Abstract
Objective
Evaluate the performance of a robotic system for CT-guided lung biopsy in comparison to the conventional manual technique.
Materials and methods
One hundred patients referred for CT-guided lung biopsy were randomly assigned to group A (robot-assisted procedure) or group B (conventional procedure). Size, distance from entry point and position in lung of target lesions were evaluated to assess homogeneity differences between the two groups. Procedure duration, dose length product (DLP), precision of needle positioning, diagnostic performance of the biopsy and rate of complications were evaluated to assess the clinical performance of the robotic system as compared to the conventional technique.
Results
All biopsies were successfully performed. The size (p = 0.41), distance from entry point (p = 0.86) and position in lung (p = 0.32) of target lesions were similar in both groups (p = 0.05). Procedure duration and radiation dose were significantly reduced in group A as compared to group B (p = 0.001). Precision of needle positioning, diagnostic performance of the biopsy and rate of complications were similar in both groups (p = 0.05).
Conclusion
Robot-assisted CT-guided lung biopsy can be performed safely and with high diagnostic accuracy, reducing procedure duration and radiation dose in comparison to the conventional manual technique.
Key Points
• CT-guided biopsy is the main procedure to obtain diagnosis in lung tumours.
• The robotic device facilitates percutaneous needle placement under CT guidance.
• Robot-assisted CT-guided lung biopsy reduces procedure duration and radiation dose.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
CT-guided lung biopsy is the procedure of choice to obtain diagnoses in patients with pulmonary lesions suggestive of malignancy at imaging [1–3]. Following the recent advances in targeted therapies, biopsy of unresectable lung lesions has also become necessary in order to assess genetic mutations in unresectable non-small cell cancers (NSCLC), with core biopsy usually being preferred to aspiration cytology owing to the larger specimens made available for molecular analysis [4]. CT-guided lung biopsy can be performed either with the step-and-shoot or the fluoroscopic technique: the step-and-shoot approach is preferred in larger, non-moving lesions, while CT-fluoroscopy is more advantageous when targeting smaller lesions or nodules in the lower lobes that are susceptible to respiratory motion [5]. Both procedures have technical limitations that should be taken into consideration; in particular the step-and-shoot technique is based on the operator’s subjective assessment of needle path and positioning and may result in increased procedure duration and complication rate, whereas CT-fluoroscopy is significantly faster and more precise but significantly raises radiation dose to both operator and patient [6, 7]. Various assisting technologies have been proposed in order to increase the diagnostic accuracy and reduce the duration of CT-guided biopsies, including external laser targeting [8] and augmented reality (i.e. with a live indirect view of anatomy by computer-generated video input) [9]. Dedicated interventional robotic systems that operate under imaging guidance also became available recently [10]. However, while these systems may theoretically represent an important step toward the automation of interventional procedures, clinical experience and comparative data with conventional techniques are still lacking or insufficient. The ROBIO™ EX (Perfint Healthcare Pvt. Ltd, Florence, OR, USA) is a CE approved robotic positioning system that facilitates percutaneous needle placement during CT-guided interventional procedures and that has been successfully tested for CT-guided biopsy and ablation on phantoms [11] and for clinical radiofrequency ablation of liver lesions [12]. The objective of this study was to evaluate the clinical performance of this system for CT-guided biopsy of lung lesions in comparison with the conventional manual technique.
Materials and methods
Patient population and study details
This was a single-centre, double-arm, non-sponsored, prospective study and received the approval of local institution review board. Between June 2013 and February 2014, 115 patients with previously diagnosed lung lesions suggestive of malignancy at chest CT, PET-CT or both were referred to the thoracic surgery department of our tertiary care hospital for histological characterization. Fifteen patients were excluded from the study population (three patients refused further diagnosis/treatment, in five patients the lesions were characterized as lung metastases following review of available imaging and in seven patients diagnosis was obtained with bronchoscopy and transbronchial biopsy). The remaining 100 patients (63 male, 37 female, age range 48–88 years, mean age 65 ± 4 years) were referred for CT-guided lung biopsy and randomly assigned to group A (robot-assisted procedure) or group B (conventional procedure). All enrolled patients gave their written informed consent to participation after being thoroughly informed of the benefits and potential risks of the procedure.
