Abstract
The thoracic duct is the body’s largest lymphatic conduit, draining upwards of 75 % of lymphatic fluid and extending from the cisterna chyli to the left jugulovenous angle. While a typical course has been described, it is estimated that it is present in only 40-60% of patients, often complicating already challenging interventional procedures. The lengthy course predisposes the thoracic duct to injury from a variety of iatrogenic disruptions, as well as spontaneous benign and malignant lymphatic obstructions and idiopathic causes. Disruption of the thoracic duct frequently results in chylothoraces, which subsequently cause an immunocompromised state, contribute to nutritional depletion, and impair respiratory function. Although conservative dietary treatments exist, the majority of thoracic duct disruptions require embolization in the interventional suite. This article provides a comprehensive review of the clinical importance of the thoracic duct, relevant anatomic variants, imaging, and embolization techniques for both diagnostic and interventional radiologists as well as for the general medical practitioner.
Key Points
• Describe clinical importance, embryologic origin, and typical course of the thoracic duct.
• Depict common/lesser-known thoracic duct anatomic variants and discuss their clinical significance.
• Outline the common causes of thoracic duct injury and indications for embolization.
• Review the thoracic duct embolization procedure including both pedal and intranodal approaches.
• Present and illustrate the success rates and complications associated with the procedure.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The thoracic duct is the largest lymphatic conduit, draining upwards of 75 % of lymphatic fluid throughout the body and carrying 1–2 L of lymphatic fluid/day [1]. It typically extends from the cisterna chyli to the left jugulovenous angle [1]. Disruption of the thoracic duct frequently results in high-output chylothoraces requiring drainage in the setting of impaired respiratory function. Persistent high-output chylothoraces are associated with significant mortality rates, as high as 25–50 %, due to the loss of plasma proteins, fat-soluble vitamins, triglycerides, lymphocytes, electrolytes, and intravascular volume [2, 3]. While conservative dietary therapies have been attempted to control output, the vast majority of patients require intervention [4]. Percutaneous thoracic duct embolization has become the standard of care for high-output chylothoraces [5].
It is estimated that the typical course of the thoracic duct is present in only 40−60 % of patients, and that variation is essentially the rule [4]. The thoracic duct, which may be imaged using magnetic resonance ductography or conventional lymphangiography, can vary at any point along its course. Documented variations include absence of the cisterna chyli, intrathoracic course, the number of ducts, location of tributaries, and point of termination [6]. Knowledge of the thoracic duct embryologic origins, typical course, and clinically significant anatomic variants is beneficial to the interventionalist performing embolization procedures in the interventional suite.
Thoracic duct embryology
The wide variation in thoracic duct anatomy arises from deviations in normal embryologic development. The lymphatic system develops at the end of the sixth week of life [7]. Lymphatic vessels, similar to blood vessels, are derived from hemangioblastic stem cells. Lymphatic clefts and sacs form around large embryologic veins and develop as evaginations from the venous system. Lymphatic clefts eventually form extensive plexuses between one another and fuse to form larger conduits including the embryonic right and left thoracic ducts [8]. The formation of lymph nodes begins predominantly in the ninth week of life, with concurrent regression of many of the previously formed plexuses. Through selective atrophy within the thorax and abdomen, a single duct remains, which drains the lymphatic fluid of the entire lower body, left head, and left arm, and empties into the confluence of the left internal jugular and subclavian veins. Lymphatic fluid of the right head and right arm empties into the corresponding location on the right [9, 10].
The lower two-thirds of the thoracic duct is formed from the embryonic right thoracic duct, and the upper one-third is formed from the embryonic left duct [1, 6]. Disturbances during the selective atrophy process as well as during lymph node formation result in extensive variation of the thoracic duct [9, 10].
