Abstract
Objectives
To investigate the technical feasibility of whole-body intravoxel incoherent motion (IVIM) imaging.
Materials and Methods
Whole-body MR images of eight healthy volunteers were acquired at 3T using a spin-echo echo-planar imaging sequence with eight b-values. Coronal parametrical whole-body maps of diffusion (D), pseudodiffusion (D*), and the perfusion fraction (Fp) were calculated. Image quality was rated qualitatively by two independent radiologists, and inter-reader reliability was tested with intra-class correlation coefficients (ICCs). Region of interest (ROI) analysis was performed in the brain, liver, kidney, and erector spinae muscle.
Results
Depiction of anatomic structures was rated as good on D maps and good to fair on D* and Fp maps. Exemplary mean D (10−3 mm2/s), D* (10−3 mm2/s) and Fp (%) values (± standard deviation) of the renal cortex were as follows: 1.7 ± 0.2; 15.6 ± 6.5; 20.9 ± 4.4. Inter-observer agreement was “substantial” to “almost perfect” (ICC = 0.80 – 0.92). The coefficient of variation of D* was significantly lower with the proposed algorithm compared to the conventional algorithm (p < 0.001), indicating higher stability.
Conclusion
The proposed IVIM protocol allows computation of parametrical maps with good to fair image quality. Potential future clinical applications may include characterization of widespread disease such as metastatic tumours or inflammatory myopathies.
Key Points
• IVIM imaging allows estimation of tissue perfusion based on diffusion-weighted MRI.
• In this study, a clinically suitable whole-body IVIM algorithm is presented.
• Coronal parametrical whole-body maps showed good depiction of anatomic details.
• Potential future applications include detection of widespread metastatic or inflammatory disease.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Diffusion-weighted magnetic resonance imaging (DWI) is widely used for disease detection and characterization of focal lesions, particularly tumours, in different organs [1]. Many tumours exhibit a diffusion restriction compared to surrounding healthy parenchyma due to their high cellularity. The degree of diffusion restriction of water molecules in tissue is quantified with the apparent diffusion coefficient (ADC) in units of mm2/s. The determination of the ADC is based on the assumption of a mono-exponential relationship between b-value and signal intensity given by the equation
where Sb is the signal intensity depending on the b-value, and S0 is the signal intensity when the b-value is zero. The b-value is a sequence parameter that adjusts the degree of diffusion weighting of the DWI sequence by different strength and duration of the diffusion gradients.
However, for low b-values, typically below 150 s/mm2, the measured signal not only reflects pure molecular water diffusion, but also “pseudodiffusion” resulting from fast moving water molecules, e.g. in the capillaries [2–7]. Based on the concept of intravoxel incoherent motion (IVIM), the contribution of both diffusion and pseudodiffusion to the signal intensity is described by a bi-exponential relationship between b-value and signal intensity according to the following equation:
where D reflects pure molecular diffusion, D* is the pseudodiffusion, and Fp is the relative perfusion fraction.
Dedicated IVIM protocols have been applied for tissue characterization in a variety of organs, including the brain [8], liver [6, 9–12], kidneys [11, 13–15], and muscles [7, 11, 16, 17]. This is the first study to present a clinically suitable whole-body IVIM algorithm. By providing more comprehensive tissue characterization, whole-body IVIM may be advantageous over conventional whole-body DWI in the assessment of metastatic disease with hypervascular tumours, such as neuroendocrine tumours, renal cell carcinoma, or melanoma.
Materials and methods
Subjects
This prospective study was performed on eight healthy volunteers (four men, four women; mean age, 30.6 years; range, 24 – 35 years; mean body height, 1.74 m; range, 1.65 – 1.86 m). Approval by the local ethics committee was obtained. All individuals gave written informed consent to the MR examination and subsequent scientific evaluation of the data sets. Three subjects (two men, one woman; age range, 24 – 31 years) underwent a second scan 4 months after the initial visit in order to assess the repeatability of D, D*, and Fp.
