosteroids.
Therefore, the purpose of this study was to compare the outcomes of patients receiving interlaminar computed tomography (CT)-guided epidural injections of the lumbar spine with particulate vs. non-particulate steroids.
Material and methods
Patients
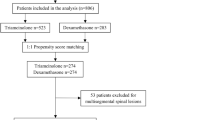
This was a comparative effectiveness retrospective outcomes study using two independent cohorts of patients referred to the radiology department of this specialized orthopaedic/rheumatology university hospital balgrist (Zürich, Switzerland) for CT-guided interlaminar epidural therapeutic injections [16, 17]. One cohort received a particulate corticosteroid preparation (i.e. 40 mg triamcinolone acetonide, Triamcort Depot, Helvepharm AG, Frauenfeld, Switzerland) and the other cohort received a non-particulate corticosteroid preparation (i.e. 4 mg dexamethasone dihydrogen phosphate, Fortecortin Inject; Merck AG, Zug, Switzerland) after our hospital changed the injection protocol on the basis of the FDA safety announcement [15]. Both cohorts include consecutive epidural injection patients registered in the radiology department outcomes database who returned follow-up postal questionnaires. Patients receiving the particulate corticosteroid preparation underwent epidural injections between October 2009 and 8 May 2014. Patients receiving the non-particulate corticosteroid preparation underwent epidural injections between 9 May 2014 and 31 October 2014. The information provided to the patients was identical in both cohorts with no discussion of the type of corticosteroid. As it appeared when entering the outcomes data from patients receiving the non-particulate corticosteroid that these outcomes were less favourable compared to patients receiving the particulate corticosteroid, the radiology department decided to perform this retrospective comparative effectiveness study (16;17).
Informed consent was obtained before the intervention. The process of the intervention, the risks and benefits were discussed in an identical fashion with the patients in both cohorts.
The study was approved by the institutional review board.
Epidural injection procedure
The lumbar interlaminar epidural injections were performed as an outpatient procedure. All intervention procedures were performed according to a standardized protocol to guarantee the consistency.
The intervention procedures were guided by CT (64-detector row CT, Philips Brilliance; Philips Medical Systems, Best, the Netherlands or 64-detector row CT, Somatom Definition AS, Siemens Healthcare, Erlangen, Germany). The patients lay prone on the examination table. The initial CT acquisition was performed over 1–2 lumbar levels cranial and caudal at the requested level. Before the procedure the best access route at the requested level was chosen by the radiologist.
Subcutaneous application of local anaesthetics was introduced under CT-fluoroscopic guidance after skin disinfection. After the needle (23 gauge 7 cm, Terumo Europe, Leuven, Belgium or 22 gauge 12.7 cm spinal needle, BD Europe, Temse, Belgium) was introduced into the interlaminar space through the ligamentum flavum, a volume of 0.5 ml iopamidol (Iopamiro 200, 200 mg of iodine per millilitre; Bracco, Milan, Italy) was injected to verify the correct epidural position of the needle. This was followed by the slow injection of 40 mg (1 ml) of the particulate steroid triamcinolone acetonide or 4 mg (1 ml) of the non-particulate steroid dexamethasone dihydrogen phosphate. Finally, 1 ml of 0.2 % ropivacaine (Naropin; Astra-Zeneca, Södertälje, Sweden) was injected in all patients.
Outcome measures
Immediately prior to the epidural injection all patients stated their current level of pain using the 11-point numerical rating scale (NRS) with 0 = no pain and 10 = intolerable pain. Fifteen minutes after the injection and while still in the radiology department all patients once again reported their pain level using the same NRS scale. Further post-injection outcome data on the pain levels were then collected via a prepaid postal questionnaire which was handed to each patient prior to leaving the radiology department. The time frames for collecting data were 1 day, 1 week and 1 month post injection. In addition to NRS pain levels, the primary outcome measure of overall ‘improvement’ was determined using the Patients Global Impression of Change (PGIC) scale at these same post-injection time points. The PGIC is a seven-item scale which includes the responses ‘much better’, ‘better’, ‘slightly better’, ‘unchanged’, ‘slightly worse’, ‘worse’ and ‘much worse’ [18]. As used in other studies, only the responses of ‘much better’ and ‘better’ were considered a clinically relevant ‘improvement’ (primary outcome) [19]. Additionally, the responses of ‘slightly worse’, ‘worse’ and ‘much worse’ all counted as worsening of the overall condition. ‘Worsening’ was a secondary outcome measure as were the pain scores.