Normal anatomic course
The thoracic duct usually arises from the cisterna chyli, designated as 200 % dilatation of the thoracic duct, at T12–L2 to the right of the aorta and behind the right crural pillar (Fig. 1). Since the lower two-thirds of the thoracic duct is formed by the embryonic right thoracic duct, the thoracic duct courses superiorly along the right anterior aspect of the vertebral column, measuring up to 2–5 mm in diameter [1]. Extending cranially, it courses between the aorta and azygos vein, entering the thorax through the aortic hiatus. The intrathoracic portion of the duct then begins coursing within the posterior mediastinum to the right of the vertebral column. At T7, the thoracic duct courses obliquely behind the oesophagus. At approximately the T5–T6 vertebral levels, the thoracic duct crosses the midline to the left and passes behind the aorta and to the left of the oesophagus as it ascends 2–3 cm above the clavicle [1]. This is the portion that is formed by the embryonic thoracic left duct. Once in the superior mediastinum, the thoracic duct courses behind the internal jugular vein, curving inferiorly to drain into the venous system at the junction of the left internal jugular and subclavian veins [6, 11, 12].
Schematic illustration (A) of the typical course of the thoracic duct arising from the cisterna chyli at T12–L2 to the right of the midline coursing cranially to enter the thorax through the aortic hiatus. The intrathoracic portion of the duct courses to right of the descending aorta, along the vertebral column and crosses midline to the left at T5–T6 ascending above the clavicle, behind the jugular vein and curving inferiorly to drain into the left jugulovenous angle. Corresponding fluorographic image (B) demonstrating the typical course of the thoracic duct (white arrows)
Significant anatomic variants
The thoracic duct may vary along any aspect of its course (Fig. 2). Many classification systems have been described to characterize the types of variation. Discussed here are variants which may have a clinically significant impact on the thoracic duct embolization procedure.
Multiple schematic illustrations showing clinically relevant variants of the cisterna chyli and thoracic duct and their relation to the descending aorta, azygos vein, subclavian veins, and spine. Highlighted variants include: complete left-sided course, complete right-sided course, proximal and distal duplications, plexiform variation, and complete absence of the cisterna chyli
Complete left-sided thoracic duct and cisterna chyli emptying into the left venous angle
In a cisterna chyli and thoracic duct with a left-sided course, the cisterna chyli and thoracic duct course along the left aspect of the vertebral column throughout its entire length (Fig. 3). Clinically, the interventionalist must be wary of attempting to cannulate the cisterna chyli, due to its close proximity to the aorta. An alternative approach to a right lumbar or intestinal lymphatic branch may be considered; however, it may be technically challenging due to small calibre lymphatics.
Schematic illustration (A) of the cisterna chyli and the thoracic duct coursing along the left aspect of the vertebral column throughout its entire length. Notice how the cisterna chyli overlies the descending aorta. Fluorographic image (B) and digitally subtracted image (C) demonstrating a left course of the entire supra-diaphragmatic thoracic duct (white arrows)
Complete right-sided thoracic duct and cisterna chyli emptying into the right venous angle
In a cisterna chyli and thoracic duct with a right-sided course, the cisterna chyli and thoracic duct course along the right aspect of the vertebral column throughout its length, without crossing the midline, terminating in the confluence of the right internal jugular and subclavian veins (Fig. 4). This should be recognized as a normal variant.
Schematic illustration (A) of the cisterna chyli and thoracic duct coursing along the vertebral column and emptying into the right jugulovenous angle. Corresponding fluorographic image (B) demonstrating a thoracic duct terminating at the right jugulovenous angle (white arrows) with abnormal contrast extravasation (white arrowhead)
Proximal and distal partial duplication of the thoracic duct
The thoracic duct may be duplicated anywhere along its course, both proximally and distally, before joining to form a single common thoracic duct and entering the left venous angle (Figs. 5 and 6). Injury to the thoracic duct may result in disruption in either or both trunks. Care must be taken to adequately embolize both trunks in cases of proximal duplication, as there is often no shared proximal trunk (Fig. 5), and proximal to the duplication in distal duplications (Fig. 6).
