Abstract
Objectives
Magnetic Resonance-guided Focused Ultrasound Surgery (MRgFUS) is a non-invasive method to treat uterine fibroids. To help determine the patient suitability for MRgFUS, we propose a new objective measure: the scaled signal intensity (SSI) of uterine fibroids in T2 weighted MR images (T2WI).
Methods
Forty three uterine fibroids in 40 premenopausal women were included in this retrospective study. SSI of each fibroid was measured from the screening T2WI by standardizing its mean signal intensity to a 0–100 scale, using reference intensities of rectus abdominis muscle (0) and subcutaneous fat (100). Correlation between the SSI and the non-perfused volume (NPV) ratio (a measure for treatment success) was calculated.
Results
Pre-treatment SSI showed a significant inverse-correlation with post treatment NPV ratio (p < 0.05). When dichotomizing NPV ratio at 45 %, the optimal cut off value of the SSI was found to be 16.0.
Conclusions
A fibroid with SSI value 16.0 or less can be expected to have optimal responses. The SSI of uterine fibroids in T2WI can be suggested as an objective parameter to help in patient selection for MRgFUS.
Key Points
• Signal intensity of fibroid in MR images predicts treatment response to MRgFUS.
• Signal intensity is standardized into scaled form using adjacent tissues as references.
• Fibroids with SSI less than 16.0 are expected to have optimal responses.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Magnetic resonance-guided focused ultrasound surgery (MRgFUS) is a non-invasive treatment modality. This approach integrates the heating capability of focused ultrasound with MRI visualization. Ultrasound energy is used to generate high temperatures and induce coagulative necrosis of the targeted lesions, while real time MR images allow monitoring of temperature changes and provide anatomical visualization for safe and effective treatment [1].
Several publications have demonstrated that MRgFUS is a safe treatment option for patients with symptomatic uterine fibroids, and that its clinical efficacy is comparable with uterine artery embolization (UAE) when achieving adequate levels of ablation, measured by the post treatment non-perfused volume ratio (NPV ratio)[2–4].
A key factor in achieving high levels of NPV ratios during MRgFUS is the correct patient selection. The fibroid absorption capability of ultrasound energy is supposed to be highly associated with treatment outcomes. Previous studies suggested that the signal intensity (SI) in T2-weighted MR images (T2WI), taken during patient MR screening, is a useful way to select the fibroids susceptible to thermal coagulation [5–7]. In those studies, fibroids were divided into two or three categories based on comparison of their SI with that of an adjacent normal tissue such as skeletal muscle or myometrium. Hypointense fibroids were reported to achieve more ablation volume than hyperintense ones and that group was accordingly proposed as an ideal category for MRgFUS. However, those categorical indications are rather subjective, and this feature of classification might lead to indifferent clinical outcomes among SI-grading categories.
In this study, we methodically calculated a scaled value of the fibroid SI, and compared it with the NPV ratio results, in order to assess whether this pre-procedural imaging parameter could predict the treatment response in terms of the NPV ratio, and if it could be used as an objective parameter to help in determining the suitability of a fibroid for MRgFUS.
Materials and methods
The study was done after the approval by the hospital’s institutional review board, and after all patients signed an informed consent form for the treatment. We retrospectively reviewed hospital records of all women with symptomatic uterine fibroids who were treated with MRgFUS between October 2008 and May 2009. We excluded all patients who had a hormonal therapy during the 6 months prior to the MRgFUS, in order to avoid a bias in their signal intensity due to the hormonal therapy. We also excluded patients with scars who were treated through a scar patch, due to potential bias of energy reflection.
As a standard of care in our hospital, in order to determine patient suitability for the MRgFUS treatment, two types of MR examinations are performed in screening: T2WI are acquired for anatomical visualization (TR 4830 ms, TE 120 ms, matrix size 256 × 144, slice thickness 5 mm, spacing 1 mm and FOV 30 cm), and T1WI are acquired for fibroid perfusion (TR 460 ms, TE 11 ms, matrix size 256 × 144, slice thickness 5 mm, spacing 1 mm and FOV 30 cm). Our criteria for the patient selection follow the guidelines described in previous literature [8]. In general, we do not treat patients with fibroids are not the source of their symptoms, patients with more than six fibroids, patients with significant intestine anterior to the uterus (which cannot be mitigated), and high SI fibroids in T2WI.
