Abstract
Objective
To compare image quality and diagnostic validity of CBCT and MSCT for distal radius fractures.
Methods
35 CBCT and 33 MSCT scans were retrospectively reviewed with a visual grading scale regarding the depiction of cortical bone, trabecular bone, articular surfaces, and soft tissue. The extent and type of artefacts was analyzed. Agreement on AO classification and measurement of cortical disruption and length of the fracture gap was determined. Fracture reduction was evaluated in post-treatment x-rays. Statistical analysis was performed with visual grading characteristics (VGC), chi square tests, and Kendall’s coefficient of concordance.
Results
CBCT performed significantly worse for cortical bone, articular surfaces, and especially soft tissue. Trabecular bone showed no significant difference. Significantly more CBCT images showed artefacts. Physics-based artefacts were the most common. CBCT scans also showed motion artefacts. There was no significant difference in agreement on AO classification. The agreement on measurements was substantial for both modalities. Slightly more fractures that had undergone MSCT imaging showed adequate reduction.
Conclusion
This initial study of an orthopaedic extremity CBCT scanner showed that the image quality of a CBCT scanner remains inferior for most structures at standard settings. Diagnostic validity of both modalities for distal radius fractures seems similar.
Key Points
• Subjectively, CBCT remains inferior to MSCT in depicting most structures.
• Similar diagnostic validity for CBCT and MSCT imaging of distal radius fractures.
• CBCT is a possible alternative to MSCT in musculoskeletal imaging.
• Visual grading characteristics (VGC) analysis proves useful in analyzing visual grading scales.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Multislice CT (MSCT) is superior to conventional radiography in sensitivity for fractures [1, 2], assessment of intraarticular fractures [3, 4], and in the accuracy of fracture measurements [5, 6]. Zbijewski et al. [7] identified three factors that limit a broader application of MSCT as an alternative to conventional x-ray imaging in musculoskeletal (MSK) imaging: MSCT imaging does not allow for weight-bearing examinations, applies more radiation dose, is more costly, and has larger spatial demands [7]. Recently cone beam computed tomography (CBCT) has been suggested as an alternative to MSCT for orthopaedic imaging of the extremities. Dedicated CBCT scanners have the possibility to (at least partially) address these limitations of MSCT scanners.
CBCT, like MSCT, works with a rotating x-ray source and an opposing detector. The main difference between the two modalities lies in the detector. While an MSCT detector consists of many detector rows, CBCT uses a two-dimensional, flat-panel detector usually of amorphous silicon thin-film transistors. The opposing x-ray source emits a pulsating conical x-ray beam, which allows the scanner to take a series of two-dimensional projections from which software reconstructs images. Due to the differences in image acquisition, CBCT scanners have lesser demand for computational power. This, as well as the smaller set-up of CBCT scanners, makes them more mobile and less costly than full-body MSCT scanners [8–10].
While the application of dedicated CBCT scanners for MSK diagnostics is fairly new, CBCT imaging has long been used in other fields. Since the late 1990s, compact CBCT scanners have found use in almost every dental field [11, 12]. CBCT has also long been used in C-arm flat-panel computed tomography (FPCT) for intraoperative imaging and in image-guided radiotherapy (IGRT) [13–15]. In more recent times, CBCT scanners have found application in ear, nose, and throat (ENT) imaging, especially in the imaging of small, high-contrast structures such as facial bones and the middle ear [16, 17]. Currently C-arm FPCT arthrography is under investigation for detection of cartilage pathologies and has shown, in part, superiority to standard CT arthrography [18, 19]. The 2011 study of the prototype of an MSK-dedicated CBCT scanner showed promising results in terms of resolution and practicability [7]. A phantom study of an MSK-dedicated CBCT scanner showed higher spatial resolution for the CBCT scanner compared to an MSCT scanner [20]. The first clinical case studies of CBCT images of wrist fractures showed similar results for CBCT and MSCT in terms of spatial resolution, allowing for easy fracture detection [21]. CBCT scanners are especially promising for weight-bearing tomography. A comparison of weight-bearing CBCT scans with non-weight-bearing MSCT scans showed significant differences in measurements of the hind foot [22]. Unfortunately, exact dosage comparisons are not very straightforward as the CT dose index (CTDI) used to approximate the effective radiation dose of MSCT scans cannot directly be applied to CBCT scanners due to different beam geometry. To date, there is no widely accepted CTDI-like dose estimation for CBCT scanners [9, 13, 23, 24]. While the radiation doses reported vary, the consensus is that the radiation dose of CBCT scans is lower than that of standard MSCT protocols yet significantly higher than that of plain radiography [25–29].