Imaging protocol
All scans were performed on a 3 Tesla whole-body MR scanner (Ingenia, Philips Healthcare, Best, the Netherlands) using a 15-channel head coil, a 32-channel flexible anterior coil, and the integrated posterior coil. Images were acquired using a spin-echo prepared echo-planar imaging (EPI) sequence in transversal orientation during free breathing. The MR protocol consisted of three (head to proximal femur) to six (whole body) stacks of axial slices, each with a field of view of 450 (right to left) x 295 (anterior to posterior) x 300 (head to feet) mm3. Imaging parameters were as follows: TR, 8,708 ms; TI, 220 ms; TE, 64 ms; voxel size, 3.5 × 3.5 × 6 mm3; EPI factor, 29; SENSE factor, 3; acquisition matrix, 128 × 81; number of signal averages, 3; b-values, 0, 10, 20, 50, 150, 300, 500, and 800 s/mm2. Fat suppression was performed using spectral selection attenuated inversion recovery (SPAIR). The baseline scanning position was set at the jugular notch, with one image stack above and the other ones below this reference level.
Post-processing
The initial images were processed for the IVIM analysis using Matlab (The MathWorks, Natick, MA, USA) implemented by A.B.. with more than 10 years of experience in MRI post-processing. Parametric maps of D, D*, and Fp were calculated on a pixel-wise basis using Matlab routines. Initial stacks of axial image data sets were linked together and reformatted in the coronal orientation. Noise was measured as the standard deviation in a region of interest (ROI) placed in the background, and noise-correction was performed on the data sets by squared subtraction according to Gudbjartsson [18].
To obtain D, D*, and Fp values, a multi-step IVIM approach was applied on the bi-exponential relationship describing the measured signal decay:
-
a)
Because of the absence of perfusion effects at higher b-values, the term reflecting pseudodiffusion in Eq. (2) can be disregarded and the signal decay is described by a mono-exponential relation. D was computed by a linear least-squares fit to the log-transformed signal intensities for b-values ≥ 150 s/mm2. D corresponds to the slope and S0’ with S0’ = S0 (1 – Fp) to the y-axis intercept of the regression line:
-
b)
The perfusion fraction Fp was then calculated from the measured signal intensity of the b0-measurement S0 and the calculated S0’:
-
c)
If Fp is ≤ 0 or ≥ 1, no pseudodiffusion is detectable and a mono-exponential fit to Eq. (1) is carried out with all b-values applying an algorithm based on the Levenberg-Marquardt algorithm recalculating D with higher precision. D* and Fp are both set to zero.
-
d)
If 0 < Fp < 1, a linear least-squares fit to the log-transformed signal intensities of the first 4 b-values was calculated with the slope denoting D*’, which contains contributions from both diffusion and pseudodiffusion.
-
e)
From D*’, the pseudodiffusion coefficient D* was calculated based on the following equation:
This algorithm is an approximation assuming nearly mono-exponential signal decay for low b-values with an effective diffusion coefficient D*’. Equation (5) can be obtained using another approximation step for low b-values with a first-order Taylor series expansion of the exponentials in Eq. (2) replacing exp(x) with (1-x). This algorithm was presumed to provide more stable IVIM results compared to the conventional bi-linear fitting algorithm [2]; this hypothesis was tested as described below.
Qualitative rating
Coronal and axial parametrical maps of D, D*, and Fp were rated qualitatively by two independent radiologists with 6 and 2 years of experience in MR imaging, respectively. A 4-point scale was used to rate the depiction of the brain, aorta, liver, renal cortex, and medulla on the different parametrical maps: 1 (excellent depiction), 2 (good depiction), 3 (fair depiction), 4 (poor depiction). Furthermore, the overall impression of image noise on D, D*, and Fp maps was defined based on another 4-point scale: 1 (very little noise), 2 (little noise), 3 (moderate noise), 4 (severe noise).
Measurement of IVIM parameters and quantitative analysis
IVIM parameters were measured by reader one in standardized ROIs on axial images in the cerebrospinal fluid (CSF, measured in the lateral ventricles; ROI size, 40 ± 3 mm2), cerebral gray matter (GM, measured in the striatum; 98 ± 9 mm2) and white matter (WM, measured in the corona radiata; 151 ± 13 mm2), liver (1398 ± 218 mm2), renal cortex (232 ± 41 mm2), renal medulla (81 ± 6 mm2), and erector spinae muscle (at the level of the kidneys; 431 ± 10 mm2). Large vascular structures such as the hepatic veins were avoided. Descriptive statistics were applied to the ROI analyses with the calculation of mean values and standard deviations.
