Abstract
Objectives
Compare variability in flow measurements by phase contrast MRI, performed at different locations in the aorta and pulmonary artery (PA) using breath-held (BH) and free-breathing (FB) sequences.
Methods
Fifty-seven patients with valvular heart disease, confirmed by echocardiography, were scanned using BH technique at 3 locations in the ascending aorta (SOV = sinus of Valsalva, STJ = sinotubular junction, ASC = ascending aorta at level of right pulmonary artery) and 2 locations in PA. Single FB measurement was obtained at STJ for aorta. Obtained metrics (SV = stroke volume, FV = forward volume, BV = backward volume, RF = regurgitant fraction) were evaluated separately for patients with aortic regurgitation (AR, n = 31) and mitral regurgitation (n = 26).
Results
No difference was noted between the two measurements in the PA. Significant differences were noted in measured SV at different aortic locations. SV measurements obtained at ASC correlated best with the measurements obtained in the PA. Strongest correlation of AR was measured at the STJ.
Conclusion
Measurements of flow volumes by phase contrast MRI differ depending on slice location. When using stroke volumes to calculate pulmonary to systemic blood flow ratio (Qp/Qs), ASC should be used. For quantifying aortic regurgitation, measurement should be obtained at STJ.
Key Points
• Aortic regurgitation can be accurately measured by MRI.
• Aortic regurgitation measurement by MRI varies according to the location where measured.
• Aortic regurgitation can also be measured by MRI without breath hold.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cardiovascular magnetic resonance (CMR) is a uniquely versatile modality for the assessment of valvular heart disease [1]. It has the advantages of no ionizing radiation and unrestricted anatomical access. As a noninvasive method, CMR has emerged as the reference standard for quantification of ventricular volumes [2], and it can similarly evaluate valvular structure and function [3]. Phase contrast CMR flow imaging (also termed velocity-encoded CMR, Q-flow or velocity mapping) is a quantitative technique to measure flow volumes through vessels. It provides more detailed information about the spatial distribution of velocities compared to echocardiography, and it offers the capability of direct quantification of blood volumes through heart valves [4]. Accurate flow measurements allow quantitation of intracardiac shunts and valvular regurgitation. CMR quantitation of aortic regurgitation (AR) has been shown to be more reproducible than echocardiography [5], and it may help in early identification of patients with AR in whom surgery could be beneficial [6].
AR by CMR has been assessed in various locations, namely the sinus of Valsalva [7], above the sinotubular junction [8] and ascending aorta [9]. It has been measured using both free-breathing techniques [4, 7, 10–13] and breath-hold techniques [14, 15]. The variability in obtained measurements as well as the interchangeability of these locations and respiratory techniques in a cohort of patients with varying severity of valvular heart disease is not known. The primary hypothesis was that measured stroke volume and regurgitant fraction would vary in the same patient depending on the distance from the aortic valve. Identifying the optimal site for aortic flow measurements has direct relevance to clinical practice in terms of how to position phase contrast flow acquisitions for evaluation of AR severity.
Material and methods
The study group consisted of 31 patients with AR and 26 patients with mitral regurgitation enrolled from January 2008 to July 2010 in a prospective study of valvular heart disease [5]. The present data represent a prespecified substudy of the previous work [5], for which the CMR protocol was created to test the above hypothesis, incorporating multiple phase contrast acquisitions to evaluate the effect of slice position on measured flow. Thus, the group of patients with pure mitral valve disease and normal aortic valve function were used as controls and compared to patients with clinically significant AR. Exclusion criteria were greater than mild regurgitation of a second valve, atrial fibrillation at the time of imaging, extreme claustrophobia and non-CMR-compatible implanted devices. The institutional review board approved the study and all patients provided written informed consent. Other imaging data on these patients have been previously reported [5].
All scanning was performed on a 1.5-Tesla MR (Achieva, Philips Medical, Best, the Netherlands). A standard body coil was used for radio-frequency transmission, and a dedicated five-channel phased array cardiac receiver coil was used for signal reception.