In this study, we investigate the clinical use of an MSK-dedicated extremity CBCT scanner by comparing the depiction of several anatomical structures, the presence of artefacts, and the diagnostic validity of routine CBCT and MSCT scans of distal radius fractures.
Materials and methods
Patient selection
The study was approved by the local ethics committee. Informed consent was waived since the study was performed retrospectively with images from routine diagnostics. All scans were performed at the University Medical Center Freiburg and were independently analyzed by three radiologists of the department (residents with 2, 3, and 3 years of experience).
Eligible for inclusion were scans of acute radius fractures within 7 days of fracture. The CBCT scans included were performed between March and July 2012. The MSCT scans chosen had been acquired between March and July 2011. Scans from the same months of two consecutive years were chosen to obtain more homogenous patient populations since fracture populations vary depending on the season. In the respective time periods, 72 CBCT and 74 MSCT scans of distal radius fractures had been performed with the selected scanners. Excluded were any scans of patients that did not have a post-treatment x-ray exam or patients under the age of 18, and any MSCT scan not performed according to the standard protocol (see “Image Acquisition”). Also excluded were scans of patients that had suffered poly-trauma or that had external fixation at the time of tomographic imaging, as they would have undergone imaging in an MSCT scanner regardless of the availability of a CBCT scanner. This reduced the two groups to 35 CBCT and 33 MSCT scans. The patients of the groups were compared with respect to age, gender, and treatment.
Image acquisition
CBCT images were acquired with the orthopaedic extremity scanner Planmed Verity® (Planmed Oy, Helsinki, Finland). All scans were performed with the standard settings of 90 kV, 6.0 mA, and a slice thickness of 0.4 mm, with the exception of one scan, which was performed at 88 kV. This scan was included, nonetheless, due to the minimal deviation from standard settings. MSCT scans were performed with a Toshiba Aquilion One® scanner (Toshiba Medical Systems Corp., Otawara, Tochigi, Japan). The scans included had been performed with the standard protocol for wrist imaging at 120 kV, 40 mAs, and a slice thickness of 1.0 mm in volume acquisition mode with a 16-cm-wide detector. For image reconstruction, the standard of each scanner was used (iterative reconstruction for the MSCT and 3D-filtered back projection for the CBCT scanner). The mean radiation dose was calculated using the CTDIvol reported by each scanner.
Image processing
For better comparability, the 0.4-mm-thick CBCT scans were interpolated using Aquarius iNtuition® (TeraRecon Inc., Foster City, CA, USA) to obtain slices of the same thickness (1.0 mm) as the MSCT scans. The method was validated using 0.4-mm- and 1.0-mm-thick MSCT images of a hand phantom (Erler-Zimmer GmbH, Germany) acquired with different scanner settings. Image pairs of reconstructed and original 1.0-mm-thick MSCT scans were formed and two observers were asked to decide if 24 image pairs were of the same image quality (i.e. same scanner settings). With a sensitivity of 95.7 % and a specificity of 92.0 %, we assumed that this method of reconstruction did not significantly influence image quality.
Axial MSCT and reconstructed 1.0-mm-thick CBCT scans were anonymized and saved in randomized order as DICOM images with their corresponding post-treatment x-ray exams. Figure 1 shows images of CBCT and MSCT scans as presented to observers.
Image evaluation
The image analysis took place under standardized conditions with dimmed lighting on RadiForce GS320® (EIZO NANAO Corp., Hakusan, Ishikawa, Japan) clinical review monitors. Images showed no evidence of the date of examination or device settings. The initial contrast was set to 500/2000 for all scans; however, observers could adjust the contrast to their liking.