In order to quantify the ‘noise’ on the parametrical maps, the coefficient of variation of D* and FP (i.e., the standard deviation divided by the mean) was calculated in the liver and kidneys. These variability measurements were compared between our whole-body algorithm and a conventional bi-exponential fitting method.
Statistical analysis
Statistical analysis was performed using commercially available software (IBM SPSS Statistics, version 19, IBM Corp., Somers, NY, USA). For the evaluation of the inter-observer agreement, intra-class correlation coefficients (ICCs; absolute agreement, two-way mixed) were calculated. An ICC of 0.21 – 0.40 was considered to be indicative of fair agreement, 0.41 – 0.60 of moderate agreement, 0.61–0.80 of substantial agreement, and an ICC greater than 0.80 to be indicative of almost perfect agreement (ICC = 1.00, perfect agreement) [19].
The coefficients of variation of D* and Fp were compared between our algorithm and the conventional fitting algorithm using paired Student t-tests with Bonferroni correction for multiple comparisons (yielding a p-value below 0.05/2 = 0.025 to indicate statistically significant differences). The repeatability of D, D*, and FP values (in all organs) was assessed in the three subjects who underwent a follow-up scan by calculating within-subject coefficients of variation.
Results
Acquisition time
Because of limited available scanner time, not all subjects were imaged from head to toe. Total scan times were 72 min (whole-body, n = 4), 48 min (head to knee, n = 2), and 36 min (head to proximal femur, n = 2), respectively.
Parametrical maps
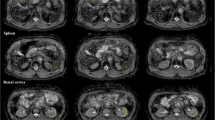
Exemplary coronal maps of D, D*, and Fp at different positions are shown in Figs. 1, 2, 3 and 4 (slice thickness 3.5 mm each). Structures of parenchymal organs could well be recognized on all three parametrical maps. The maps exhibited typical IVIM characteristics: CSF, renal pelvis, and bladder were all characterized by a high D value in the order of 2 – 3 × 10−3 mm2/s, consistent with unrestricted motion of the water molecules. On the other hand, D* and Fp were high in the renal pelvis compared to CSF, indicating faster flow of water molecules in urine being excreted compared to non-detectable flow of water molecules in the CSF. The liver was characterized by a lower D but higher Fp value compared to the renal cortex and medulla. Large vascular structures, such as the heart and the aorta, showed low D close to zero, but high D* and Fp values, consistent with predominantly fast moving water molecules.
Exemplary coronal whole-body maps of diffusion (D), pseudodiffusion (D*) and perfusion fraction (Fp) in a 31-year-old man. Slice thickness was 3.5 mm. The liver (asterisk) and the femoral vessels (arrowhead) are characterized by a low D but high D* and Fp values, indicating predominantly fast moving water molecules. On the contrary, the CSF (arrow) shows a high D but low D* and Fp values
Exemplary coronal maps of diffusion (D), pseudodiffusion (D*) and perfusion fraction (Fp) in a 27-year-old man. Slice thickness was 3.5 mm. Whereas D is high in both CSF (arrow) and renal pelvis (arrowhead), D* and Fp are considerably higher in the renal pelvis, indicating higher flow of the urine relative to the CSF. The liver is characterized by a lower D (asterisk) but higher Fp compared to the renal cortex
Exemplary coronal maps of diffusion (D), pseudodiffusion (D*) and perfusion fraction (Fp) in a 34-year-old woman. Slice thickness was 3.5 mm. Whereas the heart (arrowhead) shows high D* and Fp values, D is low, indicating predominantly fast moving water molecules. By contrast, the bladder (arrow) is characterized by a high D but low D* and Fp values
Qualitative rating
The results of the qualitative rating and the inter-observer agreement are listed in Table 1. Depiction of anatomic structures was rated good on D maps and good (aorta) to fair (brain) on D* and Fp maps. Overall image noise was rated very little on D maps, little on D* maps and moderate on Fp maps. The inter-observer agreement was substantial to almost perfect (ICC = 0.80 – 0.92).