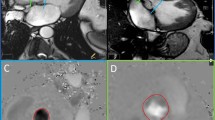
Steady state free precession (SSFP) cine images (TE 1.7 ms, TR 3.4 ms, flip angle 55°, cardiac phases 25, sense factor 2, retrospective triggering, slice thickness 6 mm, FOV 360 mm, matrix 160 × 160, acquired voxel size 2.3 × 2.3 × 6 mm) were obtained in the left ventricle outflow tract (LVOT), coronal aorta (CorAo), right ventricular outflow tract (RVOT) and the coronal plane of the pulmonary artery (CorPA). Cine SSFP stacks were obtained in the short axis (SAX) and right ventricle horizontal long axis (RHLA) plane for functional analysis of left and right ventricle respectively (two slices per breath hold, TE 1.7 ms, TR 3.4 ms, flip angle 55°, cardiac phases 25, sense factor 2, retrospective triggering, slice thickness 6 mm with gap 4 mm, FOV 360 mm, matrix 160 × 160, acquired voxel size 2.3 × 2.3 × 6 mm). By planning from the two orthogonal planes CorAo and LVOT, single slice 2D phase contrast flow images were obtained at the sinuses of Valsalva just below the coronary ostia (SOV), at the sinotubular junction (STJ) and in the tubular portion of the ascending aorta approximately 1 cm above the sinotubular junction at the approximate level of the right pulmonary artery (ASC) (Fig. 1a).
Slice positions of phase contrast acquisitions. a SSFP coronal aortic view at end-diastole using a cross-plane localizer to show the positions of SOV, STJ and ASC slice planes. b SSFP systolic phase demonstrating through-plane movement (basal descent) of the aorta during ventricular contraction (b, dotted arrow). c SSFP through the pulmonary artery, showing the two sites of phase contrast flow mapping
Each of these scans was obtained using a single end expiration breath hold with retrospective ECG gating and 45 phases per R–R interval. A single slice was also obtained in the tubular ascending aorta 1 cm above the sinotubular junction (same location as ASC, termed FB), using a non-navigated free-breathing technique. Two breath-hold phase contrast slices were obtained in the pulmonary artery (PA) by referencing from the RVOT and CorPA planes, namely at the sinus just distal to the pulmonic valve, and 1 cm distal to this (Fig. 1c). A maximum velocity (v enc) was encoded into the sequence, starting at 200 cm/s. If aliasing occurred, the maximum velocity was increased by 50 cm/s and the sequence repeated until aliasing did not occur. Scan sequence parameters used are summarized in Table 1.
Analysis was performed using commercially available software on a dedicated CMR workstation (View Forum, Philips Medical, Best, the Netherlands). For each case, the aortic lumen was manually traced (45 phases at each slice location) in a blinded fashion by two expert reviewers (AC, CHC). The inter- and intraobserver variability of these measurements has been previously reported, with excellent agreement between observers (r = 0.99) [5]. Left and right ventricle stroke volumes were calculated using short axis SSFP stack as previously published.
The obtained metrics (listed below) were evaluated separately for patients with isolated AR (n = 31), patients with isolated mitral regurgitation (n = 26) and then in combination (both patient subgroups; AR + MR, n = 57):
-
SV = stroke volume
-
FVol = forward volume
-
RVol = regurgitant volume
-
RF = regurgitant fraction
Statistics
Differences between metrics based on location were tested for statistical significance using paired t tests. Differences in classification of regurgitant fraction (mild, <30 %; moderate, 30–49 %; severe, ≥50 %) were tested for significance using the Wilcoxon matched-pairs signed rank test. A p value of less than 0.05 was considered statistically significant (two-sided). The correlation between metrics at different locations was assessed on the basis of Bland–Altman plots. Equivalence testing for regurgitant fraction was performed by determining the mean and 95 % confidence intervals of the difference in regurgitant fraction at different locations. An equivalence margin of 5 % was set, and two locations were deemed equivalent if the mean and 95 % confidence intervals of the difference in regurgitant fraction fell entirely within the equivalence margin.
Statistical analyses were performed using GraphPad Prism 6 (GraphPad Software, Inc., La Jolla, CA).
