Abstract
Objectives
To evaluate the diagnostic performance of preoperative multiparametric MRI with extracapsular extension (ECE) risk-scoring in the assessment of prostate cancer tumour stage (T-stage) and prediction of ECE at final pathology.
Materials and Methods
Eighty-seven patients with clinically localised prostate cancer scheduled for radical prostatectomy were prospectively enrolled. Multiparametric MRI was performed prior to prostatectomy, and evaluated according to the ESUR MR prostate guidelines by two different readers. An MRI clinical T-stage (cTMRI), an ECE risk score, and suspicion of ECE based on tumour characteristics and personal opinion were assigned. Histopathological prostatectomy results were standard reference.
Results
Histopathology and cTMRI showed a spearman rho correlation of 0.658 (p < 0.001) and a weighted kappa = 0.585 [CI 0.44;0.73](reader A). ECE was present in 31/87 (36 %) patients. ECE risk-scoring showed an AUC of 0.65–0.86 on ROC-curve for both readers, with sensitivity and specificity of 81 % and 78 % at best cutoff level (reader A), respectively. When tumour characteristics were influenced by personal opinion, the sensitivity and specificity for prediction of ECE changed to 61 %–74 % and 77 %-88 % for the readers, respectively.
Conclusions
Multiparametric MRI with ECE risk-scoring is an accurate diagnostic technique in determining prostate cancer clinical tumour stage and ECE at final pathology.
Key Points
• Multiparametric MRI is an accurate diagnostic technique for preoperative prostate cancer staging
• ECE risk scoring predicts extracapsular tumour extension at final pathology
• ECE risk scoring shows an AUC of 0.86 on the ROC-curve
• ECE risk scoring shows a moderate inter-reader agreement (K = 0.45)
• Multiparametric MRI provides essential knowledge for optimal clinical management
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Digital rectal examination (DRE) and transrectal ultrasound (TRUS) are traditionally used for clinical staging of prostate cancer (PCa), but both are lacking in sensitivity and specificity, and TRUS often underestimates the size and stage of the tumour [1]. Thus, prediction of extracapsular tumour extension (ECE) by DRE and TRUS has low accuracy [2, 3].
Radical prostatectomy (RP) provides great disease control for patients with localised PCa (cT1-T2), while RP for locally advanced disease (cT3) remains controversial [1, 4]. Recovery of erectile function and continence after RP is related to surgical technique and preservation of the neurovascular bundles (NVB). Accurate preoperative knowledge of tumour stage and possible ECE are crucial in achieving the best surgical, oncological, and functional result with total tumour resection, while trying to preserve both potency and continence.
Recent findings support the rapidly growing use of mp-MRI as the most sensitive and specific imaging tool for PCa staging [5]. However, the diagnostic accuracy of mp-MRI staging and prediction of ECE differs among studies [6–10], which has led to a debate regarding mp-MRI’s readiness for routine use [11]. Recently published clinical guidelines [12] from prostate MRI experts have therefore included a structured uniform reporting and scoring system (PIRADS) [11] to standardise prostatic MRI readings. Previous studies have validated the PIRADS classification for PCa detection and localisation using both targeted biopsies [13–15] and RP specimens [16] as standard reference. In addition, the guidelines also recommend using a risk score of possible ECE. Based on these recommendations, we carried out this prospective study of a patient cohort with clinically localised PCa based on DRE and TRUS findings who were scheduled for RP. The aim was to investigate the diagnostic accuracy of preoperative mp-MRI with ECE risk scoring in the assessment of tumour stage (T-stage) and prediction of ECE at final pathology.
Materials & Methods
This prospective single-institution study was approved by the Local Committee for Health Research Ethics (No. H-1-2011-066) and the Danish Data Protection Agency, and was registered at ClinicalTrials.gov (No.NCT01640262).
Patients
All patients provided written informed consent, and were prospectively enrolled between September 2011 and September 2013. Inclusion criteria required that all patients were diagnosed with clinically localised PCa determined by DRE and TRUS and scheduled for RP, based on clinical assessment of age, comorbidity, PSA, and biopsy Gleason score. No patients had prior treatment for PCa. The exclusion criteria were patients with contraindication to mp-MRI (pacemaker, magnetic implants, severe claustrophobia, previous moderate or severe reaction to gadolinium-based contrast-media, or impaired renal function with GFR <30 ml/min). No patients were excluded from RP due to preoperative mp-MRI findings.