Quantitative values and stability of IVIM algorithm
Typical signal intensities for different organs or body compartments and corresponding fitting curves are shown in Fig. 5. Without organ-specific adaptations, the algorithm provided stable IVIM parametric results for both parenchymal organs as well as fluid containing compartments. The percentage of voxels in which a monoexponential fit was applied (because FP was < 0 or > 1) was highest in the renal medulla (13 ± 7 %) and lowest in the cerebral white matter (5 ± 3 %).
Relative signal intensities at eight different b-values (0, 10, 20, 50, 150, 300, 500, and 800 mm2/s) measured on axial slices obtained from one volunteer and corresponding fitted bi-exponential signal curves in different organs/compartments. The data-points are averaged over the entire region of interest for each organ. CSF, cerebrospinal fluid; WM, white matter; GM, grey matter
The mean values and standard deviations of the IVIM parameters are summarized in Table 2 and Fig. 6. The smallest D values were found for cerebral WM, whereas the highest D values were measured in the CSF. D* and Fp values were highest in the liver.
The coefficients of variation of D* and Fp were found to be significantly lower with the proposed whole-body algorithm compared to that obtained with a conventional algorithm in both the liver (D*: 2.46 ± 0.81 vs. 4.91 ± 1.26, p < 0.001; Fp: 5.31 ± 1.45 vs. 9.42 ± 2.22, p < 0.001) and the kidneys (D*: 2.28 ± 0.88 vs. 4.59 ± 1.07, p < 0.001; Fp: 6.01 ± 1.66 vs. 10.39 ± 1.93, p < 0.001), indicating significantly lower variability and thus higher stability of the whole-body algorithm.
Repeatability
Within-subject coefficients of variation and corresponding 95 % confidence intervals were: D, 8.4 % (4.1-12.6 %); D*, 18.2 % (14.0-22.5 %); Fp, 20.2 % (16.0-24.4 %).
Discussion
This study demonstrated the technical feasibility of whole-body IVIM imaging at 3 Tesla. The proposed approach allows consistent calculation of coronal whole-body parametrical maps of good to fair image quality.
Individual IVIM protocols have been applied for tissue characterization in specific organs [6–8, 12, 14, 15, 20–30]. Our proposed whole-body IVIM algorithm differs from previously published approaches, as the requirement for a resilient algorithm for a variety of different tissues had to be addressed. It is an adaptation of an algorithm applied by Sigmund et al. [13] with the following modifications: (a) D is calculated from a linear least squares fit to the log-transformed high b-values, which is faster than the application of a mono-exponential fit. (b) If the calculation of the perfusion fraction Fp does not provide a percentage between 0 and 100 %, a mono-exponential fit to all b-values is applied to obtain more accurate D values and avoid meaningless values for Fp and D* for undetectable pseudodiffusion. (c) D* is not directly obtained from a bi-exponential fit, but from a linear least-squares fit to the low b-values and subsequent extraction of D* using the known perfusion fraction Fp and the diffusion coefficient D. This approach showed to provide a considerable increase of parameter stability with excellent description of the measurement points.
The obtained results are generally in line with former measurements in normal healthy tissue and compartments [6–10, 12–15, 17], with one important exception being the higher Fp and lower D* values in the liver. Potential explanations include the different IVIM algorithm and the lack of respiratory triggering in our protocol, which is not practical in a whole-body examination due to limited acquisition time. The free motion of the liver during image acquisition may have led to an overestimation of Fp due to breathing motion artefacts causing a subsequently lower D* in the following steps of the algorithm (the product of Fp ∙ D* was similar to that in previous studies [12]). The lack of compensation of breathing artefacts for parenchymal abdominal organs may be one disadvantage of the whole-body IVIM examination compared to dedicated organ-specific IVIM measurements. Moreover, free breathing may lead to pixel mismatch in abdominal investigations with impaired parameter value determination. Whereas DWI has proven feasible during free breathing with the phase shift not affecting image formation [31–33], this may not entirely apply for IVIM imaging.
The accuracy of D* and Fp calculation depends on the number of acquired b-values, especially those in the range of 0 – 50 [34]. Lemke et al. [35] suggested a minimum of 10 b-values for clinical applications, whereas in other studies the theoretical minimum of three b-values was used [36, 37]. In this whole-body study, images with eight different b-values were acquired to ensure acceptable accuracy and clinically viable acquisition times.