Results
Phase contrast flow images were successfully obtained in all 57 patients. Images were of diagnostic quality in all but one free-breathing sequence, which was excluded. Summaries of all metrics are presented in Table 2, and comparisons between variables in Table 3.
Analysis of pulmonary net stroke volumes revealed no significant difference between the two sites within the pulmonary artery (p = 0.57), with values of 91.7 ± 22.6 ml and 92.5 ± 22.0 ml at the proximal vs. distal sites respectively.
When compared to pulmonic sites, aortic stroke volume was found to be significantly different at all sites: SOV vs. PA proximal (p < 0.0001); STJ vs. PA proximal (p < 0.0001); ASC vs. PA proximal (p = 0.033); SOV vs. PA distal (p < 0.0001); STJ vs. PA distal (p < 0.0001); ASC vs. PA distal (p = 0.027).
For patients with isolated AR, correlations emerged between aortic and pulmonary stroke volume: SOV vs. PA proximal (r = 0.68); STJ vs. PA proximal (r = 0.66); ASC vs. PA proximal (r = 0.75). The strongest correlation between pulmonary and aortic stroke volume was found at the ascending aorta (ASC). When all patients were grouped, the correlations of SOV vs. PA proximal (r = 0.70) and ASC vs. PA proximal (r = 0.69) were strongest.
Statistically significant differences were noted between the stroke volumes obtained at the three sites within the proximal aorta: SOV (78.4 ± 18.0 ml) vs. STJ (81.7 ± 18.3 ml) (p = 0.005); SOV vs. ASC (87.1 ± 17.5 ml) (p < 0.0001); STJ vs. ASC (p = 0.0008). Box and whiskers plots for all measurements of stroke volume are shown in Fig. 2. The variability between measurements made at different locations is shown in the Bland–Altman plots in Fig. 3.
A statistically significant difference in stroke volume was found between breath-hold measurements at SOV vs. free-breathing measurements (84.5 ± 18.3 ml) (p = 0.0097). No significant difference was found between breath-hold STJ vs. FB (p = 0.34) or breath-hold ASC vs. FB (p = 0.23).
Forward volume increased at distal sites of measurement (Fig. 4), with significant differences found for SOV (110.0 ± 39.2 ml) vs. STJ (112.0 ± 39.2 ml) (p = 0.0036) and ASC (112.1 ± 39.2 ml) vs. FB (108.2 ± 36.4 ml) (p = 0.02). No significant differences were found for SOV vs. ASC (p = 0.065); SOV vs. FB (p = 0.49); STJ vs. ASC (p = 0.95); STJ vs. FB (p = 0.055).
Regurgitant fraction
RF was significantly reduced at sites progressively distal to the sinus of Valsalva (Fig. 5): SOV (23.1 ± 19.5 %) vs. STJ (20.5 ± 19.4 %) (p = 0.0003); SOV vs. ASC (17.8 ± 17.3 %) (p < 0.0001); SOV vs. FB (17.6 ± 18.3 %) (p < 0.0001). The variability between measurements made at different locations is shown in the Bland–Altman plots in Fig. 6. The aortic regurgitant volume is significantly lower at each of the four sites compared to LVSV–RVSV (left ventricle stroke volume–right ventricle stroke volume) as presented in Table 4. The strongest correlation is for STJ vs. LVSV–RVSV (r = 0.93).
Breath-hold vs free breathing
RF for breath-hold measurements vs. free breathing was found to be significantly different for SOV vs. FB (p < 0.0001) and STJ vs. FB (p = 0.0017), while no significance was found between ASC vs. FB (same slice position, p = 0.32). Equivalence testing was performed to check for equivalence between breath-hold measurements and free breathing, as shown in Fig. 7. Measurements were found to be equivalent for STJ vs. FB and ASC vs. FB.