Statistical analysis
The two cohorts were compared for differences in age using the Mann–Whitney U test for non-parametric data. Differences in sex distribution between the two cohorts were analysed using the Chi-square test. The proportion of patients reporting clinically relevant ‘improvement’ was calculated for 1 day, 1 week and 1 month post-injection and compared between the two cohorts using the Chi-square test (primary outcome). Similarly the proportion of patients reporting ‘worsening’ of their condition was also compared using the Chi-square test at the same post-injection time points. The NRS change scores (normally distributed data) were calculated for all follow-up time points and compared between the two groups using the unpaired Student’s t test. P levels lower than 0.05 were considered statistically significant.
All calculations were done with the statistical software package SPSS Statistics Version 21, IBM, Chicago, IL.
Results
There were 411 patients in the particulate steroid cohort and 120 patients in the non-particulate steroid cohort. All 531 patients returned their questionnaire for the 1 day, 1 week and 1 month pain levels and ‘improvement’. No significant age (p = 0.08) or gender (p = 0.43) differences were found between these two groups. The mean patient age for the particulate steroid group was 63.44 (SD = 16.30) years and for the non-particulate steroid group it was 66.83 (SD = 13.72) years. For the particulate steroid group 43.3 % were male and for the non-particulate group 47.9 % were male.
For the primary outcome of ‘improvement’ 51 % of the patients receiving particulate steroids reported clinically relevant ‘improvement’ at 1 week compared to 28 % of patients receiving non-particulate steroids (p = 0.0001). Very similar results were also found at the 1 month time point (p = 0.0001), also in favour of the particulate steroids (Table 1).
Additionally, patients receiving the non-particulate corticosteroid reported statistically significantly higher levels of ‘worsening’ at both 1 week (19 % vs. 7 %, p = 0.0001) and 1 month (27 % vs. 17 %) post-injection (p = 0.017) compared to those receiving the particulate corticosteroid (Table 1). The analysis of PGIC data showed similar results for clinically relevant ‘improvement’ or ‘worsening’ at 1 day after the procedure for both cohorts (Table 1), but without a statistically significant difference (p = 0.17 and p = 0.56, respectively).
There was no significant difference in the baseline NRS scores between the two cohorts or in the NRS change scores at 15 min post-injection (p = 0.22). However, highly significant differences in the NRS change scores were found at 1 day, 1 week and 1 month post-injection (p = 0.002, 0.0001 and 0.0001, respectively) with those receiving the particulate corticosteroid reporting at least twice as much pain reduction at 1 week and 1 month compared to the patients receiving the non-particulate corticosteroid (Table 2, Fig. 1). There were no significant adverse events in either cohort of patients after the interlaminar epidural injections.
Discussion
This study clearly shows a statistically significant and clinically relevant difference in overall ‘improvement’ rates as well as in the degree of pain relief at both 1 week and 1 month after interlaminar CT-guided epidural injections when comparing the patients receiving an injection of particulate versus patients receiving the non-particulate corticosteroids. The cohort receiving the particulate corticosteroids reported far better treatment outcomes. When switching from particulate to non-particulate steroids, our decision was based on safety considerations with the assumption that the treatment outcomes from the non-particulate steroids would be as good or almost as good as the particulate steroids.
The magnitude of differences in treatment outcomes (e.g. 51 % improvement after 1 week for particulate steroids vs. only 28 % for non-particulate steroids) was clearly unexpected. The NRS change scores dropped 47 % in 1 week for the particulate steroid and just 23 % for the non-particulate steroid and also the 1-month results showed a drop of the NRS change scores of 42 % for the particulate steroid and just 18 % for the non-particulate steroid. This is more than double the pain relief with the particulate steroid compared to the non-particulate steroid at 1 week and 1 month. Our results further show a significantly higher proportion of patients reporting ‘worsening’ after the injection of the non-particulate steroid compared to the injection of the particulate steroid.
Our results do not support the conclusions from a recent trial by Friedly et al. who claimed that the addition of a steroid offered minimal or no short-term benefit for epidural injections. However those authors did not report separate outcomes for particulate and non-particulate steroid preparations and used a highly variable volume of anaesthetic [19]. They even suggested that the inclusion of a corticosteroid along with the local anaesthetic in epidural injections is not needed at all and does not improve outcomes compared to the local anaesthetic alone [19]. On the basis of our study data, we disagree strongly with the report by Friedly and colleagues. While non-particulate steroids may offer only a small clinically relevant benefit, the injection of particulate steroids did offer a statistically significant and clinically relevant benefit compared to the non-particulates after epidural injections.