Multiparametric MRI
Prior to RP, all patients underwent mp-MRI using a 3.0 T MRI scanner (Achieva (n = 71 patients) and Ingenia (n = 16 patients), Philips Healthcare, Best, the Netherlands) with a pelvic phased-array coil (Philips Healthcare, Best, the Netherlands) positioned over the pelvis. We did not use an endorectal coil (ERC), due to lack of availability. If tolerated, 1 mg intramuscular Glucagon (Glucagen®, Novo Nordisk, Bagsvaerd, Denmark) injection combined with 1 mg hyoscine butylbromid (Buscopan®, Boehringer Ingelheim, Ingelheim am Rhein, Germany) intravenous injection was administered to the patient to reduce peristaltic motion. Triplanar T2W images from below the prostatic apex to above the seminal vesicles were obtained. In addition, axial diffusion-weighted images (DWI) including four b-values (b0, b100, b800, and b1400), along with reconstruction of the corresponding apparent diffusion coefficient (ADC) map (b-values 100 and 800), together with dynamic contrast-enhanced (DCE) images before, during, and after intravenous administration of 15 ml gadoterate meglumine (Dotarem 279.3 mg/ml, Guerbet, Roissy CDG, France), were performed. The contrast agent was administered using a power injector (MedRad, Warrendale, PA, USA), followed by a 20 ml saline flush injection at a flow rate of 2.5 ml/s. For imaging parameters, see Table 1.
Image analysis
All tumour-suspicious lesions were evaluated according to the PIRADS classification from ESUR [12] giving a sum of scores (range 3–15). Lesions with PIRADS summation score ≥10, equivalent to a moderate or high-risk group [15], or PIRADS overall score ≥4 [17] were considered to be possible malignant lesions and were used to predict a cT-stage based on mp-MRI (cTMRI ) according to the TNM classification [18]. Pre-contrast T1W images were used to identify post-biopsy haemorrhage as an area with high signal intensity to rule out false-positive findings on T2W. The lesion most suspicious of ECE was evaluated using the ESUR MR prostate guidelines scoring of extraprostatic disease [12], focusing on the ECE criteria (Table 2). The ECE tumour characteristics were first assessed according to the following findings: a) no sign of ECE, b) capsular abutment, c) capsular irregularity, retraction, or thickening, d) neurovascular bundle thickening, e) capsular signal loss or bulging, and f) direct sign of tumour tissue in the extraprostatic tissue. The findings were subsequently associated with the ECE risk score ranging from 0 to 5 (Fig. 1). Suspicion of possible SVI was based on the following findings: a) low T2W signal in the lumen, b) filling in of angle, and/or c) enhancement/impeded diffusion of the seminal vesicles. The ECE tumour characteristics only evaluate T2W findings, and assigning a preoperative cTMRI stage requires a definitive decision of possible ECE and/or SVI. Therefore, the suspicion of ECE was dichotomised into either organ-confined (OC) disease or ECE based on the ECE tumour characteristics and personal opinion, while incorporating functional imaging (DWI and DCE) findings. Equivocal cases with strong suspicion of ECE on mp-MRI were read as positive, and equivocal cases with low suspicion of ECE were read as negative. All mp-MRI images from each patient were analysed by a dedicated MRI prostate physician (reader A) with two years of experience in prostate mp-MRI interpretation. All imaging modalities (T2W, DWI, and DCE imaging) were interpreted simultaneously to predict cTMRI and dichotomising ECE suspicion, and only T2W imaging were used to assess ECE tumour characteristics. Qualitative visual assessment was used for DCE MRI analysis, as described in the guidelines [12]. In addition, all images were reassessed independently from reader A by a second reader B with extensive experience in general abdominal MRI, but only limited experience in prostate mp-MRI interpretation. Both readers had access to preoperative PSA and knew that the patients had biopsy-proven clinically localised PCa, but were blinded to any histopathological findings.