Currently, the main limitation of whole-body IVIM to clinical acceptance is its long scanning duration. Scan time can be improved by means of parallel imaging techniques such as GRAPPA or SENSE, as used in other IVIM studies [6, 12, 38]. We applied a relatively high SENSE factor of 3; further parallel imaging acceleration does not seem possible without image degradation. However, recent technical developments, such as simultaneous multi-slice acquisition which reduce the scan time by a factor of 2 – 4 [39, 40], raise confidence that the limitation of the long scanning duration may be overcome in the future. To further reduce scan time, acquisition of the lower limbs may be left out in most clinical settings, as usually performed in PET/CT imaging except for patients with melanoma [41, 42].
As compared to conventional whole-body DWI, a whole-body IVIM protocol provides a more comprehensive tissue characterization as it takes into account both diffusion and perfusion effects. IVIM may, therefore, be a complementary technique to whole-body DWI in the assessment of metastatic disease with tumour types exhibiting high tissue perfusion, such as neuroendocrine tumours, renal cell carcinoma, or melanoma. IVIM imaging is completely noninvasive and can be safely performed in case of concerns regarding the injection of contrast media, such as the risk of developing nephrogenic systemic fibrosis. Another potential application lies in the assessment of patients with widespread inflammatory myopathies, where whole-body MR imaging is a focus of current research [43]. Qi et al. found higher D and lower Fp values in inflamed muscle relative to normal muscular tissue [17]. However, it has to be investigated yet whether whole-body IVIM is able to depict focal lesions such as metastases accurately, as parenchymal organs show conceivable noise on the parametrical maps despite our improved algorithm.
In conclusion, whole-body IVIM imaging is feasible and allows the calculation of parametric IVIM maps of good to fair quality and repeatability. The proposed whole-body algorithm showed significantly higher stability compared to a conventional nonlinear square fitting algorithm. Applications of whole-body IVIM may include detection and characterization of widespread disease, such as metastatic tumours or inflammatory myopathies. The potential clinical value awaits further investigations.
References
Padhani AR, Liu G, Koh DM et al (2009) Diffusion-weighted magnetic resonance imaging as a cancer biomarker: consensus and recommendations. Neoplasia 11:102–125
Le Bihan D, Breton E, Lallemand D, Grenier P, Cabanis E, Laval-Jeantet M (1986) MR imaging of intravoxel incoherent motions: application to diffusion and perfusion in neurologic disorders. Radiology 161:401–407
Le Bihan D, Breton E, Lallemand D, Aubin ML, Vignaud J, Laval-Jeantet M (1988) Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology 168:497–505
Le Bihan D (1988) Intravoxel incoherent motion imaging using steady-state free precession. Magn Reson Med Off J Soc Magn Res Med / Soc Magn Reson Med 7:346–351
Turner R, Le Bihan D, Maier J, Vavrek R, Hedges LK, Pekar J (1990) Echo-planar imaging of intravoxel incoherent motion. Radiology 177:407–414
Chiaradia M, Baranes L, Van Nhieu JT et al (2013) Intravoxel incoherent motion (IVIM) MR imaging of colorectal liver metastases: are we only looking at tumor necrosis? J Magn Reso Imaging : JMRI. doi:10.1002/jmri.24172
Sasaki M, Sumi M, Van Cauteren M, Obara M, Nakamura T (2013) Intravoxel incoherent motion imaging of masticatory muscles: pilot study for the assessment of perfusion and diffusion during clenching. AJR Am J Roentgenol 201:1101–1107
Bisdas S, Koh TS, Roder C et al (2013) Intravoxel incoherent motion diffusion-weighted MR imaging of gliomas: feasibility of the method and initial results. Neuroradiology 55:1189–1196