Prior to evaluation, observers were presented with sample images of the grading scale. The 3 observers were then asked to rate the 68 scans regarding the assessment of cortical bone, trabecular bone, articular surfaces, and soft tissue using a 5-category visual grading scale (see Table 1a). They also rated the extent and main type of artefact (see Table 1b). The extent of artefacts was determined by their influence on image assessment (i.e. if observers found the diagnosis of the fracture more difficult due to the artefacts). The types of artefacts were categorized by origin as by Barrett and Keat [30]. The observers also determined the American Orthopaedic (AO) classification (see Table 1c) and measured the cortical disruption and the length of the fracture gap. References for the AO classification and the types of artefacts were available. Post-treatment x-ray exams were used to evaluate the fracture reduction as a measure for treatment outcome (see Table 1d). Lastly, the AO classification was determined by an expert (board-certified radiologist with 20 years of experience) in consensus with a radiology resident (3 years of experience, not one of the observers) to be used as a reference standard.
Statistical analysis
While semi-quantitative, subjective image analysis is well-established as a clinically relevant method, there exists no single, indisputable method for the statistical analysis [31–33]. Often, statistical tests that require nominal data are applied, although data is ordinal and grading cannot simply be transformed into numerical values [31, 34, 35]. For the statistical analysis of the five-category visual grading characteristics (VGC) analysis, as previously described by Båth and Månsson [31], was used. As a non-parametric, rank-invariant method, VGC analysis respects these limitations. The method produces a VGC curve, which describes the relationship between two modalities and is quantified by the area under the curve (AUCVGC). A 45° diagonal VGC curve shows equality between two modalities with an AUCVGC of 0.5 [31, 32]. For graphing and curve fitting, we used OriginPro® (OriginLab Corp., Northampton, MA, USA). All other statistics were performed with R [36].
Results
The patients in the two groups were comparable with respect to age, gender, and treatment (see Table 2). The mean radiation dose was 3.81 mGy (σ = 0.19) for CBCT and 5.49 mGy (σ = 1.18) for MSCT scans. A subsequent analysis of the reference standard showed slightly more type C fractures in the CBCT group (74 % of total fractures in the CBCT group vs. 67 % in the MSCT group, see supplementary table).
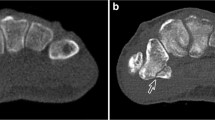
Table 3 and Fig. 2a–d show the AUCVGC of the anatomical structures investigated. A 95 % confidence interval excluding 0.5 shows a statistically significant difference in performance. Thus, the CBCT performed significantly worse in the assessment of cortical bone, articular surface, and, most notably, soft tissue. Figure 3 shows representative CBCT and MSCT images of scans with the best overall ratings. Figure 4 shows a CBCT scan with particularly poor soft tissue discrimination. There was no significant difference for trabecular bone (see Table 3 and Fig. 2a–d). The inter-rater agreement was substantial (Kendall's coefficient of concordance w = 0.605–0.676) [37].
Table 4 shows, quite apparently, that more CBCT scans were rated as having artefacts, as confirmed by Pearson's chi square test. Figure 5 shows the types of artefacts contributing to the ratings in Table 4 (percent of total ratings for each modality). The vast majority of CBCT scans were rated as having artefacts. Some of these artefacts influenced the image assessment of the CBCT scans, though diagnosis of the fracture was possible in all scans. Beam-hardening artefacts, followed by motion artefacts, were the most common in CBCT scans (see Figs. 6 and 7).
Extent and cause of artefacts: More CBCT scans showed artefacts than MSCT scans and artefacts were most often physics-based artefacts in both modalities with motion artefacts also being very common in CBCT scans. (Non-relevant artefacts = artefacts without influence on image assessment; Relevant artefacts = artefacts with influence on image assessment)
There was no significant difference in the agreement on AO classification for the two modalities (see Table 5a–b). The chi square test of inter-rater agreement for the measurements of fractures was substantial [37] for both scanners with Kendall’s coefficient of concordance w being 0.767 and 0.653 for the measurements of cortical disruption, and 0.722 and 0.758 for the measurements of the length of the fracture gap for CBCT and MSCT scans, respectively.
There was a statistically significant difference between the CBCT and MSCT groups regarding the treatment outcome. More fractures that had undergone MSCT imaging showed adequate reduction (see Table 6).
Discussion
In this clinical study, MSCT was superior to orthopaedic CBCT in the depiction of cortical bone, articular surfaces, and soft tissue. Artefacts were much more frequent among CBCT scans than MSCT scans with beam-hardening and motion artefacts being the most common types of artefacts. There was no significant difference between CBCT and MSCT regarding the agreement on AO classification and the agreement on fracture measurements was similar for both scanners.
