Abstract
Objectives
To assess dose area products (DAP) and effective doses (ED) of voiding cystourethrography (VCUG) in children using optimized protocols on a modern flat detector unit.
Methods
DAP and ED were evaluated in 651 VCUG (316 girls, median age: 2.25 years) between 2009 and 2012. DAP was analyzed in relation to patient characteristics (gender, age, presence of pathological findings) and experience of performing physician using analysis of variance. ED values were estimated using adapted conversion factors from the literature. Diagnostic image quality was validated by two experienced physicians using a 3-point scale.
Results
Median DAP/ED was 0.5 cGycm2/4.56 μSv (boys: 0.6 cGycm2/6.16 μSv; girls: 0.4 cGycm2/3.54 μSv). In 300 studies without pathologic findings DAP was 0.35 cGycm2, whereas 351 studies with pathologic findings had a median DAP of 0.7 cGycm2. No significant relationship between DAP and experience of radiologist was observed. Image validation resulted in an overall good to excellent rating.
Conclusions
DAP and ED can be markedly reduced in paediatric VCUG performed with optimized protocols on modern equipment without a noticeable decrease in diagnostic image quality.
Key points
• Voiding cystourethrography is a comprehensive examination in diagnosing vesicoureteral reflux (VUR).
• Radiation reduction is achieved in VCUG through modern equipment and optimized protocols.
• Low-dose VCUG is possible without noticeable decrease in diagnostic image quality.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Voiding cystourethrography (VCUG) is the standard examination in paediatric radiology for the detection of vesicoureteral reflux (VUR) and is often used as a complementary diagnostic tool for other pathologies of the urinary tract in children. Although alternative modalities including voiding urosonography (VUS), radionuclide cystography (RNC), and recently, magnetic resonance voiding cystourethrography (MR-VCUG) exist today, VCUG is prevalently performed due to several advantages as low cost, high availability, simple operability, and high diagnostic accuracy [1]. Among the methods using ionizing radiation, RNC has the lowest effective dose (ED), which has been reported to be 5 – 10 times lower than VCUG [2]. However, VCUG has a superior morphologic resolution in comparison to RNC [2]. Moreover, recent technical developments such as flat panel detectors (FD), grid controlled fluoroscopy, and modern dose control concepts can markedly reduce the radiation dose [2, 3]. These technologies in combination with a C-arc-based system create favourable conditions for VCUG in children.
The aim of this retrospective study was to assess dose area products (DAP) and effective doses (ED) of fluoroscopic voiding cystourethrography (VCUG) in children using optimized protocols on a modern flat detector unit and to determine variables that affect these measures.
Materials and methods
Patients
IRB approval was obtained for a retrospective study and written informed consent was obtained by all legal guardians. Inclusion criteria were: age of patient under 15 years at examination, the presence of clinical indication for VCUG according to national and international guidelines [4–6], the use of Philips MultiDiagnost Eleva FD 2.0 with the standardized filtration mode, and a complete record of the DAP.
A total of 944 VCUG studies of 729 patients who were examined from January 2009 to December 2011 were reviewed using the radiology information system (RIS) and the Picture Archiving and Communication System (PACS). Two hundred and fifty-four studies were performed as video urodynamic studies and were excluded correspondingly. Thus, according to the study criteria, 651 studies of 574 patients were included. Median age was 2.25 years ranging from 1 day to 14.8 years. Two hundred and ninety-seven studies (46 %) were obtained from 258 boys and 354 studies (54 %) from 316 girls. Median age of boys and girls was 12 months and 43.5 months, respectively (P < 0.0001). Five hundred and nine patients were examined once, 55 patients twice, nine patients received three examinations, and one patient was examined five times. All patients were examined with clinical indication for suspected VUR or as a follow-up study. An additional clinical focus on the bladder (e.g. bladder augmentation) was present in 46 patients, on the urethra in 24 patients, and another additional focus in 36 patients (e.g. reflux in seminal vesicle).
Four age classes were defined in order to calculate effective doses by adapting conversion factors according to Schultz et al. [7]. Age class 1: 0 – 1 year (234 patients), 2: 1 – 5 years (209 patients), 3: 5 – 10 years (162 patients), 4: 10 – 15 years (46 patients).
VCUG technical parameters and procedure
VCUG was performed on a C-arc-equipped flat detector unit (Philips MultiDiagnost Eleva, Philips Medical Systems; Netherlands). Technical optimization of the unit for paediatric fluoroscopy purposes, including filtration and automatic customization of the X-ray beam spectrum, shape, and pulse frequency [8], was performed before implementation in clinical practice. The examination specific parameters given in Table 2 were constant during the complete study interval.
