Abstract
Objectives
Duchenne muscular dystrophy (DMD) is the most common and severe dystrophinopathy. DMD carriers rarely present with clinical symptoms, but may suffer from cardiac involvement. Because echocardiographic findings are inconsistent and cardiac magnetic resonance imaging (CMRI) data are limited, this study sought to investigate asymptomatic carriers for cardiac abnormalities using CMRI.
Methods
Fifteen genetically confirmed DMD carriers (age, 32.3 ± 10.2 years) were prospectively examined on a 1.5T MR system. Cine, T2, and late-gadolinium-enhanced (LGE) images were acquired, and were evaluated in consensus by two experienced readers. Left ventricular (LV) parameters were analysed semiautomatically, normalized to BSA.
Results
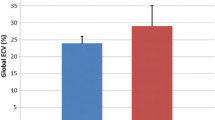
Normalized LV end-diastolic volume was increased in 7 % (73.7 ± 16.8 ml/m2; range, 48–116 ml/m2) and normalized LV end-systolic volume in 20 % (31.5 ± 13.3 ml/m2; range, 15–74 ml/m2). EF was reduced in 33 % (58.4 ± 7.6 %; range, 37–69 %) and normalized LV myocardial mass in 80 % (40.5 ± 6.8 g/m2; range, 31–55 g/m2). In 80 %, regional myocardial thinning was detected in more than one segment. In 13 % and 40 %, apical-lateral accentuation of LV non-compaction was present. LGE was found in 60 % (midmyocardial inferolateral accentuation).
Conclusions
Given the high frequency of cardiac pathologies detected by CMRI, regular cardiac risk assessment is advisable for DMD carriers. Besides clinical examination, CMRI is an excellent tool for this purpose.
Key Points
• Fifteen Duchenne muscular dystrophy carriers investigated using CMRI all showed cardiac pathologies.
• Myocardial mass reduction, regional myocardial thinning, and late gadolinium enhancement were common.
• Regular cardiac risk assessment is thus advisable in Duchenne muscular dystrophy carriers.
• Besides clinical examination, CMRI is an excellent tool for this purpose.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Duchenne muscular dystrophy (DMD) is both the most common and the most severe form of dystrophinopathy. Caused by a mutation in the dystrophin gene on chromosome Xp21.1, the disease affects approximately 1 in 3,500 live-born males [1]. In two-thirds of cases, it is inherited recessively X-linked; in one-third, it is caused by a de novo mutation. The affected males develop distinct general muscle degeneration and die at an early age, most often from respiratory (75 %) or cardiac failure (20 %). Affected adults present with a secondary dilated cardiomyopathy in 90 % of cases [2–4], which can lead to heart failure and sudden cardiac death [1, 5].
While female carriers of DMD may present asymptomatically, muscle weakness or cardiac involvement may develop in up to 50 % of cases [1]. An often asymptomatic dilated cardiomyopathy has been reported in 7–17 % of DMD carriers [6–8], and was proven to progress with age [9]. However, the literature remains contradictory whether this cardiac abnormality may lead to a higher rate of arrhythmia and heart failure [10–12].
Currently, cardiovascular magnetic resonance imaging (CMRI) data are limited, and echocardiographic findings in female DMD carriers continue to be inconsistent [13–15]. Therefore, the aim of this prospective study was to investigate cardiac abnormalities in genetically confirmed DMD carriers using CMRI in order to improve our understanding of the impairment and to assess cardiac risk in this population.
Materials and methods
Subjects
Prospective analysis and use of data was approved by the local ethics committee. Informed consent for CMRI examination, including contrast medium administration, was obtained from all participating DMD carriers. Study inclusion criteria included (a) genetic proof of heterozygous DMD carrier status and (b) a willingness to participate in the study. Exclusion criteria were any contraindications for CMRI or contrast medium administration.