In general, our study showed differences in the image quality in favour of the MSCT, most notably regarding the depiction of soft tissue. Due to the lesser importance of soft tissue depiction for fracture diagnosis, we do not see this as problematic for select orthopaedic applications for CBCT such as fracture detection. The difference between the two modalities for the depiction of cortical bone and articular surfaces was not as great as for soft tissue, but the CBCT scanner still performed significantly worse than the MSCT scanner. However, the equivalent agreement of raters on the AO classification and fracture measurements led us to the conclusion that both scanners are of similar diagnostic value for assessment of distal radius fractures. The depiction of trabecular bone showed no significant difference. Physics-based artefacts were much more common among CBCT scans. To minimize these artefacts, better correction algorithms or iterative methods for CBCT scanners would be helpful. As for the motion artefacts in the CBCT scans, we feel that adequate patient selection and an additional stabilization can sufficiently reduce motion artefacts. The evaluation of the post-treatment x-rays showed that more fractures that had undergone MSCT imaging had adequate reduction. The purpose of this evaluation was to uncover grave differences in treatment outcome, which could point to inadequate treatment planning on the basis of CBCT. Although there was a slight difference between the two groups, we do not see this as evidence for subpar treatment planning with CBCT scans. The subsequent analysis of the CBCT and MSCT groups using the reference standard showed slightly more type C fractures in the CBCT group. Due to the complexity and instability of type C fractures, we see this as a more probable explanation for the slight difference in treatment outcome.
As mentioned in the introduction, the use of MSK-dedicated CBCT scanners is fairly new and most experience comes from dedicated CBCT scanners in dental and ENT imaging. Poor depiction of soft tissue has been shown for many different CBCT scanners [8, 12, 38, 39] and is attributed to the lesser contrast resolution of CBCT scanners compared to MSCT scanners [9]. For ENT scanners, the poor soft tissue resolution is not seen as problematic for diagnosis of high-contrast pathologies in the temporal bone [40] and for image-guided surgery of the frontal recess [41]. In a recent study, the diagnostic quality of an MSK-dedicated CBCT scanner for soft tissue was rated as adequate although, as in our study, the CBCT performed significantly worse than the MSCT for soft tissue tasks [42]. The direct depiction of trabecular structure due to the high spatial resolution has been praised for ENT CBCT scanners [40]. Due to the detailed depiction of trabecular bone, CBCT scanners have been used in a method for bone density measurements in studies on anorexia nervosa [43, 44]. The study verifying this method found both the C-arm FPCT and the CBCT scanners investigated to be superior to the MSCT in the qualitative assessment of trabecular bone [45]. However, other results are more divergent: The comparison of five dental CBCT scanners with an MSCT scanner found only one CBCT scanner to be superior and the other four CBCT scanners inferior in the depiction of trabecular bone in the imaging of a dry mandible [45, 46]. As in our study, previous investigations of dental CBCT scanners and FPCT scanners found MSCT superior in depicting cortical bone [47, 48]. The aforementioned study of an MSK-dedicated CBCT scanner showed no significant difference in observer ratings between CBCT and MSCT for bone tasks in the hand, though the CBCT scanner performed significantly better than the MSCT for bone tasks in the knee [42]. As for articular surfaces, CBCT scanners have been found superior for the small articulations of the middle ear [49] and comparable to MSCT scanners in the diagnosis of temporomandibular joint pathologies [50].