Prostate cancer (white arrows) tumour characteristics corresponding to different ECE risk score groups: a) ECE risk score 1 – tumour with capsular abutment b) ECE risk score 3 – tumour with capsular thickening c) ECE risk score 4 – tumour with capsular bulging, and d) ECE risk score 5 – tumour with direct sign of ECE
Histopathological evaluation
All patients underwent RP. The surgeon was blinded to any mp-MRI findings. The specimens were coated with ink and fixed in formalin. The bases, including the bases of the seminal vesicles and the apex, were cut in sagittal sections, whereas the remaining prostate was cut into 4–5 mm sections in a plane perpendicular to the rectal surface corresponding to the plane used for axial mp-MRI. The remainder of the seminal vesicles were cut longitudinally. The slices were then further cut into microscopic sections and stained with haematoxylin and eosin. All cancerous areas, including the presence and location of any extraprostatic extension (EPE), defined as either ECE and/or SVI, were outlined based on the histopathological results by an experienced pathologist. ECE was defined as tumour cell growth into the extraprostatic tissue, and SVI was defined as tumour infiltration of the seminal vesicles. The pathological T-stage (pT) was defined using the TNM classification [18].
Statistical analysis
The patients’ clinical characteristics were calculated and compared in two groups (organ-confined and extraprostatic disease) based on the histopathological results. Continuous variables including age, PSA, and percentage positive biopsy cores were compared using the Wilcoxon rank-sum test or a Student’s t test. A Fisher’s exact test or a chi-square test were used to compare the T-stage determined by DRE (cTDRE) and TRUS (cTTRUS), the cTMRI stage, the biopsy GS, and the D’Amico risk group.
The cTMRI was compared to pT for accuracy using weighted kappa statistics and a spearman rank order calculation. A receiver operating characteristic curve (ROC-curve) with an area under the curve (AUC) value was generated to analyse the predictive accuracy of the ECE risk scoring scale in detecting ECE at pathology. An optimal risk score cutoff level, representing the best trade-off between sensitivity and specificity, was determined for the more experienced reader A. In addition, the overall diagnostic accuracy, sensitivity, specificity, positive predicted value (PPV), and negative predictive value (NPV) of mp-MRI in predicting ECE by combining ECE tumour characteristics with personal opinion and in determining SVI were calculated for both readers. Inter-reader reliability was calculated using kappa statistics [19]. A p value below 0.05 was considered significant. Statistical analysis was performed using SPSS 20.0 software (IBM Corporation, U.S.).
Results
Ninety-three patients were prospectively enrolled. However, six patients were excluded due to mp-MRI technical problems or claustrophobia. The final study population of 87 patients with a median age of 65 (range 47–74) and a median PSA of 11 (range 4.6–45) underwent mp-MRI before RP. Clinical and demographic data are presented in Table 3. There was no significant difference in PSA, cTDRE, cTTRUS, or D’Amico risk group between patients with OC or EPE disease. There was a significant difference in age, percentage of positive biopsy cores, biopsy GS, and cTMRI between the groups.
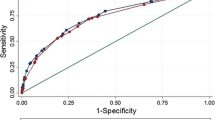
Mp-MRI identified a tumour in all 87 patients. The correlation between cTMRI and pT showed spearman rho correlations of 0.658 (p < 0.001) and 0.306 (p = 0.004), with a weighted kappa of 0.585 [CI 0.44; 0.73] and 0.22 [CI 0.09; 0.35] for reader A and reader B, respectively. The inter-reader agreement for the readers in determining cTMRI was kappa = 0.44 [CI 0.2; 0.66]. The prevalence of EPE after RP was 32/87 (37 %), including 31/87 (36 %) with ECE and 5/87 (6 %) with SVI. One patient had SVI without concomitant ECE at pathology. Each ECE tumour characteristic (Fig. 2) was stratified into ECE risk score groups (Fig. 3). Mp-MRI ECE risk scoring for the more experienced reader A showed an AUC of 0.86 on the ROC curve (Fig. 4), with sensitivity of 81 % [CI 63; 93], specificity of 78 % [CI 66; 88], and diagnostic accuracy of 79 % at the optimal risk score cutoff level ≥4 (Table 4). Using this cutoff level, 6/31 patients with ECE were missed and 12/56 patients had a false-positive mp-MRI (Fig. 3; Table 4). When mp-MRI findings were dichotomised into either OC or ECE influenced by personal opinion, the false-positive rate dropped to 7/56 patients, at the expense of increased false-negative readings, where 8/31 patients with ECE were missed, producing diagnostic accuracy of 83 %, with sensitivity, specificity, PPV, and NVP of 74 % [CI 55; 88], 88 % [CI 76; 95], 77 % [CI 56; 90], and 86 % [CI 74; 94] for prediction of ECE, respectively. For the less experienced reader B, ECE risk scoring showed an AUC of 0.65 (Fig. 4) and sensitivity, specificity, and diagnostic accuracy of 51 % [CI 33; 70], 69 % [CI 52; 78], and 61 % at the risk score cutoff level ≥4 determined by reader A. The sensitivity, specificity, and diagnostic accuracy changed to 61 % [CI 0.42; 0.78], 77 % [CI 0.64; 0.87], and 71 % when ECE risk scoring was influenced by personal opinion. The inter-reader agreement kappa value between reader A and B was 0.40 [CI 0.19; 0.60] in distinguishing OC from ECE disease and 0.45 [CI 0.21; 0.68] in agreement of ECE risk scores. Reader A detected 4/5 (80 %) patients with SVI, and one patient with pT3a had evidence of SVI (T3b) on mp-MRI due to low T2W signal intensity caused by intraluminal SV infiltration with amyloid.