Clinical procedure
VCUG was performed by staff paediatric radiologists (high level of expertise) or radiology fellows (lower level of expertise) under guidance of a senior paediatric radiologist. The operating radiologist confirmed the indication for VCUG after review of the clinical history and available previous imaging.
In the majority of patients no sedation was necessary. In a small group of young children (between 2 and 4 years old) with a high level of anxiety a mild sedation with rectal application of 0.4 mg/kg bodyweight midazolam (max. 8.2 mg for a body weight > 20 kg) was carried out before catheterization. The procedure of catheterization, the acquisition parameters, the patient and beam positioning were specified in a standard operating procedure (SOP) of the institution. The presence of a current urinary tract infection (UTI) was ruled out through dipstick urine analysis.
Imaging procedure
We began the examination with a sonography of the urinary system. Cyclic VCUG (up to four cycles) was performed following the recommendations of the ESPR workgroup [6] after informed consent of the legal guardians. The child was positioned on the table in supine position, which was usually not changed during the whole examination. Routinely, only fluoroscopic images were obtained using pulsed fluoroscopy with a frame rate of 0.5 image/s. Only in selected cases (e.g. suspicion for posterior urethral valve or in cases of complex congenital anomalies) was a radiographic image obtained. All recorded images, including the whole fluoroscopic set, were automatically stored for final interpretation by the senior radiologist.
Measurement of dose area products (DAP)
DAP was measured by the DAP meter which was set 28.2 cm from the focal spot. Calibration was carried out in cooperation with the manufacturer. The lowest DAP value given by the device was 0.1 cGycm2, values lower than 0.1 cGycm2 were displayed as 0 cGycm2. In 101 examinations (15.51 %) DAP values were lower than 0.1 cGycm2 and, therefore, displayed as 0 cGycm2. Hence, in order to avoid an underestimation of DAP, these values were substituted with the highest possible value of 0.09 cGycm2 (Table 1).
Effective dose (ED) estimation
ED values were estimated using adapted DAP-ED conversion factors based on Monte Carlo simulations obtained from Schultz et al. [7] as the examination protocol of both studies is comparable. Schultz et al. used a Philips Diagnost 88 unit with a Supertotalix 150 kV tube, 70 – 80 kV tube voltage, 2.0 mm Al inherent filtration and 1.0 mm Al added. Table 2 shows the specifications given at our institution. Conversion factors for five age groups for each gender and different projections were proposed. We used the conversion factors for AP projections as they were applicable to the examination protocol in our study [7]. The obtained conversion factors for each phantom representing an age group were set in relation to the phantom weight. Subsequently, a gender-specific formula, which states a specific dose conversion factor for every given patient weight was used to adapt conversion factors to patients’ weight, assuming average weight for each patient according to patient age [9] in case of missing documentation of patients’ weight, height, or abdominal diameter in the RIS, respectively. The received conversion factors were further multiplied by a factor of 1.5 taking into account the additional 0.3 mm Cu filtration used at our institution, based on studies by Gosch et al. [10] and Le Heron [11]. Gosch et al. performed Monte Carlo simulations to determine DAP-ED conversion factors for differing kV, projections, field positions, and filtration modalities [10]. The arithmetic mean of conversion factors for AP projections of abdominal and pelvic fields with a field size of 40 cm × 40 cm for each 70 and 80 kV for a) 4 mm Al filtration, and b) 4 mm Al + 0.3 mm Cu filtration was calculated. The ratio between a) and b) was calculated and found to be 1.5.
Assessment of image quality
In order to assess the diagnostic quality of images acquired during the examinations, 20 patients were randomly selected. A paediatric urologist (18 years of experience) and a paediatric radiologist with 8 years of experience rated image quality according to three aspects: shutter, visualization of diagnostic key findings, and noise. Every examination was rated on a 3-point scale: 1 - “adequate”, 2 - “sufficient”, and 3 - “inadequate” for shutter and visualization of diagnostic key findings, and 1 - “imperceptible”, 2 - “minor”, and 3 - “with diagnostic relevance” for noise.
Statistical analysis
In order to evaluate factors influencing DAP values analysis of variance with the factors gender (m/f), pathology (yes/no), investigator experience (fellow vs. staff paediatric radiologist), and the covariate patient age was carried out. Bonferroni post-hoc correction was performed (SPSS Statistics, version 22, IBM, Armonk, NY, USA). P-values < 0.05 were considered significant.