Between February and October 2013, 15 genetically confirmed heterozygous carriers of DMD were examined. The mean age of the subjects was 32.3 ± 10.2 years (range, 14–46 years). Mean weight was 64.3 ± 8.0 kg (range, 55–81 kg) and mean height was 164.9 ± 4.0 cm (range, 159–174 cm), resulting in a mean body surface area of 1.71 ± 0.08 m2 (range, 1.6–1.9 m2). No subject had suffered any previous heart disease.
Cardiovascular magnetic resonance imaging
All MR examinations were performed on a 1.5-Tesla system (MAGNETOM Aera; Siemens Healthcare, Erlangen, Germany). Balanced steady-state free precision (SSFP) cine sequences (repetition time (TR), 2.65 ms; echo time (TE), 1.11 ms; matrix, 156 × 192; FOV, 340 mm; flip angle, 56°) and T2-weighted turbo spin-echo (TSE) dark blood sequences (TR, 1,763 ms; TE, 60 ms; matrix, 154 × 256; FOV, 340 mm; flip angle, 180°) with 8 mm slice thickness in two-, three-, and four-chamber views and in short-axis view (2CH, 3CH, 4CH, SA) were acquired. Ten min after contrast medium injection a TI scout was performed to individually adjust TI for the following T1-weighted inversion-recovery fast low-angle shot sequences (T1W IR-FLASH; TR, 700 ms; TE, 3.3 ms; matrix, 156 × 256; FOV, 340 mm; flip angle, 25°; slice thickness, 8 mm) in 2CH, 3CH, 4CH, and SA. The contrast medium Gadobutrol (Gadovist, Bayer Healthcare, Leverkusen, Germany) was used at a dosage of 0.2 mmol Gadobutrol/kg body weight (maximum: 20 mmol) with a flow rate of 2 ml/sec.
Two radiologists with CMRI experience (>5 years and >11 years) evaluated all CMRI studies in consensus. Using the disc summation method (Argus software, Siemens Healthcare, Erlangen, Germany; standard values employed based on [16]) absolute and normalized (n) left ventricular (LV) end-diastolic volume (EDV), LV end-systolic volume (ESV), LV stroke volume (SV), LV ejection fraction (EF), and LV myocardial mass were acquired. Average end-diastolic thickness of the compact myocardium (except for segment 17), LV wall motion abnormalities, and late gadolinium enhancement (LGE) were assessed based on the 17-segment model [17]. The average end-diastolic thickness of the midventricular septum was measured in the middle of the septum. Additionally, the thinnest part of the free LV wall was measured (in segment 7 in two carriers, and in segment 11 in the remaining 13 carriers). According to Dawson et al., an average thickness of the compact myocardium ≤4 mm in one segment was considered to be reduced [18]. CMRI images were also analysed for structural LV myocardial abnormalities such as distribution of transverse relaxation time (T2) and LV non-compaction (LVNC). The T2 myocardial/skeletal muscle ratio was assessed for the septum (in which LGE was never present) and for the myocardial region corresponding to the most apparent LGE in the T1W IR-FLASH images.
For LVNC assessment, the ratio of non-compacted to compacted myocardium in short-axis slices was measured. As there is currently no consensus regarding the best diagnostic criteria for LVNC, we employed both the most commonly used Petersen criteria (cutoff of 2.3 for the ratio of non-compacted to compacted myocardium) and, to avoid overdiagnosis of LVNC, the more conservative Grothoff criteria (cutoff of 3.0 for the ratio of non-compacted to compacted myocardium) [19–22]. Further cardiac pathologies such as pericardial effusion were also registered.