Our findings are similar to several previous studies but also contrary to others. Currently, a number of studies on the use of particulate and non-particulate steroids for translaminar or transforaminal epidural injections show a broad range of results [19–21]. Manchikanti et al. [22] described in a review of the Friedly et al. paper all inconsistencies in detail and pointed out that the paper may cause confusion for both physicians and patients in terms of the benefits from epidural steroid injections for patients with spinal canal stenosis. Manchikanti et al. further stated that “the reviewed literature was improperly assessed leading to inappropriate conclusions” and that all relevant literature had not been reviewed [23].
A recent study with a small sample size (56 patients) in a randomized double-blinded controlled trial did not find any significant difference between particulate (betamethasone) and non-particulate (dexamethasone) steroids with a follow-up of pain relief at 1, 3 and 6 months. However these results showed a statistically non-significant trend for a better pain relief with the particulate steroids [24]. It would be interesting how this trend would develop with a larger number of patients. In another study, Kim and Brown reported a trend for patients receiving non-particulate steroids towards less pain relief and a shorter period of pain relief in their partially retrospective study, which supports the results of our current study [20]. However, they only had 30 patients who received fluoroscopic guided translaminar epidural injections, so it is likely that their sample size was too small to detect the clinically relevant and statistically significant difference that may have been found with a larger sample size. Furthermore there was variability in the time frames for the outcome measurements from 1 to 2 months. In using the visual analogue score for the outcome measurements, those authors did not also assess the overall improvement in the quality of everyday life as is possible with the PGIC scale.
Park et al. treated 106 patients in their study who received lumbar transforaminal epidural injections with either triamcinolone acetate or dexamethasone. Their results were similar to our study in showing the superiority of the particulate steroid triamcinolone acetonide concerning the pain relief, although their results were not supported by their secondary outcome measures which included the McGill Questionnaire and Oswestry Disability Index [25].
Two further studies compared outcomes of particulate versus non-particulate lumbar transforaminal steroid injections [21, 26]. The results of both studies showed less efficacy for non-particulate steroids compared to particulate steroids, similar to the results reported in this current study. Kennedy et al. also reported in their randomized multi-centre trial with a follow-up at 7–14 days, 3 months and 6 months that a higher number of multiple transforaminal injections for treatment using dexamethasone was required to achieve the same outcome as when using triamcinolone [21]. Similarly, Stanczak et al. reviewed the pain relief of 597 patients at 1 and 2 weeks after epidural steroid injections with either a particulate steroid (Kenalog) or a non-particulate steroid (Celestone Soluspan) with a significantly better pain reduction when using the particulate steroid [27].
However, in contrast to the results of the lumbar epidural injection studies reported above are the findings of three studies with transforaminal cervical injections: these studies reported no significant difference in the efficacy of the non-particulate steroid compared to the particulate steroid [28–30]. While Dreyfuss et al. [30] showed a slightly lower effectiveness for dexamethasone, this difference did not reach statistical significance.
Complications after spinal epidural steroid injections are rare but serious adverse events. Most complications in epidural spinal injections are reported for cervical epidural injections. Complications such as spinal cord infarction, brainstem and cerebellar infarction have occurred after direct cervical transforaminal epidural injections [31–38]. There were no serious adverse events for any of the patients in this current study.
Seven cases of paraplegia have been reported from 2002 to 2009 for foraminal or interlaminar steroid injection of the lumbar spine [39]. Five of these cases occurred in the Paris area centres between 2003 and 2008. Wybier et al. assumed that the high rate of French cases might be caused by a strong tendency of the exclusively used prednisolone acetate to conglomerate, thereby creating a higher risk for arterial embolizations to the spinal cord [40].
Benzon et al. [41] compared the particle sizes of different particulate and non-particulate steroids and concluded that the particulate steroids can occlude the spinal arteries and result in infarctions of the spinal cord, brainstem or the cerebellum [42–45]. Because of this tendency to aggregate and potentially result in infarctions, in 2011 the FDA released a safety labelling change for Kenalog (triamcinolone acetonide) stating that it is not for epidural use [14, 39]. In 2014 the FDA published a safety announcement that “injections into the epidural space may result in rare but serious adverse events, including loss of vision, stroke, paralysis, and death” [15]. The FDA stated that “the effectiveness and safety of injection of corticosteroids into the epidural space of the spine have not been established” [15].