The fact that the CBCT scans showed more artefacts was not surprising. The difference in occurrence and magnitude of physics-based artefacts between MSCT and CBCT scanners is attributed to better scatter and beam-hardening corrections of MSCT scanners as well as the iterative reconstruction in MSCT scanners [23, 30, 51]. A study of an MSK-dedicated CBCT prototype in 2011 described such cupping artefacts due to beam-hardening artefacts as possibly problematic [7], while the first clinical case studies of a CBCT scanner for wrist imaging reported only few beam-hardening artefacts [21]. An in-vitro phantom study of the same CBCT scanner as used in our study found cupping artefacts to contribute to the significantly lower homogeneity compared to an MSCT scanner [20]. Previous studies of dental CBCT scanners also found more beam-hardening artefacts in CBCT images than in MSCT images [23, 38]. Better correction algorithms and iterative methods for CBCT are currently being investigated to reduce such beam-hardening artefacts [52–54]. In our study, the CBCT scans also showed more motion artefacts. CBCT imaging is vulnerable to motion artefacts due to long scanning times [7, 21, 25] (here, the CBCT took 18 seconds, while the comparative MSCT took less than 1 second). Additionally, CBCT scanners have been reported to be particularly susceptible to motion artefacts due to their high spatial resolution [55, 56]. The differences in scanning times are due to the relatively slow cesium iodide scintillators in flat-panel detectors compared to the faster ceramic detectors in MSCT detectors [9, 40]. However, we do not see motion artefacts as being problematic if patients are carefully selected. For small children and stressed or non-compliant patients, MSCT imaging might be preferable. This was discussed in the aforementioned case studies of CBCT for wrist imaging, where fixation of the wrist was used to reduce motion artefacts [21]. In our study no fixation was used, though the scanner would likely profit from an incorporated fixation belt.
The agreement on AO classification and fracture measurements speak to a similarly reliable diagnosis and pre-operative planning with the CBCT scanner. The impact of the imaging modality on patient management could not be investigated due to differing patient populations in the two groups. In a previous comparison of CBCT and MSCT imaging for interphalangeal fractures, the CBCT scanner was found to have 100 % sensitivity and specificity for the detection of articular involvement and there was no significant difference between modalities in the number of bone fragments detected [57]. Technical studies from various applications have shown CBCT scanners to be comparable to MSCT in geometric accuracy, making them well-suited for pre-operative planning [20, 58–60].
Despite the superiority of the MSCT scanner for some anatomical structures and the fewer artefacts, we find CBCT well-suited to orthopaedic imaging when the limitations of soft tissue resolution and the susceptibility to motion artefacts are respected. We feel that MSK-dedicated scanners are a feasible alternative to MSCT imaging for fractures of the extremities in terms of fracture detection and treatment planning.
Limitations
In this study, we compared standard protocols of an orthopaedic CBCT extremity scanner and an MSCT scanner. The results are limited to this specific comparison of scanners and protocols. The influence of different scanner settings was not investigated. According to the CTDIvol calculated by the scanners, the mean radiation dose of the MSCT scans was greater than that of the CBCT scans. This is meant as an approximation of the radiation doses applied. The difficulty of reliable dose measurements for CBCT scans using the CTDIvol was discussed in the introduction. As there is no well-established alternative to the CTDI, more precise comparisons of the radiation dose of CBCT and MSCT protocols would entail dose measurements in phantoms or Monte Carlo simulations, which were not part of our study. Also the influence of the different reconstruction processes (3D filtered back projection in the CBCT and iterative reconstruction in the MSCT) was not investigated. 3D reconstructions are possible with CBCT scans as they are with MSCT scans. The quality of such reconstructions was not investigated in this study. This study was performed retrospectively with different patient populations in the CBCT and MSCT groups. It was not justifiable to examine patients with both scanners for this initial study due to the additional radiation exposure. The purpose of the inclusion and exclusion criteria was to create homogenous patient populations. The two groups proved comparable for several patient factors, so that we assume that any measurable difference in image quality is the result of the imaging modality and not due to confounding factors.
Conclusion
This initial study of an MSK-dedicated CBCT scanner showed that the image quality of an MSCT scanner remains superior for most anatomical structures when using standard protocols. CBCT scans have significantly more artefacts. However, the diagnostic validity of this CBCT scanner seems similar to that of the MSCT scanner for distal radius fractures. CBCT is a viable alternative to MSCT imaging for select orthopaedic imaging. Further studies of other orthopaedic applications and radiation dose comparisons are needed.