Statistical analysis
For statistical analysis, the IBM SPSS Statistics for Windows software package was used (Version 19.0; IBM Corp., Armonk, NY, USA). Normal distribution of the parameters was investigated using the Kolmogorov-Smirnov test. Mean values and standard deviations were calculated using normally distributed data. Categorical agreement between the presence of regional hypokinesia and LGE was calculated for every cardiac segment using Cohen’s kappa (κ < 0.00, poor agreement; κ = 0.00–0.20, slight agreement; κ = 0.20–0.40, fair agreement; κ = 0.40–0.60, moderate agreement; κ = 0.60–0.80, substantial agreement; and κ = 0.80–1.00, very good agreement). Correlation analysis was performed using Pearson, Spearman, or Chi-square tests, depending on the scale level and distribution of the compared parameters. The T2 ratios were compared using paired t tests.
Results
All DMD carriers demonstrated cardiac abnormalities. Mean nEDV was 73.7 ± 16.8 ml/m2 (range, 48–116 ml/m2; Table 1), and was increased in 7 % of the subjects. Mean nESV measured 31.5 ± 13.3 ml/m2 (range, 15–74 ml/m2), and was increased in 20 % of cases. Mean nSV was 42.2 ± 7.0 ml/m2 (range, 33–54 ml/m2), and was decreased in 47 % of cases. Compared to that in healthy adults, EF was slightly reduced in 27 % of cases and moderately reduced in 7 % (mean EF, 58.4 ± 7.6 %; range, 37–69 %). In one carrier with moderate EF reduction, the nEDV measured 121 % of the upper bound of the nEDV standard value. This carrier was thus diagnosed with dilated cardiomyopathy [23], resulting in a 7 % rate of dilated cardiomyopathy in our cohort.
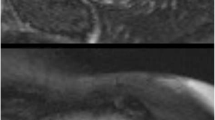
Normalized myocardial mass was reduced in 80 % of subjects (40.5 ± 6.8 g/m2; range, 31–55 g/m2). Regional LV myocardial thinning was seen in more than one segment in 80 % of cases, with apical accentuation, and found in 3.5 segments on average per DMD carrier (Figs. 1, 2, 3, and 4). The end-diastolic mean midventricular septal thickness was 7.4 ± 1.1 mm (range, 6–9 mm). The end-diastolic mean thickness of the thinnest part of the free LV compact wall was 5.6 ± 1.7 mm (range, 3–8 mm). LVNC was found in 40 % of cases using the Petersen criteria and in 13 % of cases using the more conservative Grothoff criteria, and was characterised by an apical-lateral accentuation (Figs. 3 and 4).
Forty—year-old Duchenne muscular dystrophy carrier. SSFP cine sequence short-axis apical (a, b) and T1W IR-FLASH sequence (10 min after contrast medium injection) 4CH (c) and short-axis midventricular (d). In a and b, a slightly enlarged portion of spongy myocardium and thinning of the compact left ventricular wall are present. In c and d, a streaky midmyocardial and subepicardial LGE, primarily in the lateral midventricular wall, can be seen (arrows)
Forty-year-old Duchenne muscular dystrophy carrier. SSFP cine sequence 4CH (a), short-axis midventricular (b) and apical (d), and T2 TSE dark blood sequence short-axis midventricular (c) demonstrating an end-diastolic thinning of the left ventricular wall, increased trabecularisation, and anterior midventricular and apical non-compaction
Fourteen-year-old Duchenne muscular dystrophy carrier. SSFP cine sequence midventricular (a) and apical short-axis (b) showing a slight pericardial effusion, apical non-compaction, and wall thinning. T1W IR-FLASH sequence midventricular short-axis (c and d, 10 min after contrast medium injection) demonstrating a streaky midmyocardial and subepicardial LGE in the free left ventricular wall
Regional wall hypokinesia was detected in 47 % of carriers (Fig. 2). LGE was found in 60 % of subjects, and was located predominantly in the lateral and inferolateral walls, and was detected in an average of 3.6 segments (Figs. 1, 2, 4, and 5). LGE was characterised as streaky/patchy, most commonly located in the midmyocardial region (27 %), and less often in both midmyocardial and subepicardial (20 %) or exclusively subepicardial (13 %) regions. Among all 15 carriers, LGE was found to be present in a total of 32 cardiac segments. Of these, myocardial thinning was also present in five segments, LVNC was found in one, and regional hypokinesia was seen in 11 of the 32 segments.