Looking at all the cited studies above, we can conclude that the studies with no significant difference for the lumbar epidural treatment with particulate versus non-particulate steroids had a smaller patient size than the ones with a significant difference and a better pain reduction for the particulate steroid.
Manchikanti et al. [23] criticized the FDA warning of April 2014 saying that it is not based on evidence. They showed in their review article the efficacy of epidural injections in the cervical, thoracic and lumbar spine with steroids and local anaesthetics combined or only with local anaesthetics.
Our study has limitations. A randomized clinical trial with a placebo group would have been preferable to the comparative effectiveness outcome study we performed. However, as a result of our clinical setting we are only able to provide comparative data as there would be no support for a placebo group when some evidence exists for the efficacy of epidural therapeutic injections. In this comparative effectiveness outcomes study we are using two clinical cohorts. Importantly, the patients treated in both cohorts are truly representative of the patients seen in daily clinical practice and there was no significant difference in the ages, sex proportions or baseline pain levels between the two cohorts, and the effects observed in our study showed a clear clinical and statistically significant disadvantage for the use of non-particulate steroids, confirming that our study design was suitable for detecting the differences between our two cohorts.
Another potential limitation may be due to the fact that the two cohorts only include consecutive patients who returned their postal questionnaire. We had a response rate of 35.6 % (120 of 337 patients) for the group treated with non-particulate steroids and 27.1 % (411 of 1517 patients) for the group treated with particulate steroids. We know from other studies using the database that less than 50 % of patients remember to return these questionnaires and that those who do return them tend to have worse outcomes compared to patients who forget or neglect to return their questionnaires [46, 47].
A further limitation is that we have no clinical information available for the patients included in the study. However, we are a specialized orthopaedic hospital and know that most patients with back pain referred to our hospital are chronic and an infiltration is the last attempted treatment before surgery.
Another limitation is that we have outcome data only until 1 month post injection rather than following the patients for a longer period of time.
Conclusions and outlook
After the safety warning announcement by the FDA in April 2014 [15], the radiology department in our specialized orthopaedic/rheumatology hospital changed from using particulate corticosteroids (triamcinolone acetonide) to non-particulate corticosteroids (dexamethasone dihydrogen phosphate) for the lumbar interlaminar epidural injections. This change in treatment strategy was done in May 2014. Patients treated with the new procedure using non-particulate corticosteroids had markedly worse outcomes both in terms of the percentage reporting overall ‘improvement’ as well as pain relief levels when compared to patients treated with the particulate corticosteroids before May 2014. We certainly never expected such a significant inferiority of the non-particulate steroids as shown in our results in the treatment outcomes obtained at 1 week and 1 month. Therefore, in spite of the FDA warning, this hospital, which is not located in the USA and thus not subject to their regulations, has changed back to the particulate corticosteroid preparation for interlaminar epidural steroid injections with the support from the referring clinicians. From our perspective it is not an appropriate solution to refuse the patients the more effective treatment with particulate steroids. To inform the patients about the risks and the benefits of an epidural injection with a particulate steroid and allow them to make an informed decision should be discussed as an option to provide the patients with the best treatment for pain reduction and improvement of their life quality.