References
Fotiadou A, Patel A, Morgan T, Karantanas AH (2011) Wrist injuries in young adults: the diagnostic impact of CT and MRI. Eur J Radiol 77(2):235–239
Welling RD, Jacobson JA, Jamadar DA, Chong S, Cavoili EM, Jebson PJ (2008) MDCT and radiography of wrist fractures: radiographic sensitivity and fracture patterns. AJR Am J Roentgenol 190(1):10–16
Heo YM, Roh J-Y, Kim S-B et al (2012) Evaluation of the sigmoid notch involvement in the intra-articular distal radius fractures: the efficacy of computed tomography compared with plain X-ray. Clin Orthop Surg 4(1):83–90
Pruitt DL, Gilula LA, Manske PR, Vannier MW (1994) Computed tomography scanning with image reconstruction in evaluation of distal radius fractures. J Hand Surg 19(5):720–727
Rozental TD, Bozentka DJ, Katz MA, Steinberg DR, Beredjiklian PK (2001) Evaluation of the sigmoid notch with computed tomography following intra-articular distal radius fracture. J Hand Surg 26(2):244–251
Cole RJ, Bindra RR, Evanoff BA, Gilula LA, Yamaguchi K, Gelberman RH (1997) Radiographic evaluation of osseous displacement following intra-articular fractures of the distal radius: reliability of plain radiography versus computed tomography. J Hand Surg 22(5):792–800
Zbijewski W, De Jean P, Prakash P et al (2011) A dedicated cone-beam CT system for musculoskeletal extremities imaging: design, optimization, and initial performance characterization. Med Phys 38(8):4700–4713
Dawood A, Patel S, Brown J (2009) Cone beam CT in dental practice. Br Dent J 207(1):23–28
Miracle AC, Mukherji SK (2009) Conebeam CT of the head and neck, part 1: physical principles. AJNR Am J Neuroradiol 30(6):1088–1095
Scarfe WC, Farman AG (2008) What is cone-beam CT and how does it work? Dent Clin North Am 52(4):707–730
Koong B (2010) Cone beam imaging: is this the ultimate imaging modality? Clin Oral Implant Res 21(11):1201–1208
Miracle AC, Mukherji SK (2009) Conebeam CT of the head and neck, part 2: clinical applications. AJNR Am J Neuroradiol 30(7):1285–1292
Orth RC, Wallace MJ, Kuo MD (2008) C-arm cone-beam CT: general principles and technical considerations for use in interventional radiology. J Vasc Interv Radiol 19(6):814–820
Kalender WA, Kyriakou Y (2007) Flat-detector computed tomography (FD-CT). Eur Radiol 17:2767–2779
Jaffray DA, Siewerdsen JH, Wong JW, Martinez AA (2002) Flat-panel cone-beam computed tomography for image-guided radiation therapy. Int J Radiat Oncol Biol Phys 53:1337–1349
Peltonen LI, Aarnisalo AA, Kortesniemi MK, Suomalainen A, Jero J, Robinson S (2007) Limited cone-beam computed tomography imaging of the middle ear: a comparison with multislice helical computed tomography. Acta Radiol 48(2):207–212
Dalchow CV, Weber AL, Yanagihara N, Bien S, Werner JA (2006) Digital volume tomography: radiologic examinations of the temporal bone. AJR Am J Roentgenol 186(2):416–423
Guggenberger R, Morsbach F, Alkadhi H et al (2013) C-arm flat-panel CT arthrography of the wrist and elbow: first experiences in human cadavers. Skelet Radiol 42(3):419–429
Guggenberger R, Winklhofer S, Spiczak JV, Andreisek G, Alkadhi H (2013) In vitro high-resolution flat-panel computed tomographic arthrography for artificial cartilage defect detection: comparison with multidetector computed tomography. Investig Radiol 48(8):614–621
Neubauer J, Voigt JM, Lang H et al (2014) Comparing the Image Quality of a Mobile Flat-Panel Computed Tomography and a Multidetector Computed Tomography: A Phantom Study. Investig Radiol 49(7):491–497
De Cock J, Mermuys K, Goubau J, Petegem S, Houthoofd B, Casselman JW (2012) Cone-beam computed tomography: a new low dose, high resolution imaging technique of the wrist, presentation of three cases with technique. Skelet Radiol 41(1):93–96
Hirschmann A, Pfirrmann CW, Klammer G, Espinosa N, Buck FM (2014) Upright Cone CT of the hindfoot: Comparison of the non-weight-bearing with the upright weight-bearing position. Eur Radiol 24(3):553–558
Yu L, Vrieze TJ, Bruesewitz MR et al (2010) Dose and Image Quality Evaluation of a Dedicated Cone-Beam CT System for High-Contrast Neurologic Applications. Am J Roentgenol 194(2):193–201