Discussion
The prevalence of EPE at histopathology was 37 % in patients with clinically localised PCa, confirming the fact that DRE and TRUS often underestimate the tumour extension and stage.
The prognosis of PCa is highly related to the tumour stage. We found a significant correlation between the cTMRI stage and pT for the more experienced reader A using mp-MRI for clinical staging instead of DRE and TRUS. Complete agreement between cTMRI and pT is difficult to obtain, as mp-MRI does not identify all of the small dispersed insignificant tumour foci that frequently are present within the prostate and are incorporated into the pT assessment. This can easily cause a discrepancy between a T2a/T2b stage identified on mp-MRI and a T2c stage reported at pathology for OC tumours. However, the exact stage differentiation among patients with OC tumours is of less importance, as potentially curative surgery often can be offered regardless of T2-stage. In contrast, the treatment selection of PCa strongly relies on the distinction between OC (T2) and ECE (T3) disease. ROC curve analysis of ECE risk scoring showed a high AUC (0.86), indicating high clinical value of the scoring system when interpreted by a dedicated reader, who reached sensitivity and specificity of 81 % and 78 %, respectively, at best cutoff level ≥4. A cutoff at this level includes both direct sign of tumour growth through the capsule (risk score 5 - ECE highly likely to be present) and also indirect signs such as tumour bulging, loss of capsular signal, and neurovascular bundle thickening (risk score 4 - ECE likely to be present). If the equivocal cases with cutoff level ≥ risk score 3 (capsular retraction, irregularity, or thickening) are included, the sensitivity increases to 94 %, with a decrease in specificity to 68 %, but more interestingly, the NPV increases to 95 %, indicating high clinical value to almost definitively rule out ECE at this level. However, the inter-reader agreement kappa value (0.45) signifies only moderate consistency between the readers and indicates that there are differences in the image interpretation of the individual ECE tumour characteristics.
The purpose of the ESUR PIRADS classification is to introduce a structured uniform scoring system with less subjectivity in order to standardise prostatic mp-MRI readings and facilitate consistency between readers. This approach is well suited for PCa lesion detection and localisation, as each MRI modality in each suspicious lesion is scored independently, providing a summation of all individual scores. In addition, an overall final score (range 1-5) according to the probability of clinically significant PCa being present can be assigned. Similarly, the guidelines also recommend that the probability of extraprostatic disease should be scored on a five-point risk scale, and provide individual tumour characteristics with associated risk scores for ECE, SVI, distal sphincter, and bladder neck involvement. We only evaluated the ECE criteria in this study, as we considered the a priori probability of patients having SVI, distal sphincter, or bladder neck involvement in our population with clinically localised PCa to be too low to validate a five-point risk score. The five-point risk scale is considered a continuum of risk, with higher scores corresponding to higher probability of ECE. However, ECE risk scoring does not include a risk score = 2 or findings on functional imaging. The assessment of ECE tumour characteristics is based only on T2W imaging. Previous studies have shown that functional imaging may improve detection of ECE [20–22], especially for less experienced readers [23]. Therefore, the interpretation and overall impression of possible ECE using the risk score scale may be influenced by personal opinion when incorporating functional imaging findings. This applies to our study, as the diagnostic accuracy (71 %–83 %) increased for both readers when incorporating personal opinion and functional imaging into the evaluation of ECE, and changed sensitivity and specificity to 61–74 % and 77–88 %, respectively. This overall diagnostic performance is in accordance with previous findings. A meta-analysis by Engelbrecht et al. [24] reported a combined sensitivity and specificity of 71 % in distinguishing between T2 and T3 disease on 1.5 T MRI systems. However, more recent studies at 3 T ERC MRI report higher rates, with sensitivity and specificity of 80–88 % and 95–100 %, respectively [25, 26].