Results
The median DAP over all studies was very low with a median value of 0.5 cGyxcm2 (range 0.09 – 21.1 cGyxcm2). The median DAP value in boys was 0.6 cGyxcm2 (0.09 – 21.1 cGyxcm2), and 0.4 cGyxcm2 in girls (0.09 – 10 cGyxcm2). Median DAP values and ranges for children separated by age group, gender, and pathology are given in Tables 3 and 4.
Analysis of variance revealed that DAP values were significantly influenced by patient age, gender, and the presence of pathologies. Factors that were associated with higher DAP values were male sex, the presence of a pathologic finding, and higher age. However, the investigators expertise had no influence on DAP.
Estimated EDs were very low with a median value of 4.56 μSv (range 0.45 – 157.98 μSv) with 6.16 μSv (0.66 – 87.03 μSv) in boys and 3.54 μSv (0.45 – 157.98 μSv) in girls, respectively. For ED values according to gender and age groups see Table 5.
Image quality
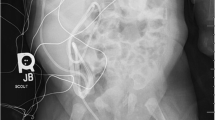
Qualitative image evaluation revealed a high level of image quality: “Shutter” was rated at an average of 1.08 – adequate (SD 0.27), “Imaging of Diagnostic Criteria” was rated at 1.35 – adequate (SD 0.66), and “Noise” at 1.43 – imperceptible (SD 0.5). No significant difference was found between the ratings of the two readers. Figures 1 and 2 show images taken from two of the selected patients.
VCUG ruled out vesicoureteral reflux in a 9-year-old boy with recurrent pyelonephritis of the left side. Six selected example images of the examination that totally contained 11 images of two micturition cycles. DAP was 3.9 cGyxcm2. a – preliminary overview, b – pre-voiding image, c – left oblique voiding image with visualization of urethra, d – right oblique voiding image, e – collimated image of the kidneys, f – post-void image without residual urine
4-year-old girl with recurrent urinary tract infection. VCUG showed vesicoureteral reflux Grade III on both sides. DAP was 0.2 cGyxcm2. a – preliminary overview; b – early filling phase with detection of VUR on the left side; c–f collimated images of ureter and the kidneys: intermediate filling phase (c), prevoiding phase (d) and images during voiding (e) revealed VUR Grade III on both sides; post-void image without residual urine (f)
Discussion
In this retrospective analysis over a period of 3 years, the DAP values of VCUG studies were evaluated with respect to differences between patient age and gender, pathological findings, and investigator’s experience. To improve sensitivity of VCUG, cyclic examinations were performed as shown in previous studies [14, 15]. In our study, the median DAP ranged from 0.1 cGycm2 in the youngest age group without pathological finding to 3.3 cGycm2 in older children with pathological findings.
In comparison to previously published data with similar age grouping [2, 7, 13, 16–18] (see Tables 4 and 5), DAP and ED values were markedly reduced. Gonzalez et al. (1995) analyzed DAP values of VCUG examinations in two centres [17]. The DAP values for the complete examination in centre 2 acquired with a conventional Siemens system with a Gigantos 1012 generator and Optilux image intensifier (five obtained radiographs, 0.3 – 1.2 min of continuous fluoroscopy) were by an approximate factor of 1000 higher than our values. Schultz et al. (1999) [7] evaluated DAP values of 84 VCUG examinations performed with a conventional Philips Diagnost 88 system (three radiographs) and estimated ED values applying Monte Carlo Simulations using mathematical phantoms. The DAP values were higher by a factor of 40 – 60, and the ED values were accordingly 20 – 100 times higher than our values. Perisinakis et al. (2006) [19] estimated ED values of 118 VCUG examinations performed with a conventional Siemens Siregraph D1 system powered by a Siemens Polydoros 50 X-ray generator with a parallax fee image intensifier TV-fluoroscopy set and a spot film device (2 – 3 radiographs, 0.2–1.4 min of intermittent fluoroscopy) and applying ATOM phantoms. Their ED values exceeded ours by a factor of 100 approximately. Pazik et al. (2007) [20] estimated ED values for five female newborns as well as stylized and tomographic phantoms using a digital Picker Vector II system with a high frequency generator (6–9 radiographs, 47.2–147.6 s of continuous fluoroscopy) and applying Monte Carlo Simulations. ED values ranged from 0.6–3.2 mSv with a mean of 1.8 mSv (SD 1.9) and exceeded ours by a factor of 250 approximately. Ward et al. (2008) [2] derived ED values of three groups with a total of 62 evaluated patient examinations using a digital Philips Easy Diagnost 90/45 system (Grid-controlled variable-rate pulsed fluoroscopy with a default pulse rate of 1.9 Hz, mean fluoroscopy time of 2.04–2.17 min) and applying Monte Carlo N-particle radiation transport code. Their values exceeded our values by a factor of 10 approximately. The most recent study of Born et al. (2013) [16] evaluated DAP values of 413 data records acquired with a digital Philips Easy Diagnost system (radiographs in exceptional cases, grid-controlled pulsed fluoroscopy with a standard pulse rate of 3 Hz, mean fluoroscopy time of 34 s) and formed four age classes according to those of official German diagnostic reference levels [21] with a total of 216 examinations. Their DAP values were still higher by an approximate factor of 4–10 than compared to our data.