Discussion
Accurate quantitation of chronic AR is a prerequisite for optimal timing of surgical intervention [14]. Patients with severe AR are at risk of heart failure and increased mortality unless valve replacement is performed before irreversible LV systolic dysfunction has occurred [7, 16]. In contrast, patients with mild to moderate AR rarely develop LV dilation or symptoms of heart failure; thus, reliable quantitation is critical for informed clinical decision-making. In addition patients with bicuspid aortic valve with anterior-posterior orientation of cusps or raphe tend to have moderate to severe aortic regurgitation [17]. Regurgitant fraction (RF) is a metric which represents a direct index of valve incompetency [11], and it has been shown to be a strong prognostic indicator in patients with AR [18, 19]. Doppler echocardiography is the most common method for quantitating AR severity, but it is limited by the requirements for a good acoustic window and the need for no regurgitation in the other cardiac valves [14]; the proximal isovelocity surface area method is rarely technically possible for aortic regurgitation, and subjective grading of chronic AR using a multiparametric approach has been found to have suboptimal interobserver consistency [20]. These limitations result in reduced echocardiographic reproducibility for the quantitation of AR [3, 5], whereas CMR has been shown to be more reproducible than echocardiography for both left and right ventricular volumes, as well as valvular quantitation [5]. Severity of AR may also be assessed by planimetry of the anatomic orifice area using CMR or retrospectively gated cardiac CT [21].
In the present study we compared AR flow metrics at three proximal aortic locations using a breath-hold technique and a single location using a free-breathing technique (as many sites use free-breathing, rather than breath-hold acquisitions). Our data confirm that phase contrast flow quantitation in the aorta is dependent on slice positioning, with a significant increase in net stroke volume and forward flow, and a significant decrease in backward flow and calculated regurgitant fraction, when measuring at sites progressively distal to the aortic valve. This remains true for both patients with isolated AR as well as patients with MR (acting as controls). This is an important observation when considering the accuracy and reproducibility of flow measurements, as genuine changes in valvular regurgitation are an important factor in determining the optimal timing of valve replacement.
There are several potential explanations for the effects of CMR slice location on quantitating aortic regurgitation. First, the aorta is an elastic structure that moves and changes shape during the cardiac cycle. Changes in aortic geometry as a result of both through-plane movement (basal descent, Fig. 1b) and the elastic change in aortic area due to intrinsic pulsatility certainly affect measured flow volumes, particularly those occurring over only part of the cardiac cycle (e.g. FVol, RVol, RF). These geometric changes are clearly position dependent. In our patient cohort, mean annular descent, defined as the change in distance from the aortic valve to the measurement plane during the cardiac cycle, was 9.9 ± 3.4 mm (range 4.0–20.1 mm). For an aortic diameter of 3 cm (area 7.1 cm2), the blood volume potentially moving through our “fixed” phase contrast acquisition plane during systolic basal descent (or similarly diastolic ascent) is 7.1 × 0.99 cm3, or approximately 7 ml. This is in the order of 8–9 % of our measured stroke volumes, and it may account for some of the differences observed. Second, turbulence close to the valve can cause intravoxel dephasing and loss of signal during high systolic flow, which may contribute to greater loss of forward flow at proximal sites closer to the valve. Third, changes in intrathoracic pressure with respiration may be influential, as previous studies have shown that aortic and pulmonary artery flow measurements performed with large volume inspiratory breath holding are lower than measurements performed during free-breathing [22]. Finally, when an imaging slice is located above the sinotubular junction, the measured regurgitant volume will in theory include resting coronary blood flow [7], which is typically about 250 ml min−1 (0.8 ml min−1 g−1 of heart muscle), representing 5 % of cardiac output [23].
Other studies using in vitro phantom experiments and a small cohort of patients have shown similar differences in measurement of regurgitant volume as the slice location is moved distal from the sinus of Valsalva [7]. These findings had not been validated because of a lack of any other reliable quantitative clinical method [7]. Whether this finding is true in a larger cohort of valve disease patients had not been previously shown. Our data support the observation that detection of stroke volume and regurgitant fraction differs on the basis of measurement slice position, and suggest that the highest regurgitant fraction is measured at the SOV.