Dyvorne HA, Galea N, Nevers T et al (2013) Diffusion-weighted imaging of the liver with multiple b values: effect of diffusion gradient polarity and breathing acquisition on image quality and intravoxel incoherent motion parameters–a pilot study. Radiology 266:920–929
Patel J, Sigmund EE, Rusinek H, Oei M, Babb JS, Taouli B (2010) Diagnosis of cirrhosis with intravoxel incoherent motion diffusion MRI and dynamic contrast-enhanced MRI alone and in combination: preliminary experience. J Magn Reso Imaging : JMRI 31:589–600
Yamada I, Aung W, Himeno Y, Nakagawa T, Shibuya H (1999) Diffusion coefficients in abdominal organs and hepatic lesions: evaluation with intravoxel incoherent motion echo-planar MR imaging. Radiology 210:617–623
Andreou A, Koh DM, Collins DJ et al (2013) Measurement reproducibility of perfusion fraction and pseudodiffusion coefficient derived by intravoxel incoherent motion diffusion-weighted MR imaging in normal liver and metastases. Eur Radiol 23:428–434
Sigmund EE, Vivier PH, Sui D et al (2012) Intravoxel incoherent motion and diffusion-tensor imaging in renal tissue under hydration and furosemide flow challenges. Radiology 263:758–769
Ichikawa S, Motosugi U, Ichikawa T, Sano K, Morisaka H, Araki T (2013) Intravoxel incoherent motion imaging of the kidney: alterations in diffusion and perfusion in patients with renal dysfunction. Magn Reson Imaging 31:414–417
Rheinheimer S, Stieltjes B, Schneider F et al (2012) Investigation of renal lesions by diffusion-weighted magnetic resonance imaging applying intravoxel incoherent motion-derived parameters–initial experience. Eur J Radiol 81:e310–e316
Morvan D (1995) In vivo measurement of diffusion and pseudo-diffusion in skeletal muscle at rest and after exercise. Magn Reson Imaging 13:193–199
Qi J, Olsen NJ, Price RR, Winston JA, Park JH (2008) Diffusion-weighted imaging of inflammatory myopathies: polymyositis and dermatomyositis. J Magn Reso Imaging : JMRI 27:212–217
Gudbjartsson H, Patz S (1995) <Gudbjartsson_1995.pdf> Magn Reson Med Off J Soc Magn Res Med / Soc Magn Reson Med 34:910–914
Kundel HL, Polansky M (2003) Measurement of observer agreement. Radiology 228:303–308
Yoon JH, Lee JM, Yu MH, Kiefer B, Han JK, Choi BI (2013) Evaluation of hepatic focal lesions using diffusion-weighted MR imaging: comparison of apparent diffusion coefficient and intravoxel incoherent motion-derived parameters. J Magn Reso Imaging : JMRI. doi:10.1002/jmri.24158
Ichikawa S, Motosugi U, Ichikawa T, Sano K, Morisaka H, Araki T (2013) Intravoxel incoherent motion imaging of focal hepatic lesions. J Magn Reso Imaging : JMRI 37:1371–1376
Chow AM, Gao DS, Fan SJ et al (2012) Liver fibrosis: an intravoxel incoherent motion (IVIM) study. J Magn Reso Imaging : JMRI 36:159–167
Guiu B, Petit JM, Capitan V et al (2012) Intravoxel incoherent motion diffusion-weighted imaging in nonalcoholic fatty liver disease: a 3.0-T MR study. Radiology 265:96–103
Klauss M, Lemke A, Grunberg K et al (2011) Intravoxel incoherent motion MRI for the differentiation between mass forming chronic pancreatitis and pancreatic carcinoma. Investig Radiol 46:57–63
Dopfert J, Lemke A, Weidner A, Schad LR (2011) Investigation of prostate cancer using diffusion-weighted intravoxel incoherent motion imaging. Magn Reson Imaging 29:1053–1058
Sigmund EE, Cho GY, Kim S et al (2011) Intravoxel incoherent motion imaging of tumor microenvironment in locally advanced breast cancer. Magn Reson Med Off J Soc Magn Res Med / Soc Magn Reson Med 65:1437–1447
Bokacheva L, Kaplan JB, Giri DD et al (2013) Intravoxel incoherent motion diffusion-weighted MRI at 3.0 T differentiates malignant breast lesions from benign lesions and breast parenchyma. J Magn Reson Imaging: JMRI. doi:10.1002/jmri.24462
Federau C, Meuli R, O’Brien K, Maeder P, Hagmann P (2013) Perfusion measurement in brain gliomas with intravoxel incoherent motion MRI. AJNR American journal of neuroradiology. doi:10.3174/ajnr.A3686