There was no correlation found between EF values and the frequently reduced normalized myocardial mass (r = −0.026, p = 0.928), the presence of myocardial thinning (r = 0.193, p = 0.491), or the presence of LGE (r = −0.063, p = 0.824). Likewise, no relationship was found between the normalized myocardial mass and the presence of LGE (r = −0,157, p = 0.575) or between myocardial thinning and regional hypokinesia (p = 0.605). Hypokinesia and LGE (considering all cardiac segments) were significantly correlated (κ = 0.422, p < 0.001).
Visually, no increased myocardial T2 signal was detected in the T2 images. The mean T2 myocardial/skeletal muscle ratio of the septum was 1.74 ± 0.51, and in the LV myocardial region with the most obvious LGE, the mean ratio was 1.76 ± 0.91. There was no significant difference in T2 ratio between the two regions (p = 0.684), and thus no sign of myocardial oedema. Additionally, pericardial effusion was present in 13 % of all subjects (Fig. 4).
Discussion
Male DMD patients frequently suffer from secondary dilated cardiomyopathy, and cardiac death is a major cause of mortality in this population [1–5]. In these patients, cardiomyocytes are primarily replaced by connective tissue and fat in the inferobasal and lateral left ventricular wall [1]. Yilmaz et al. assume that this is due to a diffuse membranous dystrophin defect of the cardiomyocytes, resulting in reduced resistance of the structurally impaired cardiomyocytes to mechanical strain that is known to occur in the inferolateral wall [4, 24]. The mechanism of cardiac damage, however, is not completely understood.
Silva et al. were the first to report a midmyocardial/subepicardial LGE detected by CMRI in patients with DMD and Becker muscular dystrophy (BMD), which was located primarily in the lateral LV wall [3]. Puchalski et al. described a subepicardial LGE of the inferobasal LV wall [25]. A comparative CMRI investigation of an affected DMD patient and its carrier mother reported a similar LGE pattern in both [14]. In other studies, LVNC located predominantly in the apical segments was reported in up to 28 % of DMD patients by Statile et al., and in up to 19 % of DMD and BMD patients by Kimura et al. [15, 26]. Kimura et al. also reported significantly higher EDV and ESV values and significantly lower EF values in DMD patients with LVNC, and described a faster deterioration of cardiac function in this group [26].
In contrast to DMD patients, female carriers of DMD clinically may appear unimpaired. They can present with a wide range of clinical involvement, from a lack of symptoms to severe impairment, including heart failure [1], which raises the question of cardiac risk assessment in this population.
We investigated 15 genetically confirmed DMD carriers using CMRI, and found cardiac pathologies in all. A reduced normalized myocardial mass was seen in 80 % of cases, regional LV myocardial thinning in 80 %, LGE in 60 %, reduced EF in 33 %, and LVNC in 13 % and 40 % (using Grothoff and Petersen criteria, respectively). In contrast to previously reported rates of 50–84 % for the diagnosis of cardiac involvement in DMD carriers using ECG, echocardiography, myocardial scintigraphy, and CMRI [1, 27], our results demonstrate a much higher frequency of cardiac pathologies, which were present in all investigated DMD carriers.
Left ventricular volumes and EF
In our cohort, nEDV was increased in 7 % and nESV was increased in 20 % of carriers. EF was slightly reduced in 27 % and moderately reduced in 7 %, and the rate of dilated cardiomyopathy was 7 %. Grain et al. reported echocardiographic assessment of cardiomyopathy in 7 % in their DMD and BMB carriers, and Hoogerwaard et al. noted an 8 % rate of dilated cardiomyopathy in their DMD carriers [6, 7], which corresponds well with the current findings. Comi et al. reported a higher rate, 17 %, of clinically, electrocardiographically, and echocardiographically proven cardiomyopathy in DMD and BMD carriers, and Ueda et al. reported combined increased EDV and reduced EF of 75 % in their DMD carriers [6, 8, 12]. Considering the progression of cardiac pathology in DMD carriers with age, the relatively low rate of dilated cardiomyopathy in our cohort compared to those of Comi et al. and Ueda et al. may be explained, in part, by the low age of the DMD carriers in our cohort [6, 8, 12]. In several case studies, congestive heart failure, severe reduction in EF (up to 20 %), and increased EDV have also been reported in DMD carriers [28–30].