Pauwels R, Theodorakou C, Walker A et al (2012) Dose distribution for dental cone beam CT and its implication for defining a dose index. Dento Maxillo Facial Radiol 41(7):583–593
Coppenrath E, Draenert F, Lechel U et al (2008) Cross-sectional imaging in dentomaxillofacial diagnostics: dose comparison of dental MSCT and NewTom 9000 DVT. RöFo 180(5):396–401
Roberts JA, Drage NA, Davies J, Thomas DW (2009) Effective dose from cone beam CT examinations in dentistry. Br J Radiol 82:35–40
Ludlow JB, Davies-Ludlow LE, Brooks SL (2003) Dosimetry of two extraoral direct digital imaging devices: new Tom cone beam CT and Orthophos Plus DS panoramic unit. Dento Maxillo Facial Radiol 32(4):229–234
Loubele M, Bogaerts R, van Dijck E et al (2009) Comparison between effective radiation dose of CBCT and MSCT scanners for dentomaxillofacial applications. Eur J Radiol 71(3):461–468
Silva MA, Wolf U, Heinicke F, Bumann A, Visser H, Hirsch E (2008) Cone-beam computed tomography for routine orthodontic treatment planning: a radiation dose evaluation. Am J Orthod Dentofac Orthop 133(5):640, e1–e5
Barrett JF, Keat N (2004) Artifacts in CT: recognition and avoidance. Radiographics 24(6):1679–1691
Bath M, Mansson LG (2007) Visual grading characteristics (VGC) analysis: a non-parametric rank-invariant statistical method for image quality evaluation. Br J Radiol 80:169–176
Ludewig E, Richter A, Frame M (2010) Diagnostic imaging--evaluating image quality using visual grading characteristic (VGC) analysis. Vet Res Commun 34(5):473–479
Smedby O, Fredrikson M (2010) Visual grading regression: analysing data from visual grading experiments with regression models. Br J Radiol 83:767–775
Pittayapat P, Galiti D, Huang Y et al (2013) An in vitro comparison of subjective image quality of panoramic views acquired via 2D or 3D imaging. Clin Oral Investig 17(1):293–300
Burmeister HP, Baltzer PAT, Möslein C et al (2011) Visual grading characteristics (VGC) analysis of diagnostic image quality for high resolution 3 Tesla MRI volumetry of the olfactory bulb. Acad Radiol 18(5):634–639
R Core Team (2014) R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. URL http://www.R-project.org/
Landis JR, Koch GG (1977) The measurement of observer agreement for categorical data. Biometrics 33(1):159–174
Kyriakou Y, Kolditz D, Langner O, Krause J, Kalender W (2011) Digital volume tomography (DVT) and multislice spiral CT (MSCT): an objective examination of dose and image quality. RöFo 183(2):144–153
Fakhran S, Alhilali L, Sreedher G et al (2014) Comparison of simulated cone beam computed tomography to conventional helical computed tomography for imaging of rhinosinusitis. Laryngoscope 124(9):2002–2006
Gupta R, Grasruck M, Suess C et al (2006) Ultra-high resolution flat-panel volume CT: fundamental principles, design architecture, and system characterization. Eur Radiol 16(6):1191–1205
Rafferty MA, Siewerdsen JH, Chan Y et al (2005) Investigation of C-arm cone-beam CT-guided surgery of the frontal recess. Laryngoscope 115(12):2138–2143
Demehri S, Muhit A, Zbijewski W et al (2015) Assessment of image quality in soft tissue and bone visualization tasks for a dedicated extremity cone-beam CT system. Eur Radiol 25(6):1742–1751
Walsh CJ, Phan CM, Misra M et al (2010) Women with anorexia nervosa: finite element and trabecular structure analysis by using flat-panel volume CT. Radiology 257:167–174
Bredella MA, Misra M, Miller KK et al (2008) Distal radius in adolescent girls with anorexia nervosa: trabecular structure analysis with high-resolution flat-panel volume CT. Radiology 249:938–946
Liang X, Jacobs R, Hassan B et al (2010) A comparative evaluation of Cone Beam Computed Tomography (CBCT) and Multi-Slice CT (MSCT). Eur J Radiol 75(2):265–269
Phan CM, Macklin EA, Bredella MA et al (2011) Trabecular structure analysis using C-arm CT: comparison with MDCT and flat-panel volume CT. Skelet Radiol 40(8):1065–1072
Loubele M, Guerrero ME, Jacobs R, Suetens P, van Steenberghe D (2007) A comparison of jaw dimensional and quality assessments of bone characteristics with cone-beam CT, spiral tomography, and multi-slice spiral CT. Int J Oral Maxillofac Implant 22(3):446–454