MRgFUS
The procedures were performed with the ExAblate 2000 system (InSightec Ltd., Haifa, Israel) integrated with a 1.5 T HDxt MRI (General Electric Healthcare, Milwaukee, WI, USA). Details of MRgFUS procedure have been described in previous publications [3, 9]. In brief, after patients were positioned prone on the ExAblate treatment table, the radiologist defined the region of treatment (ROT) so to cover as much of the fibroid volume as possible, while avoiding healthy tissues. Multiple sonications (focal energy deliveries) are then performed while MR images give real time feedback on temperature and anatomy. At the end of the treatment, contrast-enhanced T1WI are obtained to assess NPV in the fibroids.
Calculations of SI and NPV ratios
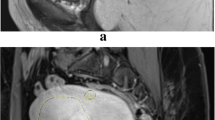
To calculate the SI of the fibroid, a sagittal T2WI, which encompassed the largest section of the fibroid, was selected. Three regions of interest (ROI) figures were placed over the centre of the fibroid, the rectus abdominis muscle, and the subcutaneous fat layer. ROIs included the maximum area of the tissues while avoiding the surrounding structures from which partial volume averaging could occur (Fig. 1).
The fibroid SI was then compared with the muscle and fat layer and transformed into a 0–100 scale through the following formula. Zero represents the intensity of the rectus abdominis muscle and 100 represents the intensity of fat.
Fibroid volume was measured slice-by-slice on the planning T2WI acquired prior to the treatment, and the NPV was measured slice-by-slice on the contrast-enhanced T1WI acquired immediately after the treatment. The NPV ratio was then calculated as the NPV divided by the fibroid volume.
Results
Between October 2008 and May 2009, 62 patients with symptomatic uterine fibroids were treated using MRgFUS in our institute. Of them, 13 patients were excluded due to a scar in the abdomen and nine patients were excluded as they had received hormonal therapy up to 6 months before the treatment. In total, forty women were included in this study. The mean age of the patients was 38.2 years ± 5.4 and the mean body mass index was 22.0 kg/m2 ± 2.5 (Table 1). Three patients had two fibroids and the other 37 patients had a single fibroid.
All patients completed MRgFUS in a single session, with mean sonication time of 141.3 min ± 51.4. The mean fibroid volume was 145.7 cm3 ± 124.3, and the mean NPV ratio was 62.2 % ± 19.1 %. The mean SSI was 14.0 ± 11.6 (range 0–45.5). Linear regression analysis between the SSI square root (resulting normal distribution) and the NPV ratio showed significant inverse correlation (p < 0.001)(Fig. 2).
In addition, all fibroids with SSI of 16.0 or less resulted in NPV ratios of more than 45 %, while only 47 % of the fibroids with SSI more than 16 resulted in NPV ratios of more than 45 % (p < 0.001).
Discussion
Studies of MRgFUS treatment for symptomatic uterine fibroid have shown that the post treatment NPV ratio is highly correlated with volume and symptom reduction [1, 2, 7]. Therefore ,the NPV ratio is considered the immediate predictor of the treatment’s success.
Fibroid T2WI SI is reported to be related to the treatment response of conservative modalities such as gonadotropin releasing hormone (GnRH) agonist, UAE, and MRgFUS [8, 10, 11]. The signal intensity can reflect proliferative activity and histological characteristics such as cellular density and fibre constitution [10, 12].
Various studies have shown the correlation between the fibroid SI and the NPV ratio. Lenard et al., retrospectively assessed the imaging predictors of treatment success in MR images [6] . They categorized fibroids into two groups comparing them to the SI of the skeletal muscle on T2WI as a reference. Restricted by early regulatory and technological limitations, they still showed that the NPV ratio was significantly lower in the hyperintense fibroids compared with hypointense ones (13.6 % ± 12.7 vs 19.9 % ± 13.2, P < 0.05).
In a prospective study by Funaki et al., fibroids were classified into three groups based on the SIs of the skeletal muscle and myometrium [5]. Fibroids having the highest SI (equal to or higher than that of myometrium), presented a NPV ratio of 33.3 %, significantly lower than the other two groups.
In this study, we suggest using the SSI, which is an objective quantitative measure that correlates well with the NPV ratio. The SSI of a fibroid is measured in a standardized method compared to the rectus abdominis muscle and the subcutaneous fat, each of which shows the extreme value of SI in a pelvic MRI. This way, the SSI is not biased from visual subjective measurement.
We showed that the pretreatment SSI correlates well with the NPV ratio, the measurement of the treatment success. Based on existing data, we also suggested a SSI cut-off value of 16, which results in NPV ratios higher than 45 %. An NPV ratio of 45 % was previously discussed as a determinant of treatment success [7, 10]. This way, physicians can integrate the SSI value in decision-making regarding patient suitability, taking this factor into account with other factors such as number of fibroids, location, size, and patient age.