Schematic illustration (A) of the thoracic duct partially duplicated proximally near its origin off the cisterna chyli and then joining to form a single vessel that extends toward the left jugulovenous angle. Fluorographic images (B and C) demonstrating partial proximal duplicated ducts (white arrows)
Schematic illustration (A) of thoracic duct partially duplicated distally and then joining to form a single vessel that terminates at the left jugulovenous angle. Fluorographic image (B) demonstrating a partial distal duplication of the thoracic duct (white arrows). Free spillage of contrast (white arrowhead) from the upper thoracic duct denotes the site of thoracic duct injury
Plexiform variation of the thoracic duct
The thoracic duct may form a complex network of channels that eventually combine to terminate in a common trunk at the left venous angle (Fig. 7). Advancing a guidewire or catheter in a plexiform variant may be challenging due to the small calibre and tortuous multiple trunks. The difficulty in accessing the complex network of channels often results in technical failure. A retrograde approach (cranial to caudal) via a left upper extremity vein and accessing the thoracic duct at the confluence of the left internal jugular and subclavian veins may be considered in this instance [13–15].
Absence of the cisterna chyli
The cisterna chyli is defined as a 200 % dilatation of the thoracic duct [8]. In some cases, lumbar and intestinal lymphatic branches combine to form a normal-calibre thoracic duct without a focal dilatation that meets criteria for a cisterna chyli (Fig. 8). When there is no cisterna chyli, it may be technically challenging, but certainly not impossible, to access the thoracic duct, due to the small calibre of the thoracic duct and even smaller diameter of the lumbar and intestinal branches. With persistence, the thoracic duct may be accessed directly; however, a retrograde approach (cranial to caudal) via a left upper extremity vein and accessing the thoracic duct at the left internal jugular and subclavian veins may also be considered in this case.
Thoracic duct disruption and injury
The two main mechanisms of thoracic duct injury and chylous extravasation include (1) direct trauma or laceration of the lymphatic vessels and (2) occlusion of the thoracic duct, with concurrent development of delicate leaky collateral ducts [16, 17].
Traumatic injury is the more common cause of thoracic duct injury and chylous extravasation [18]. Its lengthy course and frequent variation predispose the thoracic duct to injury from a variety of iatrogenic causes, including thoracic, cardiac, and head and neck surgeries [19]. Injury during oesophageal resection is the most common iatrogenic cause of thoracic duct disruption and chylothoraces, with an incidence rate of approximately 4 % [18]. It is prudent to note, however, that thoracic duct injury may occur in less invasive procedures, such as central line placement, particularly when a subclavian approach is taken [20].
Non-traumatic occlusion and leakage of the thoracic duct is much less common, and may arise from both malignant and benign aetiologies (Table 1). Malignant processes include lymphoma, oesophageal adenocarcinoma, primary lung malignancies, and mesothelioma [21]. Benign causes of obstruction range from systemic disease and infection to primary lymphatic vessel diseases and idiopathic causes [21]. Congenital aetiologies include atresia of the thoracic duct and pleural thoracic duct fistula [22]. Idiopathic causes exist as well and account for the majority of non-traumatic chylothoraces [4].
Indications for embolization
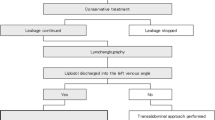
In cases of chylous output less than 1 L/day, conservative management with dietary restriction is a reasonable initial approach [23]. Patients are generally advised to consume a low-fat diet rich in medium-chained triglycerides, as these are readily absorbed directly into the portal system [18]. Alternatively, lipid-free total parenteral nutrition with associated bowel rest may be considered [18]. Other potentially beneficial medical management options include chemotherapy or radiation in the setting of malignant chylothoraces, or medication infusions such as somatostatin or octreotide [18]. In cases where chylous output exceeds 1 L/day, however, patients often require thoracic duct embolization or surgical ligation [24]. Thoracic duct embolization, and less often surgical management, is the preferred method for addressing post-traumatic or post-surgical chylothoraces, as the incidence of persistent chylothoraces after esophagectomy may be as high as 50 % with conservative management, but drops to 10 % with intervention [25, 26].