The mp-MRI interpretation can be affected by the way the physician analyses the images when incorporating personal opinion, especially when deciding on possible ECE in equivocal cases. Until recently, RP was restricted to patients with localised PCa, while patients with high suspicion of ECE and/or SVI were referred for radiation therapy. This might influence the physician to value high specificity with a low number of false-positive readings, so no patients with equivocal mp-MRI findings would be ruled out of possible curative surgery. There has been an increasing interest in performing RP in selected patients with locally advanced disease, as some studies have shown promising results [27–30]. If this tendency continues, the value of high-sensitivity readings increases, such that the surgeon is directed to the site of possible ECE to avoid positive surgical margins. Therefore, the ECE risk score cutoff level in Table 4 might be altered to either value high sensitive or high specific readings, depending on the clinical situation. Mp-MRI findings can then be combined with clinical findings and nomograms, and increase the overall pre-therapeutic diagnostic staging accuracy [31, 32].
We evaluated the reader performance of two readers with different experience in mp-MRI prostate interpretation. Overall, the more experienced reader A had significantly higher performance than reader B in the assessment of ECE, using both ECE risk scoring and personal opinion, and was more accurate in predicting the pathological stage. Furthermore, our results indicate that the overall interpretation of possible ECE in our hands should not rely only on ECE risk scoring in its current form, but whenever possible, should also incorporate functional imaging, especially for less experienced readers. It is evident that mp-MRI interpretation has a considerable learning curve, and is a specialised task that requires substantial dedication and experience to achieve acceptable diagnostic results [7, 33]. A dedicated reader education program on prostate mp-MRI interpretation is associated with a statistically significant increase in diagnostic accuracy [34].
This study was designed so as not to exclude any patients from RP based on any preoperative mp-MRI findings, in order to have the prostatectomy specimen from all patients as a standard reference for correlation. Further studies are now needed to address the clinical therapeutic consequences of performing staging of mp-MRI.
This study has some limitations. We included only patients with clinically localised disease at the time of surgery. This might have caused a selection bias, as all patients with clinically locally advanced disease had already been excluded from surgery – and therefore this study – which could cause an overestimation of mp-MRI specificity and an underestimation of sensitivity. Moreover, the readers knew that the patients had clinically localised disease and were not blinded to PSA during the mp-MRI readings; however, we find this more reflective of everyday clinical practice. We used only a pelvic-phased-array coil for staging purposes, and despite promising results at 3.0 T MRI, the use of an ERC might have improved image quality and staging accuracy [26, 35], and is recommended by the ESUR MR prostate guidelines [12] as part of optimal requirements.
Conclusions
Multiparametric MRI with ECE risk scoring by a dedicated reader is an accurate diagnostic technique for determining prostate cancer tumour stage and ECE at final pathology. However, further studies must investigate whether functional imaging should be included in the ECE risk scoring scale, and if so, how to weigh the individual findings in order to increase the diagnostic accuracy of the scoring system.