Federau C, O’Brien K, Meuli R, Hagmann P, Maeder P (2013) Measuring brain perfusion with intravoxel incoherent motion (IVIM): initial clinical experience. J Magn Reso Imaging : JMRI. doi:10.1002/jmri.24195
Kim HS, Suh CH, Kim N, Choi CG, Kim SJ (2013) Histogram analysis of intravoxel incoherent motion for differentiating recurrent tumor from treatment effect in patients with glioblastoma: initial clinical experience. AJNR Am J Neuroradiol. doi:10.3174/ajnr.A3719
Kwee TC, Takahara T, Ochiai R, Nievelstein RA, Luijten PR (2008) Diffusion-weighted whole-body imaging with background body signal suppression (DWIBS): features and potential applications in oncology. Eur Radiol 18:1937–1952
Bammer R (2003) Basic principles of diffusion-weighted imaging. Eur J Radiol 45:169–184
Takahara T, Imai Y, Yamashita T, Yasuda S, Nasu S, Van Cauteren M (2004) Diffusion weighted whole body imaging with background body signal suppression (DWIBS): technical improvement using free breathing, STIR and high resolution 3D display. Radiat Med 22:275–282
Cohen AD, Schieke MC, Hohenwalter MD, Schmainda KM (2014) The effect of low b-values on the intravoxel incoherent motion derived pseudodiffusion parameter in liver. Magn Reson Med Off J Soc Magn Res Med / Soc Magn Reson Med. doi:10.1002/mrm.25109
Lemke A, Stieltjes B, Schad LR, Laun FB (2011) Toward an optimal distribution of b values for intravoxel incoherent motion imaging. Magn Reson Imaging 29:766–776
Concia M, Sprinkart AM, Penner AH et al (2013) Diffusion-weighted magnetic resonance imaging of the pancreas: diagnostic benefit from an intravoxel incoherent motion model-based 3 b-value analysis. Investig Radiol. doi:10.1097/RLI.0b013e3182a71cc3
Penner AH, Sprinkart AM, Kukuk GM et al (2013) Intravoxel incoherent motion model-based liver lesion characterisation from three b-value diffusion-weighted MRI. Eur Radiol 23:2773–2783
Lemke A, Laun FB, Simon D, Stieltjes B, Schad LR (2010) An in vivo verification of the intravoxel incoherent motion effect in diffusion-weighted imaging of the abdomen. Magn Reson Med Off J Soc Magn Res Med / Soc Magn Reson Med 64:1580–1585
Kong XZ (2014) Association between in-scanner head motion with cerebral white matter microstructure: a multiband diffusion-weighted MRI study. PeerJ 2:e366
Chang HC, Guhaniyogi S, Chen NK (2014) Interleaved diffusion-weighted improved by adaptive partial-Fourier and multiband multiplexed sensitivity-encoding reconstruction. Magn Reson Med Off J Soc Magn Res Med / Soc Magn Reson Med. doi:10.1002/mrm.25318
Niederkohr RD, Rosenberg J, Shabo G, Quon A (2007) Clinical value of including the head and lower extremities in 18F-FDG PET/CT imaging for patients with malignant melanoma. Nucl Med Commun 28:688–695
Querellou S, Keromnes N, Abgral R et al (2010) Clinical and therapeutic impact of 18F-FDG PET/CT whole-body acquisition including lower limbs in patients with malignant melanoma. Nucl Med Commun 31:766–772
Malattia C, Damasio MB, Madeo A et al (2013) Whole-body MRI in the assessment of disease activity in juvenile dermatomyositis. Ann Rheum Dis. doi:10.1136/annrheumdis-2012-202915
Acknowledgements
The scientific guarantor of this publication is Andreas Boss. The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article. The authors state that this work has not received any funding. No complex statistical methods were necessary for this paper. Institutional review board approval was obtained. Written informed consent was obtained from all subjects (patients) in this study. Methodology: prospective, experimental, performed at one institution.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Filli, L., Wurnig, M.C., Luechinger, R. et al. Whole-body intravoxel incoherent motion imaging. Eur Radiol 25, 2049–2058 (2015). https://doi.org/10.1007/s00330-014-3577-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-014-3577-z