If thoracic duct embolization is unsuccessful, several surgical options are available, including ligation of the thoracic duct under direct visualization, surgical pleurodesis, pleurectomy, and formation of a pleuroperitoneal shunt [18, 21]. Surgical ligation, the first-line surgical treatment, when technically successful may carry significant morbidity and mortality, with rates of 38 % and 2.1 %, respectively [3]. As a result, percutaneous thoracic duct embolization has become the standard of care for clinically significant chyle leaks, with upwards of 74 % success and with few complications [5].
Pre-procedural imaging
Contrast-enhanced multidetector computed tomography using 1-mm thin slices with axial and coronal multiplanar reformations may be used to visualize the thoracic duct and cisterna chyli. Many authors, however, advocate the use of magnetic resonance ductography in embolization planning due to improved visualization (Fig. 9) [4]. Heavily T2-weighted images should be obtained in axial and coronal projections, with and without fat saturation, and with maximum-intensity reconstructions of the thoracoabdominal region. With the use of a long repetition time (greater than 9,000 ms) and a relatively long effective echo time of 400 ms or greater, lymphatic fluid maintains a high signal intensity, while blood, which has a shorter T2 signal, is attenuated. The location of the cisterna chyli, if present, and the course and configuration of the thoracic duct may be characterized before conventional lymphangiography and thoracic duct embolization. Okuda et al. visualized the course of the thoracic duct with magnetic resonance ductography in 94 % of patients and identified anatomic variants in 14 % of those individuals [27]. Early recognition of thoracic duct variation is paramount in pre-procedural planning, especially when an alternative approach must be sought.
Thoracic duct embolization procedure
The thoracic duct embolization procedure is routinely performed in an angiography suite, with active participation of an interventional radiologist, nurse, and radiologic technologist.
Pedal lymphangiography
Intravenous moderate procedural sedation is administered and prophylactic intravenous antibiotics are given for skin flora. Approximately 0.5 mL of an indicator dye such as methylene blue (American Regent, Shirley, NY, USA) or isosulfan blue (Tyco Healthcare, Montreal, Canada) is injected intradermally into the first and third web spaces of the foot to facilitate visualization of lymphatic vessels (Fig. 10). After local anaesthesia with 1–2 % lidocaine, a 2-cm incision is made along the dorsum of the foot. A lymphatic duct is identified, skeletonized by blunt dissection, isolated with silk ties, and cannulated with a 30-gauge lymphangiography needle.
Ethiodized oil (Ethiodol; Savage Laboratories, Melville, NY, USA; or Lipiodol Ultra-Fluide; Guerbet, Roissy, France) is infused with a power injector through the lymphangiography needle at a rate of 8–10 mL/h (Fig. 11). Fluoroscopic spot images of the lower extremity are obtained every 10–30 min to monitor the progression of the ethiodized oil throughout the lower extremity. Evaluation is terminated when the cisterna chyli is opacified by the ethiodized oil.
Intranodal lymphangiography
As an alternative to the traditional pedal access, the inguinal nodes may be accessed percutaneously under direct ultrasound visualization using a 21–25-gauge needle (Fig. 12). The ultrasound-guided needle tip is optimally positioned in the transitional zone at the junction of the cortex and hilum of the targeted node. Ethiodized oil is then hand-injected at a rate of about 1 mL every 5 min. Serial upward fluoroscopic spot images are obtained until the cisterna chyli is opacified.
The intranodal approach is now preferred, as it is significantly shorter than the pedal approach given that it bypasses the legs. Also, because of its close proximity to the targeted retroperitoneal lymphatic ducts, the intranodal approach often results in better opacification of the thoracic duct [25]. Moreover, it is often technically easier than the pedal approach. Intranodal access does, however, require prominent inguinal nodes, without significant distortion from prior procedures, such as what may be seen after femoral-femoral bypass surgery.