The main limitation of the study is the fact that we used pre-screened data from commercial treatments, which includes mainly fibroids that have low SI values in efforts to select suitable patients. Therefore we lack the SSI data of high intensities. In addition, we included fibroids with heterogeneous signal intensities, which might have different characteristics from uniform intensities (such as high fibre content).
Despite the limitations, the SSI seems to be an objective and useful measure to predict the NPV ratio following MRgFUS. Larger scale studies, taking into account wider SSI distributions, wider NPV ratios, and additional covariates, can help in validating the prediction abilities of the SSI, and determine its use in patient selection for MRgFUS treatment of uterine fibroid.
References
McDannold N, Tempany CM, Fennessy FM et al (2006) Uterine leiomyomas: MR imaging-based thermometry and thermal dosimetry during focused ultrasound thermal ablation. Radiology 240:263–272
Stewart EA, Gostout B, Rabinovici J, Kim HS, Regan L, Tempany CM (2007) Sustained relief of leiomyoma symptoms by using focused ultrasound surgery. Obstet Gynecol 110:279–287
LeBlang SD, Hoctor K, Steinberg FL (2010) Leiomyoma shrinkage after MRI-guided focused ultrasound treatment: report of 80 patients. AJR Am J Roentgenol 194:274–280
Yoon SW, Kim KA, Whang YY, Lee C, Cha SH, Lee SY et al (2008) Magnetic resonance imaging-guided focused ultrasound surgery for uterine fibroids: Initial experience in Korea. Korean J Obstet and Gynecol 51:5
Funaki K, Fukunishi H, Funaki T, Sawada K, Kaji Y, Maruo T (2007) Magnetic resonance-guided focused ultrasound surgery for uterine fibroids: relationship between the therapeutic effects and signal intensity of preexisting T2-weighted magnetic resonance images. Am J Obstetrics Gynecol 196:184 e1-6
Lenard ZM, McDannold NJ, Fennessy FM et al (2008) Uterine leiomyomas: MR imaging-guided focused ultrasound surgery–imaging predictors of success. Radiology 249:187–194
Machtinger R, Inbar Y, Cohen-Eylon S, Admon D, Alagem-Mizrachi A, Rabinovici J (2012) MR-guided focus ultrasound (MRgFUS) for symptomatic uterine fibroids: predictors of treatment success. Hum Reprod 27:3425–3431
Yoon SW, Lee C, Cha SH et al (2008) Patient selection guidelines in MR-guided focused ultrasound surgery of uterine fibroids: a pictorial guide to relevant findings in screening pelvic MRI. Eur Radiol 18:2997–3006
Fennessy FM, Tempany CM, McDannold NJ et al (2007) Uterine leiomyomas: MR imaging-guided focused ultrasound surgery–results of different treatment protocols. Radiology 243:885–893
Oguchi O, Mori A, Kobayashi Y, Horiuchi A, Nikaido T, Fujii S (1995) Prediction of histopathologic features and proliferative activity of uterine leiomyoma by magnetic resonance imaging prior to GnRH analogue therapy: correlation between T2-weighted images and effect of GnRH analogue. J Obstet Gynaecol 21:107–117
Cura M, Cura A, Bugnone A (2006) Role of magnetic resonance imaging in patient selection for uterine artery embolization. Acta Radiol 47:1105–1114
Swe TT, Onitsuka H, Kawamoto K, Ueyama T, Tsuruchi N, Masuda K (1992) Uterine leiomyoma: correlation between signal intensity on magnetic resonance imaging and pathologic characteristics. Radiat Med 10:235–242
Acknowledgements
The authors thank Cha Bok Chung and Eun Joo Baek for their help in managing the patients during MRgFUS treatment. We also thank Suk Hee Kim and Seon Ha Yun for their help in preparing the manuscript.
The scientific guarantor of this publication is Professor Sang-Wook Yoon. The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article. The authors state that this work has not received any funding. One of the authors has significant statistical expertise. Institutional Review Board approval was obtained.
Written informed consent was waived by the Institutional Review Board. Methodology: retrospective, observational study, performed at one institution.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Park, H., Yoon, SW. & Sokolov, A. Scaled signal intensity of uterine fibroids based on T2-weighted MR images: a potential objective method to determine the suitability for magnetic resonance-guided focused ultrasound surgery of uterine fibroids. Eur Radiol 25, 3455–3458 (2015). https://doi.org/10.1007/s00330-015-3806-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-015-3806-0