References
Manchikanti L, Pampati V, Boswell MV, Smith HS, Hirsch JA (2010) Analysis of the growth of epidural injections and costs in the Medicare population: a comparative evaluation of 1997, 2002, and 2006 data. Pain Physician 13:199–212
Harkness EF, Macfarlane GJ, Silman AJ, McBeth J (2005) Is musculoskeletal pain more common now than 40 years ago?: two population-based cross-sectional studies. Rheumatology (Oxford) 44:890–895
Karamouzian S, Ebrahimi-Nejad A, Shahsavarani S, Keikhosravi E, Shahba M, Ebrahimi F (2014) Comparison of two methods of epidural steroid injection in the treatment of recurrent lumbar disc herniation. Asian Spine J 8:646–652
Friedly J, Nishio I, Bishop MJ, Maynard C (2008) The relationship between repeated epidural steroid injections and subsequent opioid use and lumbar surgery. Arch Phys Med Rehabil 89:1011–1015
Abdi S, Datta S, Trescot AM et al (2007) Epidural steroids in the management of chronic spinal pain: a systematic review. Pain Physician 10:185–212
Krych AJ, Richman D, Drakos M et al (2012) Epidural steroid injection for lumbar disc herniation in NFL athletes. Med Sci Sports Exerc 44:193–198
Parr AT, Diwan S, Abdi S (2009) Lumbar interlaminar epidural injections in managing chronic low back and lower extremity pain: a systematic review. Pain Physician 12:163–188
Conn A, Buenaventura RM, Datta S, Abdi S, Diwan S (2009) Systematic review of caudal epidural injections in the management of chronic low back pain. Pain Physician 12:109–135
Buenaventura RM, Datta S, Abdi S, Smith HS (2009) Systematic review of therapeutic lumbar transforaminal epidural steroid injections. Pain Physician 12:233–251
Price CM, Rogers PD, Prosser AS, Arden NK (2000) Comparison of the caudal and lumbar approaches to the epidural space. Ann Rheum Dis 59:879–882
Cohen SP, Bicket MC, Jamison D, Wilkinson I, Rathmell JP (2013) Epidural steroids: a comprehensive, evidence-based review. Reg Anesth Pain Med 38:175–200
Abram SE (1999) Treatment of lumbosacral radiculopathy with epidural steroids. Anesthesiology 91:1937–1941
Derby R, Lee SH, Date ES, Lee JH, Lee CH (2008) Size and aggregation of corticosteroids used for epidural injections. Pain Med 9:227–234
FDA - US Food and Drug Administration. Shoulder. Kenalog-10 (triamcinolone acetonide) injection and Kenalog-40 (triamcinolone acetonide) injection. http://www.fda.gov/safety/medwatch/safetyinformation/ucm262876.htm. Accessed 15 Feb 2015
US Food and Drug Administration FDA (2014) FDA drug safety communication: FDA requires label changes to warn of rare but serious neurologic problems after epidural corticosteroid injections for pain. http://www.fda.gov/downloads/Drugs/DrugSafety/UCM394286.pdf. Accessed 15 Feb 2015
Tinetti ME, Studenski SA (2011) Comparative effectiveness research and patients with multiple chronic conditions. N Engl J Med 364:2478–2481
Neumann PJ (2013) Communicating and promoting comparative-effectiveness research findings. N Engl J Med 369:209–211
Bensler S, Sutter R, Pfirrmann CW, Peterson CK (2015) Long term outcomes from CT-guided indirect cervical nerve root blocks and their relationship to the MRI findings - a prospective Study. Eur Radiol 25:3405–3413
Friedly JL, Comstock BA, Turner JA et al (2014) A randomized trial of epidural glucocorticoid injections for spinal stenosis. N Engl J Med 371:11–21
Kim D, Brown J (2011) Efficacy and safety of lumbar epidural dexamethasone versus methylprednisolone in the treatment of lumbar radiculopathy: a comparison of soluble versus particulate steroids. Clin J Pain 27:518–522
Kennedy DJ, Plastaras C, Casey E et al (2014) Comparative effectiveness of lumbar transforaminal epidural steroid injections with particulate versus nonparticulate corticosteroids for lumbar radicular pain due to intervertebral disc herniation: a prospective, randomized, double-blind trial. Pain Med 15:548–555
Manchikanti L, Candido KD, Kaye AD et al (2014) Randomized trial of epidural injections for spinal stenosis published in the New England Journal of Medicine: further confusion without clarification. Pain Physician 17:E475–E488
Manchikanti L, Candido KD, Singh V et al (2014) Epidural steroid warning controversy still dogging FDA. Pain Physician 17:E451–E474
Denis I, Claveau G, Filiatrault M, Fugere F, Fortin L (2015) Randomized double-blind controlled trial comparing the effectiveness of lumbar transforaminal epidural injections of particulate and nonparticulate corticosteroids for lumbosacral radicular pain. Pain Med 16:1697–1708
Park CH, Lee SH, Kim BI (2010) Comparison of the effectiveness of lumbar transforaminal epidural injection with particulate and nonparticulate corticosteroids in lumbar radiating pain. Pain Med 11:1654–1658
Lee SE, Kim DS, Lee WK et al (2010) Application of the Epstein criteria for prediction of clinically insignificant prostate cancer in Korean men. BJU Int 105:1526–1530