Cisterna chyli access and cannulisation
Prophylactic administration of intravenous antibiotics may be given for gastrointestinal flora. Under fluoroscopic guidance, the opacified cisterna chyli, prominent retroperitoneal duct, or lumbar or intestinal branches are accessed using a 21-gauge 15-cm needle and a right paramedian transabdominal approach (Fig. 13). An 0.018-inch guidewire is advanced through the needle into the thoracic duct, and a 3 French dilator with stiffening cannula is inserted into the proximal thoracic duct.
Thoracic duct lymphangiography
The guidewire is removed, and iodinated contrast material is injected to evaluate thoracic duct anatomy, any potential variation, and the level and degree of contrast extravasation (Fig. 14).
Thoracic duct embolization
Over the guidewire, the dilator is exchanged for a 3 French microcatheter and advanced into the distal thoracic duct. With the microcatheter advanced past the site of injury, micro-coils are deployed proximally along the entire course of the thoracic duct, both proximal and distal to the leak (Fig. 15). A 2:1 or 1:1 mixture of ethiodized oil and N-butyl cyanoacrylate glue with tantalum powder (TruFill; Codman and Shurtleff, Raynham, MA, USA) is used to complete the embolization of the distal thoracic duct and to seal the cisterna chyli.
Procedural success rates
Thoracic duct embolization technical success rates are as high as 79 % [29]. Technical failure tends to be related to difficulty with pedal lymphangiography, and concomitant poor opacification of the retroperitoneal lymphatic system [29]. Adoption of the intranodal approach, however, has the potential to significantly improve technical success rates [28].
Clinical success rates, based on the three largest series, range from 71 to 73.8 % [5, 26, 27]. Clinical success is guided by the underlying aetiology of the chylothoraces. According to Pamarthi et al., success was higher in cases of traumatic versus non-traumatic chylothoraces, with clinical success rates of 62 % and 13 %, respectively [29]. The type of surgical intervention also affected the clinical outcome, with pneumonectomy patients having clinical success rates of 82 % and pleurectomy patients having success rates of 47 % [29].
Procedural complications
As with all invasive procedures, there are some inherent risks to thoracic duct embolization, including pain, infection, and bleeding [30]. These may be minimized with the appropriate moderate procedural sedation, judicious use of local anaesthetic, adequate post-procedure pain medications, appropriate prophylactic antibiotic coverage, and careful procedural technique.
Serious complications reported in the literature include methylene blue skin necrosis, anaphylaxis to ethiodized oil, ethiodized oil emboli to the pulmonary and portal vein vasculature, and ethiodized oil extravasation into the soft tissues (Fig. 16) [31]. Many of these complications can be minimized by limiting the volume of ethiodized oil infused [32]. The incidence of major complications is rare. One series of 120 thoracic duct embolizations in 105 patients reported no major complications, and noted minor complications in only 6.7 % of patients [29].
A single photograph (A) and two computed tomography images (B and C) demonstrating potential thoracic duct embolization complications including: methylene blue skin blistering and necrosis (A), non-target pulmonary artery embolization (B) (white arrow), and non-target portal vein embolization (C) (white arrows)
Delayed complications have also been reported. Laslett et al. found that 14 % of patients experienced symptoms related to their procedure after an average of 34 months. Such delayed complications, including protein-losing enteropathy, chronic diarrhoea, chylous ascites, and lymphedema, are theorized to be the result of redistribution of high-volume flow following thoracic duct occlusion [33, 34]. However, delayed complications have been reported as mild and not disabling [33, 34].