With regards to the pulmonary valve, a previous study has suggested the ideal location for pulmonic flow assessment to be close to the pulmonic valve plane [24]. Pulmonic stroke volume did not significantly differ at the two main pulmonary artery locations measured (p = 0.57), strongly suggesting that flow values at these sites are a good approximation of total forward blood flow in this cohort of patients without intracardiac shunts, and giving some validation of scan-to-scan measurements with our technique. This makes sense in that these patients had no pulmonic valvular disease, and thus relatively laminar flow through the proximal pulmonary artery that is not highly sensitive to small displacements in measurement location. In the unfortunate absence of any true “gold standard” for measuring net forward flow, the average flow in these pulmonic measurements was used as the best comparator for aortic net flow (in the absence of intracardiac shunt), providing an internal control for comparison of stroke volumes. How the presence of pulmonic valve pathology (such as substantial pulmonary regurgitation in patients with tetralogy of Fallot) would influence pulmonic measurements is beyond the scope of this work and is the subject of a separate study [25].
Comparison between net pulmonic and aortic stroke volume correlated best at the ascending aorta, thus making this the likely most accurate aortic location for measuring net total stroke volume. Use of more proximal sampling sites may lead to an under-representation of net aortic stroke volume and therefore an inaccurate pulmonary to systemic blood flow ratio (Qp/Qs). The most suitable explanation for this phenomenon is that significant intravoxel dephasing occurs as a result of turbulence around the aortic valve leaflets (both normal but more so when diseased), causing a loss of signal and resultant underestimation of fast forward flow close to the valve. This finding becomes relevant when performing CMR to quantitate known intracardiac shunts (Qp/Qs), such as in patients with atrial or ventricular septal defects, or when comparing the systemic forward volume (FVol) measured from phase contrast vs. that from LV functional analysis (i.e. stack of short axis cine images) in order to derive regurgitant volumes in patients with mitral regurgitation [8]. In such cases the ascending aorta plane should be used to avoid under-reporting of forward flow. Cyclical motion of the aorta (distension, contraction, descent) does not factor into the measurement of net stroke volume (unlike it does for forward or reverse flow alone), as this quantity is integrated over the entire cardiac cycle.
Some studies have demonstrated a significant decrease in flow volumes using the breath-holding technique, believed to be due to alterations in physiological flow from changes in intrathoracic pressure [22]. Free-breathing images take longer to acquire, and the evaluation of this sequence can be limited by motion and breathing artefacts, although they may be particularly useful in patients with difficulty in ECG gating or breath-holding [14]. However in these patients it would take even a longer to acquire these non-breath-held acquisitions as the irregular R–R intervals are rejected by the arrhythmia rejection window and repeat acquisition is obtained when the R–R intervals are regular. The present data suggest that the FB sequence is a reasonable substitute for net stroke volume in patients unable to perform breath-hold sequences for Qp/Qs calculations, as we noted no significant difference in net stroke volume, backward volume or regurgitant fraction at the single location (ASC) evaluated (Table 3).
At many centres, CMR studies use a single flow measurement obtained in the aorta and pulmonary artery. However, our data suggest that when quantitating AR, the regurgitant fraction obtained at the sinotubular junction (STJ) should be reported. The regurgitant fraction measured here has the best correlation with regurgitation volumes calculated by subtracting right ventricle stroke volume from left ventricle stroke volume. However, the LV − RV stroke volume method alone is less reproducible than flow measurements for quantitation of regurgitant fractions [5, Appendix]. Until a method to account for flow errors and geometric correction can be obtained (4-dimensional flow may offer this potential [26]), it can be recommended to perform phase contrast acquisitions in both the STJ (quantitate aortic regurgitation) and ASC (calculate Qp/Qs) slice planes. Since these are single slice, single breath-hold acquisitions, adding a second phase contrast scan for the aorta will add only minimal scan time while improving the accuracy of flow quantitation. Results of the present study suggest that, in the absence of pulmonary valvular disease, either of the two pulmonary artery locations can be used.