This comparison of DAP and ED values in VCUG studies in children from the past 20 years shows a continuous reduction in radiation exposure, which is based on several reasons. For our study, we assume the advanced and specially optimized flat detector unit to be the most influential factor. This system provides special paediatric dose curves and less radiation-on time [8] and, therefore, reduces radiation dose. A further important issue in radiation-saving might be the automatic digital storage of all obtained images including complete fluoroscopy, thus repeating of exposure can be reduced to a minimum. Moreover, transient or inconclusive findings (e.g. low-grade reflux) will be assessed with maximal possible sensitivity giving more confidence for final impression also in cases of limited experienced radiologists, because the senior staff can review the complete examination later.
Statistical analysis further showed a significant influence on DAP by age, gender, and pathology. Children with pathological findings showed higher DAP values compared to healthy children. This may be explained by more extensive imaging of the detected pathologies. The significant differences in DAP values between boys and girls with pathological findings were presumably due to diverse anatomic and pathologic conditions resulting in higher DAP values in boys.
The distribution of patients’ age differs between the genders (see Table 1): Boys receiving VCUG are overall younger than girls, which is assumed to be due to the differential pathologies that occur gender dependent. According to literature, 80 % of VUR discovered at VCUG conducted with indication of UTI were females at the average age of 2–3 years, whereas 80 % of VUR, which were discovered evaluating prenatal hydronephrosis, are male children [22].
The investigators expertise, however, did not affect DAP. We propose a special training prior to the first examination, and performing the first five examinations under supervision of a senior paediatric radiologist. Clearly specified acquisition parameters in the SOP of our institution lead to a high level of confidence in all examiners, and thus to a high reproducibility of examinations. In addition the automatic fluoroscopic image storage might be also an important issue, as mentioned before.
The standardization of dedicated imaging can, therefore, not only help to ensure state-of-the-art patient care, but also to reduce radiation burden as already discussed in the recommendations of ESPR before [6].
A total of 651 cases included in this study are an adequately high number and considerably higher than in other studies regarding the DAP (Table 4). The gender distribution of the analyzed records is representative. The fact that a small number of patients were examined more than once is not a limiting factor since this occurs in clinical routine and is, therefore, an inevitable part of a representative study. With regard to DAP values, it is likely that patients who undergo more than one examination raise the average DAP value because children are examined repeatedly if they show pathologies, and our data analysis indicates that children with pathological findings feature higher DAP values.
The median calculated ED in our patients was 4.56 μSv, which is noticeably lower than the values of RNC recently reported in literature. According to the guidelines of the European Association of Nuclear Imaging, the effective dose for RNC ranges between 10 and 30 μSv, whereas effective dose values of the 2010 North American consensus guidelines are even higher (20 to 60 μSv) [23]. Particularly with respect to the better anatomical depiction in comparison to RNC, VCUG still remains the modality of choice in complex disorders. In addressing uncomplicated cases, VUR can also be sufficiently diagnosed by VUS.
Image evaluation resulted in an overall good to excellent rating. Since the images were randomly selected, they can be expected to be representative for the entire study. The images were reviewed according to qualitative attributes, which is sensible and indicated in this case. The aspects chosen for evaluation, shutter, visualization of diagnostic key findings, and noise, are substantial ones with respect to diagnostic image quality. Our results are supported by those of previous studies suggesting that fluoroscopically captured images are adequate in documenting the absence of VUR on VCUG examinations [12].