Pre-procedure
All procedures were performed by the same radiologist (MA, 8 years of experience in CT-guided interventions, including more than 300 lung biopsies) on a 128-MDCT dual-source scanner (Somatom Definition, Siemens, Erlangen, Germany). A standard inspiratory breath-hold scan of the chest (100 kV, 100 mAs, detector configuration 128 × 1 mm, slice thickness 1 mm, reconstruction interval 1 mm) was acquired in all cases prior to biopsy, in order to confirm the presence and to assess the position of the target lesion. Patients were laid on a vacuum stabilization mattress and positioned in order to reduce at minimum the intrapleural path of the needle, as well as to avoid critical lung structures (vessels, bronchi and fissures). Local anaesthesia was performed with 10 mL of 1 % lidocaine along the projected path of the biopsy needle into the soft tissues, down to the epipleural space. In all cases an 18-G, 150/200-mm-long modified Menghini end-cutting needle (SURECUT, TSK Laboratory, Tochigi-Shi, Japan) was used for tissue sampling. Targeting CT scans were acquired with a low-dose interventional protocol (100 kV, 50 mAs, detector configuration 128 × 1 mm, slice thickness 1 mm, reconstruction interval 1 mm).
Conventional biopsy technique
All conventional biopsies were performed with the step-and-shoot technique to assess needle positioning and angulation. The z-axis extension of targeting scans was limited to include only the needle and the target lesion. A minimum of two scans (before the pleura and into the lesion) was required to target lesions adjacent to the chest wall and a minimum of three scans (before the pleura, midway to the lesion, into the lesion) was required for deeper lesions. Additional scans and multiplanar reconstructions were performed in real time when necessary for needle adjustment. Once the needle tip was in position, biopsy was performed with a combination of aspiration and push/rotation movements.
Robot-assisted biopsy technique
Positioning and docking of the robotic system were performed as previously described [11], with the arm and planning console located to the side of the CT bed (left or right, depending on the required access) and firmly coupled to ground metal plates on the floor to ensure stability. A preliminary inspiratory breath-hold CT of the chest was performed using a Breath Hold® respiratory belt coupled to a light sign (Medspira, Minneapolis, USA) mounted on a flexible arm, in order to monitor the extent of chest movement and instruct patients to maintain and reproduce proper apnoea (Fig. 1). Images were then exported over a local area network to the ROBIO™ EX workstation for biopsy planning. The centre of the target lesion and the entry point on the skin were determined by the operator, while the angulations of the needle, the depth of the target and the needle path were automatically calculated by the workstation and displayed in real time (Fig. 2). Each parameter was readily modifiable by the operator in order to avoid critical structures, such as ribs, bronchi and vessels. Once the plan was confirmed, the CT table was moved to the coordinates displayed on the workstation and the robotic arm was activated and positioned for biopsy execution. A plastic holder with a disposable bush was placed at the end effector of the robotic arm to guide needle insertion. Subsequently, the needle was manually inserted through the chest wall directly into the lesion in a single pass, while the patient maintained breath-hold to the same extent as that of the initial positioning CT scan, guided by the light sign coupled to the respiratory belt. After decoupling the needle from the end effector and retraction of the robotic arm, needle positioning was confirmed with a further CT scan (Figs. 3 and 4) and adjustments were performed if required. Biopsy was then performed similarly to the conventional approach.
Patient preparation. Respiratory belt placed in patients sight (a, arrow) and coupled to the light sign (b, arrow). The belt registers the extent of chest movement at each respiratory act and displays this as coloured dots. More dots light up with wider respiratory movements. The patients were asked to control their breath during the procedure trying to avoid lighting of the outer dots
Biopsy planning on the ROBIO™ EX workstation. Target lesion in the lower right lobe at contrast-enhanced CT (a, arrow), surrounded by atelectasis (a, arrowhead). The entry point on the skin (b, arrow) and centre of target lesion (c, arrow) are determined by the operator. The angulations and insertion path of the needle are automatically calculated by the workstation and displayed in real time
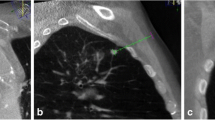
Positioning confirmation after control scan, immediately before biopsy. Adjacent slices demonstrate overlapping between the planned needle path (green line) and the actual needle position at the end of insertion. Robot-assisted biopsy allowed correct sampling of tumour tissue avoiding atelectasis. Final histological diagnosis was adenocarcinoma
Data analysis
The homogeneity assessment of the two groups included evaluation of the size, distance from entry point and position in lung of target lesions. The size and distance from entry point were compared between the two groups with the unpaired sample t test. Differences in the location (according to lobar anatomy) of target lesions between the two groups were assessed with the Mann–Whitney U test.