Conclusions
Thoracic duct injury and chylous extravasation may result from myriad iatrogenic injuries as well as from malignant and benign obstructions. High-output chylous leakage requires intervention in order to prevent serious morbidity and mortality. Given their minimally invasive nature and lower risk of serious complications, embolization procedures completed by an interventional radiologist are favoured over surgical approaches. Variations in thoracic duct anatomy, including, but not limited to, the presence or absence of the cisterna chyli to the intrathoracic course of the thoracic duct, present challenges to the interventional radiologist during embolization. Together, the knowledge of thoracic duct embryology, typical anatomic course, anatomic variation, pre-procedural imaging, and the embolization procedure as presented in this review will allow successful treatment, with minimal morbidity and mortality.
References
Skandalakis JE, Skandalakis LJ, Skandalakis PN (2007) Anatomy of the lymphatics. Surg Oncol Clin N Am 16:1–16
Campagna J, Jain R (2010) Thoracic duct embolization. In: Kessel D, Ray C (eds) Transcatheter embolization and therapy. Springer, London, pp 471–472
Cope C (2004) Management of chylothorax via percutaneous embolization. Curr Opin Pulm Med 10:311–314
Chen E, Itkin M (2011) Thoracic duct embolization for chylous leaks. Semin Interv Radiol 28:63–74
Cope C, Kaiser LR (2002) Management of unremitting chylothorax by percutaneous embolization and blockage of retroperitoneal lymphatic vessels in 42 patients. J Vasc Interv Radiol 13:1139–1148
Phang K, Bowman M et al (2014) Review of thoracic duct anatomical variations and clinical implications. Clin Anat 27:637–644
Van Der Putte SCJ, Van Limborgh J (1980) The embryologic development of the main lymphatics in man. Actca Morphol Neerl Scand 18:323–335
Loukas M, Wartmann CT, Louis RG Jr et al (2007) Cisterna chyli: a detailed anatomic investigation. Clin Anat 20:683–688
Kwon SS, Falk A, Mitty HA (2002) Thoracic duct injury associated with left internal jugular vein catheterization: anatomic considerations. J Vasc Interv Radiol 13:337–339
Chen H, Shoumura S, Emura S (2006) Bilateral thoracic ducts with coexistent persistent left superior vena cava. Clin Anat 19:350–353
Cha EM, Sirijintakam P (1976) Anatomic variation of the thoracic duct and visualization of mediastinal lymph nodes: a lymphographic study. Radiology 119:45–48
Kausel HW, Reeve TX, Stein AA et al (1957) Anatomic and pathologic studies of the thoracic duct. J Thorac Surg 34:631–641
Koike Y, Hirai C, Nishimura J et al (2013) Percutaneous transvenous embolization of the thoracic duct in the treatment of chylothorax in two patients. J Vasc Interv Radiol 24:135–137
Mittleider D, Dykes TA, Cicuto KP, Amberson SM, Leusner CR (2008) Retrograde cannulation of the thoracic duct and embolization of the cisterna chyli in the treatment of chylous ascites. J Vasc Interv Radiol 19:285–290
DeArment MC, May GR, Malik SW (2003) Idiopathic chylothorax successful treatment with percutaneous retrograde catheterization and embolization of the duct. Chest 124;4:274S
Dunn RP (1975) Primary chylopericardium: a review of the literature and an illustrated case. Am Heart J 89:369–377
Itkin M, Swe NM, Shapiro SE, Shrager JB (2009) Spontaneous chylopericardium: delineation of the underlying anatomic pathology by CT lymphangiography. Ann Thorac Surg 87:1595–1597
Nair SK, Petko M, Hayward MP (2007) Aetiology and management of chylothorax in adults. Eur J Cardiothorac Surg 32:362–369
Doerr CH, Allen MS, Nichols FC III, Ryu JH (2005) Etiology of chylothorax in 203 patients. Mayo Clin Proc 80:867–870
Kurekci E, Kaye R, Koehler M (1998) Chylothorax and chylopericardium: a complication of a central venous catheter. J Pediatr 132:1064–1066