However, the reduced image quality of fluoroscopy compared to radiographic images could have an impact in diagnosis of intrarenal reflux (IRR), but the clinical significance as well as the therapeutic management of IRR remains controversial. Kim et al. report a higher rate of scarring in patients with prophylactic antibiotic therapy compared to patients after surgery and, therefore, suggest the patients with IRR should be managed more actively [24]. In contrast, Boubnova et al. as well as Fukui et al. could show that under medical management, the prognosis for IRR is not different from high-grade VUR without IRR [25, 26]. As the therapeutic consequence of intrarenal reflux varies between institutions the relevance of this specific diagnosis is blurry. For the given more aggressive treatment of VUR of grade III or higher at our institution, it remains irrelevant and therefore might be disregarded.
Limitations
There were several limitations to our study. First of all, we did not conduct dosage measurements with an additional external dosimeter, which could ensure a higher accuracy of measured dose values. However, since the equipment is regularly controlled according to regulations of the National Federal Office for Radiation Protection, an adequate accuracy of values can be assumed.
For estimation of ED we further used conversion factors adapted to patients’ weight that were calculated assuming average weight for each patient according to patient age [9], in cases of missing documentation of patients’ weight, height, or abdominal diameter in the RIS, respectively. This represents a potential source of error leading to imprecision in ED estimation. In fact, an aberrance of body weight from the average in children seems to be a small error considering that the ED is an imprecise dimension which can solely be estimated using conversion factors which are dependent on tube voltage, filtration, projection, and field size and position. Patient’s cooperation has a possible influence on DAP/ED, and as we did not document this fact we have to mention that the evaluation of the influence of cooperation on DAP/ED was not possible.
Concerning the comparison to existing data in literature, several limitations have to be taken into account: differences in chosen age classes, in composition of patient groups, as well as equipment and examination protocols between different institutions might lead to discrepancies in DAP and ED values and complicate the comparison of results. Furthermore, a certain overestimation of the DAP seems likely, as about 15 % of our measured values were less than 0.1 cGyxcm2 and were substituted for 0.09 cGyxcm2.
In conclusion, the results of our study suggest that the use of modern equipment and optimized protocols adapted to paediatric patients allow for a considerable reduction in resulting DAP and ED values without a noticeable decrease in diagnostic image quality in paediatric VCUG compared to ten years ago.
Abbreviations
- VCUG:
-
Voiding cystourethrography
- VUR:
-
Vesicoureteral reflux
- VUS:
-
Voiding urosonography
- RNC:
-
Radionuclide cystography
- MR-VCUG:
-
Magnetic resonance voiding cystourethrography
- DAP:
-
Dose area product
- ED:
-
Effective dose
- IRB:
-
Institutional review board
- FD:
-
Flat panel detector
- RIS:
-
Radiology information system
- PACS:
-
Picture Archiving and Communication System
References
Ammenti A, Cataldi L, Chimenz R et al (2012) Febrile urinary tract infections in young children: recommendations for the diagnosis, treatment and follow‐up. Acta Paediatr 101:451–457
Ward VL, Strauss KJ, Barnewolt CE et al (2008) Pediatric radiation exposure and effective dose reduction during voiding cystourethrography1. Radiology 249:1002–1009
Ward VL, Barnewolt CE, Strauss KJ et al (2006) Radiation exposure reduction during voiding cystourethrography in a pediatric porcine model of vesicoureteral reflux. Radiol Radiol Soc N Am 238:96–106
Hernanz-Schulman C, Coley - American College of Radiology. ACR–SPR Practice Guideline for the Performance of Voiding Cystourethrography in Children Res. 33 – 2009. American College of Radiology Web site2009 [February 3, 2015]; Available from: http://www.acr.org/~/media/ACR/Documents/PGTS/guidelines/Voiding_Cystourethrography.pdf
Roberts KB, Revised AAP (2012) Guideline on UTI in febrile infants and young children. Am Fam Physician 86:940–946
Riccabona M, Avni FE, Blickman JG et al (2008) Imaging recommendations in paediatric uroradiology: minutes of the ESPR workgroup session on urinary tract infection, fetal hydronephrosis, urinary tract ultrasonography and voiding cystourethrography, Barcelona, Spain, June 2007. Pediatr Radiol 38:138–145