In order to demonstrate statistically significant differences (p < 0.01) of clinical and technical performance between the conventional biopsy approach and the robot-assisted technique, the following parameters were evaluated in the two groups:
-
Procedure duration (including planning time) and dose length product (DLP) were compared with the unpaired sample t test.
-
Number of needle adjustments was compared with the unpaired sample t test.
-
Planar and craniocaudal deviations of the needle tip from the planned target were calculated in millimetres and compared between the two groups with the unpaired sample t test. Multiplanar reformatted images were used for the evaluation of z-axis deviation.
-
Orbital and craniocaudal angular deviations at the target from the projected needle path were calculated in degrees (°) for robot-assisted biopsies only. Multiplanar reformatted images were used for the evaluation of craniocaudal angular deviation.
-
Diagnostic performance of the biopsy procedure was evaluated qualitatively (diagnostic/non-diagnostic sampling) and compared with the Mann–Whitney test.
-
The rate of complications in the two groups was evaluated following the clinical practice guidelines of the Society of Interventional Radiology [13] (no complications/minor complications/major complications) and compared with the Mann–Whitney U test.
Results
All biopsies were successfully performed under CT guidance in both groups. Lesions size (p = 0.41), distance from entry point (p = 0.86) and lesions location (p = 0.32) were similar in the two groups. Full results of the homogeneity assessment of the two groups are given in Table 1.
In group A procedure duration was significantly shorter (p = 0.001), DLP was lower (p = 0.001) and just occasional needle adjustments were required as compared to group B (p = 0.000). Planar and craniocaudal deviations of the needle tip from the planned target were similar in both groups (p = 0.05), while the orbital (transversal on the x-axis) and craniocaudal (longitudinal on the z-axis) angular deviations from the projected needle path in robot-assisted biopsies were 2 ± 1° and 2.5 ± 0.5° (Fig. 5). The diagnostic performance of CT-guided biopsies was similar in the two groups (p = 0.05), with four patients in group A and three patients in group B requiring re-biopsy due to inadequate quality of the biopsy sample. The rate of complications was comparable in the two groups (p = 0.05); there were three (6 %) cases of pneumothorax in group A and two (4 %) cases of pneumothorax in group B requiring chest tube drainage and prolonged hospitalization. Minor complications (including small pneumothorax not requiring therapy and self-limiting peri-lesional haemorrhages) occurred in two (4 %) cases in group A and four (7 %) in group B. Full results of the assessment of the clinical and technical performance of the two groups are given in Table 2.
Biopsy of a deep solitary lung nodule in the right upper lobe. The maximum transverse diameter of the nodule was 10 mm and its craniocaudal size was 15 mm. Planning CT demonstrates the desired needle path and tip positioning (a, arrowhead). Control CT scan after needle positioning shows a slight angular deviation of the needle tip resulting in a 1.5-mm deviation from the planned path (b, arrowhead). Notwithstanding the deviation, biopsy was successfully performed without further needle adjustments, achieving final histological diagnosis of adenocarcinoma
Discussion
Imaging-guided interventional techniques currently represent a fundamental tool in diagnosis and treatment of oncologic pathologies. Among the various guidance modalities, CT is the method of choice in the chest region owing to its excellent spatial and contrast resolution for the visualization of lung parenchyma, airways and cardiovascular structures that safely allows biopsy of lung and mediastinal lesions, percutaneous tube placement and thermal ablation of lung tumours. The conventional technique for CT-guided interventional procedures requires a trial-and-error method with the step-and-shoot approach, or the application of a real-time fluoroscopic monitoring in order to visualize and modify the path of needles and percutaneous probes. Even if the clinical performance of conventional approaches is highly reliable in expert hands [4–7], these methods present well-known technical limitations and their successful application depends significantly on operators’ manual skill and experience. In order to reduce such operator dependence, several assisting devices have been developed and tested in clinical practice, including external laser [8] or optical [14] targeting systems that project and/or guide the needle path onto the skin surface, electromagnetic tracking with image fusion [15] and augmented reality system under infrared guidance that display a real-time simulation of needle movements [9]. Preliminary reports are encouraging, but it should be noted that the