Stuby F, Seethaler AC, Shiozawa T et al (2011) Evaluation of image quality of two different three-dimensional cone-beam-scanners used for orthopedic surgery in the bony structures of the pelvis in comparison with standard CT scans. Z Orthop Unfall 149(6):659–667
Dahmani-Causse M, Marx M, Deguine O, Fraysse B, Lepage B, Escude B (2011) Morphologic examination of the temporal bone by cone beam computed tomography: comparison with multislice helical computed tomography. Eur Ann Otorhinolaryngol Head Neck Dis 128(5):230–235
Zain-Alabdeen EH, Alsadhan RI (2012) A comparative study of accuracy of detection of surface osseous changes in the temporomandibular joint using multidetector CT and cone beam CT. Dento Maxillo Facial Radiol 41(3):185–191
Agten CA, Sutter R, Pfirrmann CWA (2014) CT and MRI of hip arthroderplasty. Radiologe 54(7):715–725
Wang AS, Stayman JW, Otake Y et al (2014) Soft-tissue imaging with C-arm cone-beam CT using statistical reconstruction. Phys Med Biol 59(4):1005–1026
Zbijewski W, Gang GJ, Xu J et al (2014) Dual-energy cone-beam CT with a flat-panel detector: effect of reconstruction algorithm on material classification. Med Phys 41(2):021908
Makeev A, Glick SJ (2013) Investigation of statistical iterative reconstruction for dedicated breast CT. Med Phys 40(8):081904
Schulze R, Heil U, Gross D et al (2011) Artefacts in CBCT: a review. Dento Maxillo Facial Radiol 40(5):265–273
Jaju PP, Jain M, Singh A, Gupta A (2013) Artefacts in cone beam CT. Open J Stomatol 3(5):292–297
Faccioli N, Foti G, Barillari M, Atzei A, Mucelli RP (2010) Finger fractures imaging: accuracy of cone-beam computed tomography and multislice computed tomography. Skelet Radiol 39(11):1087–1095
Suomalainen A, Kiljunen T, Käser Y, Peltola J, Kortesniemi M (2009) Dosimetry and image quality of four dental cone beam computed tomography scanners compared with multislice computed tomography scanners. Dento Maxillo Facial Radiol 38(6):367–378
Loubele M, van Assche N, Carpentier K et al (2008) Comparative localized linear accuracy of small-field cone-beam CT and multislice CT for alveolar bone measurements. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 105(4):512–518
Kobayashi K, Shimoda S, Nakagawa Y, Yamamoto A (2004) Accuracy in measurement of distance using limited cone-beam computerized tomography. Int J Oral Maxillofac Implant 19(2):228–231
Acknowledgments
The scientific guarantor of this publication is Elmar Kotter, M.D., Prof. of Radiology; Vice chairman, Dept. of Radiology, University Medical Center Freiburg, Germany. The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article. The authors state that this work has not received any funding.
Gerta Rücker, PhD, of the Institute of Medical Biometry and Medical Statistics, University Medical Center Freiburg, and Pau Jorba, M.Sc., of the Physics Department E21, TU Munich, kindly provided assistance for the statistical analysis. Institutional review board approval was obtained from the ethics committee of the University of Freiburg, Germany. Written informed consent was not required for this study as it was a retrospective study of medical imaging obtained in routine diagnostics. No study subjects or cohorts have been previously reported. Methodology:
Retrospective, diagnostic or prognostic study, performed at one institution.
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article is available at http://dx.doi.org/10.1007/s00330-016-4371-x.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
Supplementary Table: Frequency of AO classification fracture types in CBCT and MSCT groups (5AO classification according to reference standard) (PNG 12 kb)
Rights and permissions
About this article
Cite this article
Lang, H., Neubauer, J., Fritz, B. et al. A retrospective, semi-quantitative image quality analysis of cone beam computed tomography (CBCT) and MSCT in the diagnosis of distal radius fractures. Eur Radiol 26, 4551–4561 (2016). https://doi.org/10.1007/s00330-016-4321-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-016-4321-7