Limitations
First, the sample is of moderate size and did not include a cohort of normal subjects without valvular disease. Second, the free-breathing sequence was obtained in a single position in the ascending aorta; therefore how FB measurements would vary at the other locations is unknown. Third, phantom acquisitions to correct for background offset errors were not acquired [27]. This, however, is not routinely clinically performed on this MRI unit, and it was not feasible in this study on clinical patients. Finally, the flow acquisitions were performed on a single commercially available MRI system, and whether these observations would be similar for different vendors, or at different field strengths, requires further investigation.
Conclusion
Measurements of aortic flow by phase contrast imaging differ depending on slice location. For pure stroke volume comparisons (Qp/Qs, or deriving mitral regurgitant volume using the comparison of left ventricular and aortic stroke volumes) the acquisition should be made in the tubular portion of ascending aorta at the level of the pulmonary artery. In the presence of inefficient ECG gating due to arrhythmia or poor breath hold due to dyspnoea, a free-breathing sequence appears to be a reasonable alternative to calculate stroke volume. When specifically assessing patients with known aortic valvular regurgitation, in order to reproducibly quantitate the degree of AR, the greatest regurgitant fraction is calculated from the sinus of Valsalva position, closer to the valve plane. However the strongest correlation between the measured aortic regurgitation and calculated aortic regurgitation (left ventricle stroke volume − right ventricle stroke volume) is at the sinotubular junction and should be the location used. For patients undergoing serial CMR examinations to evaluate for interval change, it is important to note and attempt to duplicate the location of prior measurements before reporting a real change in flow volumes.
References
Kramer CM (ed) (2010) Multimodality imaging in cardiovascular medicine. Demos Medical, New York
Kutty S, Whitehead KK, Natarajan S, Harris MA, Wernovsky G, Fogel MA (2009) Qualitative echocardiographic assessment of aortic valve regurgitation with quantitative cardiac magnetic resonance: a comparative study. Pediatr Cardiol 30:971–977
Cawley PJ, Otto CM (2009) Valvular regurgitation: does cardiovascular magnetic resonance provide additional information compared to echocardiography? Minerva Cardioangiol 57:521–535
Kozerke S, Scheidegger MB, Pedersen EM, Boesiger P (1999) Heart motion adapted cine phase-contrast flow measurements through the aortic valve. Magn Reson Med 42:970–978
Cawley PJ, Hamilton-Craig C, Owens DS et al (2013) Prospective comparison of valve regurgitation quantitation by cardiac magnetic resonance imaging and transthoracic echocardiography. Circ Cardiovasc Imaging 6:48–57
Myerson SG, d'Arcy J, Mohiaddin R et al (2012) Aortic regurgitation quantification using cardiovascular magnetic resonance: association with clinical outcome. Circulation 126:1452–1460
Chatzimavroudis GP, Walker PG, Oshinski JN, Franch RH, Pettigrew RI, Yoganathan AP (1997) Slice location dependence of aortic regurgitation measurements with MR phase velocity mapping. Magn Reson Med 37:545–551
Devos DG, Kilner PJ (2010) Calculations of cardiovascular shunts and regurgitation using magnetic resonance ventricular volume and aortic and pulmonary flow measurements. Eur Radiol 20:410–421
Pouleur AC, le Polain de Waroux JB, Goffinet C et al (2008) Accuracy of the flow convergence method for quantification of aortic regurgitation in patients with central versus eccentric jets. Am J Cardiol 102:475–480
Bogren HG, Buonocore MH (1999) Complex flow patterns in the great vessels: a review. Int J Card Imaging 15:105–113
Honda N, Machida K, Hashimoto M et al (1993) Aortic regurgitation: quantitation with MR imaging velocity mapping. Radiology 186:189–194
Kilner PJ, Gatehouse PD, Firmin DN (2007) Flow measurement by magnetic resonance: a unique asset worth optimising. J Cardiovasc Magn Reson 9:723–728