Abbreviations
- DRE:
-
Digital rectal examination
- TRUS:
-
Transrectal ultrasound
- PCa:
-
Prostate cancer
- EPE:
-
Extraprostatic tumour extension
- RP:
-
Radical prostatectomy
- cT:
-
Clinical tumour stage
- NVB:
-
Neurovascular bundle
- MRI:
-
Magnetic resonance imaging
- Mp-MRI:
-
Multiparametric MRI
- PIRADS:
-
Prostate imaging reporting and data system
- T2W:
-
T2-weighted
- DWI:
-
Diffusion-weighted imaging
- ADC:
-
Apparent diffusion coefficient
- DCE:
-
Dynamic contrast-enhanced
- ECE:
-
Extracapsular extension
- SVI:
-
Seminal vesicle invasion
- GS:
-
Gleason score
- ERC:
-
Endorectal coil
References
Heidenreich A, Bastian PJ, Bellmunt J et al (2014) EAU guidelines on prostate cancer. part 1: screening, diagnosis, and local treatment with curative intent-update 2013. Eur Urol 65:124–137
Mullerad M, Hricak H, Kuroiwa K et al (2005) Comparison of endorectal magnetic resonance imaging, guided prostate biopsy and digital rectal examination in the preoperative anatomical localization of prostate cancer. J Urol 174:2158–2163
Grossfeld GD, Chang JJ, Broering JM et al (2001) Under staging and under grading in a contemporary series of patients undergoing radical prostatectomy: results from the Cancer of the Prostate Strategic Urologic Research Endeavor database. J Urol 165:851–856
Soulié M (2008) What is the role of surgery for locally advanced disease? Eur Urol Suppl 7:400–405
Sciarra A, Barentsz J, Bjartell A et al (2011) Advances in magnetic resonance imaging: how they are changing the management of prostate cancer. Eur Urol 59:962–977
Fütterer JJ, Engelbrecht MR, Jager GJ et al (2007) Prostate cancer: comparison of local staging accuracy of pelvic phased-array coil alone versus integrated endorectal-pelvic phased-array coils. Local staging accuracy of prostate cancer using endorectal coil MR imaging. Eur Radiol 17:1055–1065
Mullerad M, Hricak H, Wang L, Chen H-N, Kattan MW, Scardino PT (2004) Prostate cancer: detection of extracapsular extension by genitourinary and general body radiologists at MR imaging. Radiology 232:140–146
Brajtbord JS, Lavery HJ, Nabizada-Pace F, Senaratne P, Samadi DB (2011) Endorectal magnetic resonance imaging has limited clinical ability to preoperatively predict pT3 prostate cancer. BJU Int 107:1419–1424
Hegde JV, Chen M-H, Mulkern RV, Fennessy FM, D’Amico AV, Tempany CMC (2013) Preoperative 3-Tesla multiparametric endorectal magnetic resonance imaging findings and the odds of upgrading and upstaging at radical prostatectomy in men with clinically localized prostate cancer. Int J Radiat Oncol Biol Phys 85:101–107
Renard-Penna R, Rouprêt M, Comperat E et al (2013) Accuracy of high resolution (1.5 tesla) pelvic phased array magnetic resonance imaging (MRI) in staging prostate cancer in candidates for radical prostatectomy: results from a prospective study. Urol Oncol 31:448–454
Heidenreich A (2011) Consensus criteria for the use of magnetic resonance imaging in the diagnosis and staging of prostate cancer: not ready for routine use. Eur Urol 59:495–497
Barentsz JO, Richenberg J, Clements R et al (2012) ESUR prostate MR guidelines 2012. Eur Radiol 22:746–757
Portalez D, Mozer P, Cornud F et al (2012) Validation of the European Society of Urogenital Radiology scoring system for prostate cancer diagnosis on multiparametric magnetic resonance imaging in a cohort of repeat biopsy patients. Eur Urol 62:986–996
Röthke M, Blondin D, Schlemmer H-P, Franiel T (2013) PI-RADS classification: structured reporting for MRI of the prostate. Röfo 185:253–261
Boesen L, Noergaard N, Chabanova E et al (2014) Early experience with multiparametric magnetic resonance imaging-targeted biopsies under visual transrectal ultrasound guidance in patients suspicious for prostate cancer undergoing repeated biopsy. Scand J Urol. doi:10.3109/21681805.2014.9
Rosenkrantz AB, Kim S, Lim RP et al (2013) Prostate cancer localization using multiparametric MR imaging: comparison of Prostate Imaging Reporting and Data System (PI-RADS) and Likert scales. Radiology 269:482–492
Roethke MC, Kuru TH, Schultze S et al (2014) Evaluation of the ESUR PI-RADS scoring system for multiparametric MRI of the prostate with targeted MR/TRUS fusion-guided biopsy at 3.0 Tesla. Eur Radiol 24:344–352
Sobin L, Gospodarowicz MWC (2009) TNM classification of malignant tumours. Urological tumours, 7th edn. Wiley-Blackwell, Hoboken
Landis JR, Koch GG (1977) The measurement of observer agreement for categorical data. Biometrics 33:159–174