success of these technologies is highly dependent on the integration between the assisting software/hardware, the CT system and the operator, with increased complexity and costs as compared to conventional techniques. Moreover, with the approaches mentioned above the dependence on operator experience is reduced but not completely eliminated, not mentioning the need for adequate training. On the other hand, the use of medical robots for surgical or imaging-guided procedures allows extremely accurate tool guidance with stable access, leading to increased precision, accuracy and reproducibility in a variety of applications, including percutaneous ablations, biopsies, orthopaedic fixture placement, hollow viscera or solid organ access [10]. While earlier robots required extensive installation and were often cumbersome to operate, being time consuming and economically disadvantageous [16, 17], more recent systems, such as the ROBIO™ EX, require minimal effort to be mounted and registered to the imaging device [11], reducing the complexity of the procedure. Also the fully automated movement of the robotic arm represents a relevant advantage that removes the need for manual or joystick adjustments in the pretreatment phase that are necessary with other devices [18, 19] and may further complicate the clinical workflow. From a clinical point of view, our study demonstrated in a large patient population that the presented robotic system facilitates CT-guided lung biopsies, with results that are substantially in line with previous reports on biopsies in phantoms [11] and clinical radiofrequency ablation of liver lesions [12]. It should be considered that, apart from these two preliminary studies performed with the same robotic platform, there is no literature evidence of large clinical series of robot-assisted CT-guided interventions, in particular for what regards chest procedures; hence, an indirect comparison with the performance of different robotic devices is currently impossible. In our single-centre experience, the precision in lesion targeting, the diagnostic performance of the biopsy sampling and the rate of complications in the robot-assisted procedures were comparable to those of conventional biopsies, with accurate needle positioning and very few adjustment required even in lesions as small as 15 mm, but the use of the robot significantly reduced procedure duration and radiation dose in comparison to the unassisted technique. This observation is particularly relevant, since in our study all procedures were performed by an operator with previous experience of more than 300 conventional CT-guided lung biopsies and, notwithstanding this expertise, significant reduction of procedure duration and radiation dose were in any case obtained in robot-assisted procedures as compared to the conventional technique. In this regard, future work should aim to evaluate when and how operators with different levels of experience may benefit from robot assistance in daily clinical routine, and assess potential differences in the clinical performance of robot-assisted procedures between expert and non-expert radiologists. Moreover, even if a dedicated cost-analysis is currently unavailable, it could be speculated that the use of interventional robotic systems will be probably even more beneficial in clinical settings in which financial resources or time for appropriate training of interventional radiologists is lacking, pushing less expert, non-interventional operators to perform simple imaging-guided procedures. Even if these preliminary results are encouraging, this study has some limitations. First, the sample size was not determined in advance with a power analysis in order to increase the relevance of the statistical evaluation. Moreover, a statistical subanalysis based on the anatomic characteristics of the target lesions (size, distance to pleura and position in lung) was not performed; hence we cannot provide clustered data on system performance for the biopsy of smaller and hardly accessible lesions, which should be the ideal target for robot-assisted procedures. Last, an independent evaluation of the status of the lung parenchyma surrounding the target lesions was not available in order to assess the influence of local pulmonary factors (emphysema, fibrosis, bronchiectases) on the rate of complications in the two groups, even if this parameter was probably not influential, since our complication rates do not differ from those reported in the literature [4–7]. Notwithstanding these limitations, the results of our study demonstrate that robot-assisted CT-guided lung biopsy is a safe and accurate interventional technique that can reduce procedure duration and radiation dose in comparison to the conventional manual approach even in expert hands. Further studies are needed to confirm these data and to evaluate the performance of robot-assisted interventional procedures in other clinical scenarios.