Wall thinning and LVNC
In 80 % of DMD carriers we found regional wall thinning in more than one segment. Apical-lateral accentuation of LVNC was found in 13 % of DMD carriers using the Grothoff criteria and in 40 % using the Petersen criteria. Apically accentuated LVNC was recently described in 19–28 % of DMD patients, and was interpreted as muscular degeneration versus compensatory remodelling [15, 31]. Finsterer et al. noted one case of hypertrabecularisation in a BMD carrier [32], but this is the first report of LVNC in DMD carriers.
Myocardial mass
Reduced normalized myocardial mass was detected in 80 % of cases, which conflicts with recently published CMRI data. Barison et al. noted one DMD carrier with dilated cardiomyopathy (reduced EF, increased EDV) and lateral patchy LGE who had normal LV mass, and Walcher et al. reported four DMD carriers with normal LV mass [30]. This obvious difference is presumably due to the high frequency of regional wall thinning (80 %) and LVNC (13 % and 40 % using Grothoff and Petersen criteria, respectively) in our cohort.
Delayed gadolinium enhancement and hypokinesia
LGE was detected in 60 % of DMD carriers and was accentuated midmyocardially in the lateral and inferolateral LV wall. In several recent case reports, LGE in DMD carriers was reported to be located predominantly in the inferolateral subepicardial region [14, 30, 33–35], presumably induced by exaggerated mechanical stress in this region [4, 36]. Furthermore, regional hypokinesia was frequently observed in segments with LGE, and the presence of both pathologies was correlated. This finding conclusively demonstrates that the presence of LGE represents myocardial fibrosis, which impairs cardiac contractility.
Our study is not without limitations. Due to the strict inclusion criteria, our screened cohort was relatively small. We included only genetically confirmed heterozygous DMD carriers. However, given the distinct findings in our study, we consider our data representative.
In summary, we found cardiac pathologies in all 15 investigated female carriers of Duchenne muscular dystrophy. The most frequent and obvious findings were reduced normalized myocardial mass, regional LV myocardial thinning, and LGE. Due to the high frequency of cardiac pathologies detected by CMRI, a regular cardiac risk assessment in DMD carriers is advisable. In addition to clinical examination, CMRI was proven to be an excellent tool for this purpose.