Stanczak J, Blankenbaker DG, De Smet AA, Fine J (2003) Efficacy of epidural injections of Kenalog and Celestone in the treatment of lower back pain. AJR Am J Roentgenol 181:1255–1258
Shakir A, Ma V, Mehta B (2013) Comparison of pain score reduction using triamcinolone vs. dexamethasone in cervical transforaminal epidural steroid injections. Am J Phys Med Rehabil 92:768–775
Lee JW, Park KW, Chung SK et al (2009) Cervical transforaminal epidural steroid injection for the management of cervical radiculopathy: a comparative study of particulate versus non-particulate steroids. Skeletal Radiol 38:1077–1082
Dreyfuss P, Baker R, Bogduk N (2006) Comparative effectiveness of cervical transforaminal injections with particulate and nonparticulate corticosteroid preparations for cervical radicular pain. Pain Med 7:237–242
Bose B (2005) Quadriparesis following cervical epidural steroid injections: case report and review of the literature. Spine J 5:558–563
Hodler J, Boos N, Schubert M (2013) Must we discontinue selective cervical nerve root blocks? Report of two cases and review of the literature. Eur Spine J 22:S466–S470
Wallace MA, Fukui MB, Williams RL, Ku A, Baghai P (2007) Complications of cervical selective nerve root blocks performed with fluoroscopic guidance. AJR Am J Roentgenol 188:1218–1221
Rozin L, Rozin R, Koehler SA et al (2003) Death during transforaminal epidural steroid nerve root block (C7) due to perforation of the left vertebral artery. Am J Forensic Med Pathol 24:351–355
Ludwig MA, Burns SP (2005) Spinal cord infarction following cervical transforaminal epidural injection: a case report. Spine (Phila Pa 1976) 30:E266–E268
Meyer HJ, Monticelli F, Kiesslich J (2005) Fatal embolism of the anterior spinal artery after local cervical analgetic infiltration. Forensic Sci Int 149:115–119
Popescu A, Lai D, Lu A, Gardner K (2013) Stroke following epidural injections–case report and review of literature. J Neuroimaging 23:118–121
Ziai WC, Ardelt AA, Llinas RH (2006) Brainstem stroke following uncomplicated cervical epidural steroid injection. Arch Neurol 63:1643–1646
US Food and Drug Administration FDA (2011) FDA - US Food and Drug Administration. Shoulder. Kenalog-10 (triamcinolone acetonide) injection and Kenalog-40 (triamcinolone acetonide) injection. http://www.fda.gov/safety/medwatch/safetyinformation/ucm262876.htm. Accessed 15 Jan 2015
Wybier M, Gaudart S, Petrover D, Houdart E, Laredo JD (2010) Paraplegia complicating selective steroid injections of the lumbar spine. Report of five cases and review of the literature. Eur Radiol 20:181–189
Benzon HT, Chew TL, McCarthy RJ, Benzon HA, Walega DR (2007) Comparison of the particle sizes of different steroids and the effect of dilution: a review of the relative neurotoxicities of the steroids. Anesthesiology 106:331–338
Tiso RL, Cutler T, Catania JA, Whalen K (2004) Adverse central nervous system sequelae after selective transforaminal block: the role of corticosteroids. Spine J 4:468–474
Rathmell JP, Aprill C, Bogduk N (2004) Cervical transforaminal injection of steroids. Anesthesiology 100:1595–1600
Huntoon MA (2005) Anatomy of the cervical intervertebral foramina: vulnerable arteries and ischemic neurologic injuries after transforaminal epidural injections. Pain 117:104–111
Rathmell JP, Benzon HT (2004) Transforaminal injection of steroids: should we continue? Reg Anesth Pain Med 29:397–399
Lechmann M, Peterson CK, Pfirrmann CW, Hodler J (2013) Lumbar nerve root injections: a prospective cohort outcomes study comparing age- and gender-matched patients who returned an outcomes-based postal questionnaire with patients who did not return the postal questionnaire. Skeletal Radiol 42:1429–1435
Kremer S, Pfirrmann CW, Hodler J, Peterson CK (2012) Imaging-guided lumbar facet injections: is there a difference in outcomes between low back pain patients who remember to return a postal questionnaire and those who do not? Insights Imaging 3:411–418
Acknowledgments
The scientific guarantor of this publication is Susanne Bensler. The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
The authors state that this work has not received any funding. No complex statistical methods were necessary for this paper. Institutional review board approval was obtained. Written informed consent was obtained from all subjects (patients) in this study. Methodology: retrospective, diagnostic or prognostic study, performed at one institution.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bensler, S., Sutter, R., Pfirrmann, C.W.A. et al. Is there a difference in treatment outcomes between epidural injections with particulate versus non-particulate steroids?. Eur Radiol 27, 1505–1511 (2017). https://doi.org/10.1007/s00330-016-4498-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-016-4498-9