Laffon E, Galy-Lacour C, Laurent F, Ducassou D, Marthan R (2003) MRI quantification of the role of the reflected pressure wave on coronary and ascending aortic blood flow. Physiol Meas 24:681–692
Wittlinger T, Dzemali O, Bakhtiary F, Moritz A, Kleine P (2008) Hemodynamic evaluation of aortic regurgitation by magnetic resonance imaging. Asian Cardiovasc Thorac Ann 16:278–283
Baltes C, Hansen MS, Tsao J et al (2008) Determination of peak velocity in stenotic areas: echocardiography versus k-t SENSE accelerated MR Fourier velocity encoding. Radiology 246:249–257
Bonow RO, Carabello BA, Chatterjee K et al (2006) ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to revise the 1998 guidelines for the management of patients with valvular heart disease) developed in collaboration with the Society of Cardiovascular Anesthesiologists endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. J Am Coll Cardiol 48:e1–148
Shin HJ, Shin JK, Chee HK, Kim JS, Ko SM (2015) Characteristics of aortic valve dysfunction and ascending aorta dimensions according to bicuspid aortic valve morphology. Eur Radiol 25:2103–2114
Detaint D, Messika-Zeitoun D, Maalouf J et al (2008) Quantitative echocardiographic determinants of clinical outcome in asymptomatic patients with aortic regurgitation: a prospective study. JACC Cardiovasc Imaging 1:1–11
Croft CH, Lipscomb K, Mathis K et al (1984) Limitations of qualitative angiographic grading in aortic or mitral regurgitation. Am J Cardiol 53:1593–1598
Dahiya A, Bolen M, Grimm RA, Rodriguez LL, Thomas JD, Marwick TH (2012) Development of a consensus document to improve multireader concordance and accuracy of aortic regurgitation severity grading by echocardiography versus cardiac magnetic resonance imaging. Am J Cardiol 110:709–714
Goffinet C, Kersten V, Pouleur AC et al (2010) Comprehensive assessment of the severity and mechanism of aortic regurgitation using multidetector CT and MR. Eur Radiol 20:326–336
Sakuma H, Kawada N, Kubo H et al (2001) Effect of breath holding on blood flow measurement using fast velocity encoded cine MRI. Magn Reson Med 45:346–348
Hall JE, Guyton AC (2011) Coronary circulation, Chap. 21. In: Guyton and Hall textbook of medical physiology. Saunders/Elsevier, Philadelphia, pp xix, 1091 p
Reid SA, Walker PG, Fisher J et al (2002) The quantification of pulmonary valve haemodynamics using MRI. Int J Cardiovasc Imaging 18:217–225
Rahman EA, Koshy M, Neill J et al (2014) Quantitation of pulmonary regurgitation in repaired tetralogy of Fallot by cardiac magnetic resonance – it matters where you measure! Global Heart 9:e143–144
Markl M, Frydrychowicz A, Kozerke S, Hope M, Wieben O (2012) 4D flow MRI. J Magn Reson Imaging 36:1015–1036
Gatehouse PD, Rolf MP, Graves MJ et al (2010) Flow measurement by cardiovascular magnetic resonance: a multi-centre multi-vendor study of background phase offset errors that can compromise the accuracy of derived regurgitant or shunt flow measurements. J Cardiovasc Magn Reson 12:5
Acknowledgments
The scientific guarantor of this publication is Jeffery H. Maki. The authors of this manuscript declare relationships with the following companies: none. This study has received funding by research grants from the Society for Cardiovascular Angiography and Interventions, General Electric, and the John L. Locke Jr. Charitable Trust. Dr. Christian H. Craig was supported by the Smart Futures Fellowship early career grant and the Washington-Queensland Trans-Pacific Fellowship Trust. Timothy M. Baran kindly provided statistical advice for this manuscript. Institutional review board approval was obtained. Written informed consent was obtained from all subjects (patients) in this study. Some study subjects or cohorts have been previously reported (Circ Cardiovasc Imaging 6:48–57, 2003). Methodology: prospective, observational, performed at one institution.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chaturvedi, A., Hamilton-Craig, C., Cawley, P.J. et al. Quantitating aortic regurgitation by cardiovascular magnetic resonance: significant variations due to slice location and breath holding. Eur Radiol 26, 3180–3189 (2016). https://doi.org/10.1007/s00330-015-4120-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-015-4120-6