Rosenkrantz AB, Chandarana H, Gilet A et al (2013) Prostate cancer: utility of diffusion-weighted imaging as a marker of side-specific risk of extracapsular extension. J Magn Reson Imaging 38:312–319
Chong Y, Kim CK, Park SY, Park BK, Kwon GY, Park JJ (2014) Value of diffusion-weighted imaging at 3 T for prediction of extracapsular extension in patients with prostate cancer: a preliminary study. AJR Am J Roentgenol 202:772–777
Bloch BN, Furman-Haran E, Helbich TH et al (2007) Prostate cancer: accurate determination of extracapsular extension with high-spatial-resolution dynamic contrast-enhanced and T2-weighted MR imaging–initial results. Radiology 245:176–185
Fütterer JJ, Engelbrecht MR, Huisman HJ et al (2005) Staging prostate cancer with dynamic contrast-enhanced endorectal MR imaging prior to radical prostatectomy: experienced versus less experienced readers. Radiology 237:541–549
Engelbrecht MR, Jager GJ, Laheij RJ, Verbeek ALM, van Lier HJ, Barentsz JO (2002) Local staging of prostate cancer using magnetic resonance imaging: a meta-analysis. Eur Radiol 12:2294–2302
Fütterer JJ, Heijmink SWTPJ, Scheenen TWJ et al (2006) Prostate cancer: local staging at 3-T endorectal MR imaging–early experience. Radiology 238:184–191
Heijmink SWTPJ, Fütterer JJ, Hambrock T et al (2007) Prostate cancer: body-array versus endorectal coil MR imaging at 3 T–comparison of image quality, localization, and staging performance. Radiology 244:184–195
Ward JF, Slezak JM, Blute ML, Bergstralh EJ, Zincke H (2005) Radical prostatectomy for clinically advanced (cT3) prostate cancer since the advent of prostate-specific antigen testing: 15-year outcome. BJU Int 95:751–756
Hsu C-Y, Joniau S, Oyen R, Roskams T, Van Poppel H (2007) Outcome of surgery for clinical unilateral T3a prostate cancer: a single-institution experience. Eur Urol 51:121–128, discussion 128–9
Mitchell CR, Boorjian SA, Umbreit EC, Rangel LJ, Carlson RE, Karnes RJ (2012) 20-Year survival after radical prostatectomy as initial treatment for cT3 prostate cancer. BJU Int 110:1709–1713
Spahn M, Briganti A, Capitanio U et al (2012) Outcome predictors of radical prostatectomy followed by adjuvant androgen deprivation in patients with clinical high risk prostate cancer and pT3 surgical margin positive disease. J Urol 188:84–90
Wang L, Hricak H, Kattan MW, Chen H-N, Scardino PT, Kuroiwa K (2006) Prediction of organ-confined prostate cancer: incremental value of MR imaging and MR spectroscopic imaging to staging nomograms. Radiology 238:597–603
Shukla-Dave A, Hricak H, Akin O et al (2012) Preoperative nomograms incorporating magnetic resonance imaging and spectroscopy for prediction of insignificant prostate cancer. BJU Int 109:1315–1322
Ruprecht O, Weisser P, Bodelle B, Ackermann H, Vogl TJ (2012) MRI of the prostate: interobserver agreement compared with histopathologic outcome after radical prostatectomy. Eur J Radiol 81:456–460
Garcia-Reyes K, Passoni NM, Palmeri ML et al (2014) Detection of prostate cancer with multiparametric MRI (mpMRI): effect of dedicated reader education on accuracy and confidence of index and anterior cancer diagnosis. Abdom Imaging. doi:10.1007/s00261-014-019
Turkbey B, Merino MJ, Gallardo EC et al (2014) Comparison of endorectal coil and nonendorectal coil T2W and diffusion-weighted MRI at 3 Tesla for localizing prostate cancer: correlation with whole-mount histopathology. J Magn Reson Imaging 39:1443–1448
Acknowledgments
The scientific guarantor of this publication is Henrik S. Thomsen. The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article. The authors state that this work has not received any funding. No complex statistical methods were necessary for this paper. Institutional Review Board approval was obtained. Written informed consent was obtained from all subjects (patients) in this study. Approval from the institutional animal care committee was not required because the study was not on animals. Methodology: prospective, diagnostic, or prognostic study, performed at one institution.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Boesen, L., Chabanova, E., Løgager, V. et al. Prostate cancer staging with extracapsular extension risk scoring using multiparametric MRI: a correlation with histopathology. Eur Radiol 25, 1776–1785 (2015). https://doi.org/10.1007/s00330-014-3543-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-014-3543-9