Abbreviations
- CMRI:
-
Cardiovascular magnetic resonance imaging
- DMD:
-
Duchenne muscular dystrophy
- EDV:
-
End-diastolic volume
- ESV:
-
End-systolic volume
- LGE:
-
Late gadolinium enhancement
- LV:
-
Left ventricular
- LVNC:
-
Left ventricular non-compaction
- n:
-
Normalized
References
Finsterer J, Stollberger C (2003) The heart in human dystrophinopathies. Cardiology 99:1–19
Maron BJ, Towbin JA, Thiene G et al (2006) Contemporary definitions and classification of the cardiomyopathies: an American Heart Association Scientific Statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention. Circulation 113:1807–1816
Silva MC, Meira ZM, Gurgel Giannetti J et al (2007) Myocardial delayed enhancement by magnetic resonance imaging in patients with muscular dystrophy. J Am Coll Cardiol 49:1874–1879
Yilmaz A, Sechtem U (2012) Cardiac involvement in muscular dystrophy: advances in diagnosis and therapy. Heart 98:420–429
Fayssoil A, Nardi O, Orlikowski D, Annane D (2013) Cardiac asynchrony in Duchenne muscular dystrophy. J Clin Monit Comput 27:587–589
Hoogerwaard EM, Bakker E, Ippel PF et al (1999) Signs and symptoms of Duchenne muscular dystrophy and Becker muscular dystrophy among carriers in the netherlands: a cohort study. Lancet 353:2116–2119
Grain L, Cortina-Borja M, Forfar C, Hilton-Jones D, Hopkin J, Burch M (2001) Cardiac abnormalities and skeletal muscle weakness in carriers of Duchenne and becker muscular dystrophies and controls. Neuromuscul Disord 11:186–191
Comi LI, Nigro G, Politano L, Petretta VR (1992) The cardiomyopathy of Duchenne/becker consultants. Int J Cardiol 34:297–305
Schade van Westrum SM, Hoogerwaard EM, Dekker L et al (2011) Cardiac abnormalities in a follow-up study on carriers of Duchenne and Becker muscular dystrophy. Neurology 77:62–66
Stollberger C, Blazek G, Wegner C, Finsterer J (2012) Neurological comorbidity affects prognosis in left ventricular hypertrabeculation/noncompaction. Heart Lung 41:594–598
Holloway SM, Wilcox DE, Wilcox A et al (2008) Life expectancy and death from cardiomyopathy amongst carriers of Duchenne and Becker muscular dystrophy in scotland. Heart 94:633–636
Ueda Y, Kawai H, Adachi K, Naruo T, Saito S (1995) Cardiac dysfunction in female gene carriers of Duchenne muscular dystrophy. Rinsho Shinkeigaku 35:1191–1198
Mavrogeni S, Papavasiliou A, Skouteli E, Magoutas A, Dangas G (2010) Cardiovascular magnetic resonance imaging evaluation of two families with Becker muscular dystrophy. Neuromuscul Disord 20:717–719
Yilmaz A, Gdynia HJ, Ludolph AC, Klingel K, Kandolf R, Sechtem U (2010) Images in cardiovascular medicine. Cardiomyopathy in a DuchenneDuchenne muscular dystrophy carrier and her diseased son: similar pattern revealed by cardiovascular MRI. Circulation 121:e237–e239
Statile CJ, Taylor MD, Mazur W et al (2013) Left ventricular noncompaction in Duchenne muscular dystrophy. J Cardiovasc Magn Reson 15:67-429X-15-67
Maceira AM, Prasad SK, Khan M, Pennell DJ (2006) Normalized left ventricular systolic and diastolic function by steady state free precession cardiovascular magnetic resonance. J Cardiovasc Magn Reson 8:417–426
Cerqueira MD, Weissman NJ, Dilsizian V et al (2002) Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation 105:539–542
Dawson DK, Maceira AM, Raj VJ, Graham C, Pennell DJ, Kilner PJ (2011) Regional thicknesses and thickening of compacted and trabeculated myocardial layers of the normal left ventricle studied by cardiovascular magnetic resonance. Circ Cardiovasc Imaging 4:139–146
Kawel N, Nacif M, Arai AE et al (2012) Trabeculated (noncompacted) and compact myocardium in adults: the multi-ethnic study of atherosclerosis. Circ Cardiovasc Imaging 5:357–366
Grothoff M, Pachowsky M, Hoffmann J et al (2012) Value of cardiovascular MR in diagnosing left ventricular non-compaction cardiomyopathy and in discriminating between other cardiomyopathies. Eur Radiol 22:2699–2709