Schultz F, Geleijns J, Holscher H, Weststrate J, Zonderland H, Zoetelief J (1999) Radiation burden to paediatric patients due to micturating cystourethrography examinations in a Dutch children’s hospital. Br J Radiol 72:763–772
Stueve D (2006) Management of pediatric radiation dose using Philips fluoroscopy systems DoseWise: perfect image, perfect sense. Pediatr Radiol 36:216–220
Ogden CL, Kuczmarski RJ, Flegal KM et al (2002) Centers for Disease Control and Prevention 2000 growth charts for the United States: improvements to the 1977 National Center for Health Statistics version. Pediatrics 109:45–60
Gosch D, Gosch K, Kahn T (2007) Conversion coefficients for estimation of effective dose to patients from dose area product during fluoroscopy x-ray examinations. Röfo 179:1035–1042
Le Heron J (1992) Estimation of effective dose to the patient during medical x-ray examinations from measurements of the dose-area product. Phys Med Biol 37:2117
Fefferman NR, Sabach AS, Rivera R et al (2009) The efficacy of digital fluoroscopic image capture in the evaluation of vesicoureteral reflux in children. Pediatr Radiol 39:1179–1187
Fotakis M, Athanasopoulou EM, Psarrakos K, Economou I (2003) Radiation doses to paediatric patients up to 5 years of age undergoing micturating cystourethrography examinations and its dependence on patient age: a Monte Carlo study. Br J Radiol 76:812–817
Papadopoulou F, Efremidis SC, Oiconomou A et al (2002) Cyclic voiding cystourethrography: is vesicoureteral reflux missed with standard voiding cystourethrography? Eur Radiol 12:666–670
Paltiel HJ, Rupich RC, Kiruluta HG (1992) Enhanced detection of vesicoureteral reflux in infants and children with use of cyclic voiding cystourethrography. Radiology 184:753–755
Born M, Spiller L, Bachour H, Heydweiller A, Franke I (2013) Dose area product of pediatric VCUG with regard to the strongly lowered German diagnostic reference levels. Röfo 185:262–267
González L, Vañó E, Ruiz M (1995) Radiation doses to paediatric patients undergoing micturating cystourethrography examinations and potential reduction by radiation protection optimization. Br J Radiol 68:291–295
Merkle EM, Aschoff AJ, Vogel J, Merk J, Bachor R, Brambs HJ (1997) Radiation exposure in digital micturition cystourethrography in children. How much exposure by fluoroscopy? Der Urologe Ausg A 36:181–185
Perisinakis K, Raissaki M, Damilakis J, Stratakis J, Neratzoulakis J, Gourtsoyiannis N (2006) Fluoroscopy-controlled voiding cystourethrography in infants and children: are the radiation risks trivial? Eur Radiol 16:846–851
Pazik FD, Staton RJ, Williams JL, Arreola MM, Hintenlang DE, Bolch WE (2007) Organ and effective doses in newborns and infants undergoing voiding cystourethrograms (VCUG): a comparison of stylized and tomographic phantoms. Med Phys 34:294–306
Publication of updated diagnostic reference levels for diagnostic and interventional X-ray examinations. German Federal Office for Radiation Protection Web site2010 [February 3, 2015]; Available from: http://www.bfs.de/de/ion/medizin/diagnostik/drw_roentgen.pdf
Kliegman R (2011) Nelson textbook of pediatrics. Elsevier/Saunders
Lassmann M, Treves ST (2014) Paediatric radiopharmaceutical administration: harmonization of the 2007 EANM paediatric dosage card (version 1.5.2008) and the 2010 North American consensus guidelines. Eur J Nucl Med Mol Imaging 41:1036–1041
Kim SW, Im YJ, Hong CH, Han SW (2010) The clinical significance of intrarenal reflux in voiding cystourethrography (VCUG). Korean J Urol 51:60–63
Boubnova J, Sergent-Alaoui A, Deschenes G, Audry G (2011) Evolution and prognosis value of intrarenal reflux. J Pediatr Urol 7:638–643
Fukui S, Watanabe M, Yoshino K (2013) Intrarenal reflux in primary vesicoureteral reflux. Int J Urol Off J Jpn Urol Assoc 20:631–636
Acknowledgments
The scientific guarantor of this publication is Jürgen F Schäfer. The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article. The authors state that this work has not received any funding. One of the authors has significant statistical expertise. Institutional Review Board approval was obtained. Written informed consent was obtained from all subjects (patients) in this study. Methodology: retrospective, observational, performed at one institution.
Author information
Authors and Affiliations
Corresponding author
Additional information
Sara Y. S. Linke and Ilias Tsiflikas contributed equally to this work.
Rights and permissions
About this article
Cite this article
Linke, S.Y.S., Tsiflikas, I., Herz, K. et al. Ultra low-dose VCUG in children using a modern flat detectorunit. Eur Radiol 26, 1678–1685 (2016). https://doi.org/10.1007/s00330-015-3996-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-015-3996-5