McGrath EE, Blades Z, Anderson PB (2010) Chylothorax: aetiology, diagnosis, and therapeutic options. Respir Med 104:1–8
Lopez-Gutierrez JC, Tovar JA (2014) Chylothorax and chylous ascites: management and pitfalls. Semin Pediatr Surg 23:298–302
Marts BC, Naunheim KS, Fiore AC, Pennington DG (1992) Conservative versus surgical management of chylothorax. Am J Surg 164:532–534
Thompson KJ, Kernstine KH, Grannis FW, Mojica P Jr, Falabella A (2008) Treatment of chylothorax by robotic thoracic duct ligation. Ann Thorac Surg 85:334–336
Bolger C, Walsh T, Tanner W, Hennessy T (1991) Chylothorax after oesophagectomy. Br J Surg 78:587–588
Orringer M, Bluett M, Deeb G (1988) Aggressive treatment of chylothorax complicating transhiatal esophagectomy without thoracotomy. Surgery 104:726–750
Okuda I, Udagawa H, Takahashi J et al (2009) Magnetic resonance-thoracic ductography: imaging aid for thoracic surgery and thoracic duct depiction based on embryological considerations. Gen Thorac Cardiovasc Surg 57:640–646
Itkin M (2014) Lymphatic Intervention is a new frontier of IR. J Vasc Interv Radiol 25:1404–1405
Pamarthi V, Stecker MS, Schenker MP, Baum RA, Killoran TP, Suzuki Han A et al (2014) Thoracic duct embolization and disruption for treatment of chylous effusions: experience with 105 patients. J Vasc Interv Radiol 25:1398–1404
Itkin M, Kucharczuk JC, Kwak A, Trerotola SO, Kaiser LR (2010) Nonoperative thoracic duct embolization for traumatic thoracic duct leak: experience in 109 patients. J Thorac Cardiovasc Surg 139:584–589
Lee EW, Shin JH, Ko HK, Park J, Kim SH, Sung KB (2014) Lymphangiography to treat postoperative lymphatic leakage: a technical review. Korean J Radiol 15:724–732
Guermazi A, Brice P, Hennequin C, Sarfati E (2003) Lymphography: an old technique retains its usefulness. Radiographics 23:1541–1558
Nadolski G, Itkin M (2013) Thoracic duct embolization for the management of chylothoraces. Curr Opin Pulm Med 19:380–386
Laslett D, Trerotola SO, Itkin M (2012) Delayed Complications following technically successful thoracic duct embolization. J Vasc Interv Radiol 23:76–79
Acknowledgments
The authors thank Rachel L. Johnson for her generous assistance in developing the schematic diagrams. The authors would also like to thank Dr. Beth A. Ripley for preparation of the volume-rendered thoracic duct and cisterna chyli images (Fig. 9).
This manuscript is based on the electronic presentations entitled: “When, Why, and How of Thoracic Duct Embolization” and “Imaging and Embolization: Illustration of Variable Thoracic Duct Anatomy for Thoracic Duct Embolization Pre-Procedure Planning,” both of which were presented at the European Society of Radiology in 2015. The electronic presentation entitled “When, Why, and How of Thoracic Duct Embolization” was selected as presentation of the month.
The guarantor of this manuscript is Dr. Alisa Suzuki-Han. The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article. The authors state that this work has not received any funding. No complex statistical methods were necessary for this paper. Institutional review board approval was not required because this is a review paper.
Author information
Authors and Affiliations
Corresponding author
Additional information
Drs. Johnson and Chick contributed equally to this manuscript and share first authorship.
Rights and permissions
About this article
Cite this article
Johnson, O.W., Chick, J.F.B., Chauhan, N.R. et al. The thoracic duct: clinical importance, anatomic variation, imaging, and embolization. Eur Radiol 26, 2482–2493 (2016). https://doi.org/10.1007/s00330-015-4112-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-015-4112-6