Gati S, Rajani R, Carr-White GS, Chambers JB (2014) Adult left ventricular noncompaction: reappraisal of current diagnostic imaging modalities. JACC Cardiovasc Imaging 7:1266–1275
Petersen SE, Selvanayagam JB, Wiesmann F et al (2005) Left ventricular non-compaction: insights from cardiovascular magnetic resonance imaging. J Am Coll Cardiol 46:101–105
Thomas DE, Wheeler R, Yousef ZR, Masani ND (2009) The role of echocardiography in guiding management in dilated cardiomyopathy. Eur J Echocardiogr 10:iii15–iii21
Yildiz H, Tanay E, Rösch S, Sechtem U, Yilmaz A (2012) Ursache der spezifischen Kardiomyopathie bei Muskeldystrophie-Patienten: Genetisch bedingte Strukturschwäche vs. Myokarditis? – Antworten durch einen Geschwistervergleich. Oral presentation at the German cardiac annual conference in Mannheim
Puchalski MD, Williams RV, Askovich B et al (2009) Late gadolinium enhancement: precursor to cardiomyopathy in Duchenne muscular dystrophy? Int J Cardiovasc Imaging 25:57–63
Kimura K, Takenaka K, Ebihara A et al (2013) Prognostic impact of left ventricular noncompaction in patients with Duchenne/Becker muscular dystrophy—prospective multicenter cohort study. Int J Cardiol 168:1900–1904
Politano L, Nigro V, Nigro G et al (1996) Development of cardiomyopathy in female carriers of Duchenne and Becker muscular dystrophies. JAMA 275:1335–1338
Tunteeratum A, Witoonpanich R, Phudhichareonrat S et al (2009) Congestive heart failure with rhabdomyolysis and acute renal failure in a manifesting female carrier of Duchenne muscular dystrophy with duplication of dystrophin gene. J Clin Neuromuscul Dis 11:49–53
Cheng VE, Prior DL (2013) Peripartum cardiomyopathy in a previously asymptomatic carrier of Duchenne muscular dystrophy. Heart Lung Circ 22:677–681
Walcher T, Steinbach P, Spiess J et al (2011) Detection of long-term progression of myocardial fibrosis in Duchenne muscular dystrophy in an affected family: a cardiovascular magnetic resonance study. Eur J Radiol 80:115–119
Finsterer J, Stollberger C, Feichtinger H (2007) Non-compaction on autopsy in Duchenne muscular dystrophy. Cardiology 108:161–163
Finsterer J, Stollberger C, Wexberg P, Schukro C (2012) Left ventricular hypertrabeculation/non-compaction in a Duchenne/Becker muscular dystrophy carrier with epilepsy. Int J Cardiol 162:e3–e5
Finsterer J, Stollberger C, Avanzini M, Bastovansky A, Wexberg P (2011) Late gadolinium enhancement as subclinical myocardial involvement in a manifesting Duchenne carrier. Int J Cardiol 146:231–232
Barison A, Aquaro GD, Passino C et al (2009) Cardiac magnetic resonance imaging and management of dilated cardiomyopathy in a Duchenne muscular dystrophy manifesting carrier. J Neurol 256:283–284
Walcher T, Kunze M, Steinbach P et al (2010) Cardiac involvement in a female carrier of Duchenne muscular dystrophy. Int J Cardiol 138:302–305
Yilmaz A, Gdynia HJ, Ponfick M, Ludolph AC, Rosch S, Sechtem U (2011) The proteoglycan-dystrophin complex in genetic cardiomyopathies—lessons from three siblings with limb-girdle muscular dystrophy-2I (LGMD-2I). Clin Res Cardiol 100:611–615
Acknowledgements
The scientific guarantor of this publication is Thomas Schlosser. The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article. The authors state that this work has not received any funding. No complex statistical methods were necessary for this paper. Institutional review board approval was obtained. Written informed consent was obtained from all subjects in this study. No study subjects have been previously reported. Methodology: prospective cross-sectional study, performed at one institution.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schelhorn, J., Schoenecker, A., Neudorf, U. et al. Cardiac pathologies in female carriers of Duchenne muscular dystrophy assessed by cardiovascular magnetic resonance imaging. Eur Radiol 25, 3066–3072 (2015). https://doi.org/10.1007/s00330-015-3694-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-015-3694-3