References
MacMahon H, Austin JH, Gamsu G, Herold CJ, Jett JR, Naidich DP, Patz EF Jr, Swensen SJ, Fleischner Society (2005) Guidelines for management of small pulmonary nodules detected on CT scans: a statement from the Fleischner Society. Radiology 237:395–400
Naidich DP, Bankier AA, MacMahon H, Schaefer-Prokop CM, Pistolesi M, Goo JM, Macchiarini P, Crapo JD, Herold CJ, Austin JH, Travis WD (2013) Recommendations for the management of subsolid pulmonari nodules detected at CT: a statement from the Fleischner Society. Radiology 266:304–317
Gould MK, Donington J, Lynch WR, Mazzone PJ, Midthun DE, Naidich DP, Wiener RS (2013) Evaluation of individuals with pulmonary nodules: when is it lung cancer? Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 143:e93S–e120S
Tuna T, Ozkaya S, Dirican A, Findik S, Atici AG, Erkan L (2013) Diagnostic efficacy of computed tomography-guided transthoracic needle aspiration and biopsy in patients with pulmonary disease. Onco Targets Ther 6:1553–1557
Lal H, Neyaz Z, Nath A, Borah S (2012) CT-guided percutaneous biopsy of intrathoracic lesions. Korean J Radiol 13:210–226
Kim GR, Hur J, Lee SM, Lee HJ, Hong YJ, Nam JE, Kim HS, Kim YJ, Choi BW, Kim TH, Choe KO (2011) CT fluoroscopy-guided lung biopsy versus conventional CT-guided lung biopsy: a prospective controlled study to assess radiation doses and diagnostic performance. Eur Radiol 21:232–239
Prosch H, Stadler A, Schilling M, Bürklin S, Eisenhuber E, Schober E, Mostbeck G (2012) CT fluoroscopy-guided vs. multislice CT biopsy mode-guided lung biopsies: accuracy, complications and radiation dose. Eur J Radiol 81:1029–1033
Hong CW, Xu S, Imbesi KL, Wood BJ (2013) Integrated laser-guided CT biopsy. Clin Imaging 37:1135–1137
Grasso RF, Faiella E, Luppi G, Schena E, Giurazza F, Del Vescovo R, D'Agostino F, Cazzato RL, Beomonte Zobel B (2013) Percutaneous lung biopsy: comparison between an augmented reality CT navigation system and standard CT-guided technique. Int J Comput Assist Radiol Surg 8:837–848
Kettenbach J, Kronreif G, Melzer A, Fichtinger G, Stoianovici D, Cleary K (2007) Ultrasound-, CT- and MR-guided robot-assisted interventions. In: Neri E, Caramella D, Bartolozzi C (eds) Image processing in radiology: current applications. Springer, Heidelberg, pp 391–404
Koethe Y, Xu S, Velusamy G, Wood BJ, Venkatesan AM (2014) Accuracy and efficacy of percutaneous biopsy and ablation using robotic assistance under computed tomography guidance: a phantom study. Eur Radiol 24(3):723–730
Abdullah BJ, Yeong CH, Goh KL, Yoong BK, Ho GF, Yim CC, Kulkarni A (2014) Robot-assisted radiofrequency ablation of primary and secondary liver tumours: early experience. Eur Radiol 24:79–85
Sacks D, McClenny TE, Cardella JF, Lewis CA (2003) Society of Interventional Radiology clinical practice guidelines. J Vasc Interv Radiol 14:S199–S202
von Jako CR, Zuk Y, Zur O, Gilboa P (2013) A novel accurate minioptical tracking system for percutaneous needle placement. IEEE Trans Biomed Eng 60:2222–2225
Krücker J, Xu S, Glossop N, Viswanathan A, Borgert J, Schulz H, Wood BJ (2007) Electromagnetic tracking for thermal ablation and biopsy guidance: clinical evaluation of spatial accuracy. J Vasc Interv Radiol 18:1141–1150
Tovar-Arriaga S, Tita R, Pedraza-Ortega JC, Gorrostieta E, Kalender WA (2011) Development of a robotic FD-CT-guided navigation system for needle placement-preliminary accuracy tests. Int J Med Robot 7:225–236
Yanof J, Haaga J, Klahr P (2001) CT-integrated robot for interventional procedures: preliminary experiment and computer human interfaces. Comput Aided Surg 6:352–359
Schulz B, Eichler K, Siebenhandl P et al (2012) Accuracy and speed of robotic assisted needle interventions using a modern cone beam computed tomography intervention suite: a phantom study. Eur Radiol 23:198–204
Su L-M, Stoianovici D, Jarrett TW et al (2002) Robotic percutaneous access to the kidney: comparison with standard manual access. J Endourol 16:471–475
Acknowledgments
The scientific guarantor of this publication is Dr. Michele Anzidei. The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article. The authors state that this work has not received any funding. Dr. Fulvio Zaccagna kindly provided statistical advice for this manuscript. Institutional review board approval was obtained. Written informed consent was obtained from all subjects (patients) in this study. No study subjects or cohorts have been previously reported. Methodology: prospective, randomised controlled trial, performed at one institution.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Anzidei, M., Argirò, R., Porfiri, A. et al. Preliminary clinical experience with a dedicated interventional robotic system for CT-guided biopsies of lung lesions: a comparison with the conventional manual technique. Eur Radiol 25, 1310–1316 (2015). https://doi.org/10.1007/s00330-014-3508-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-014-3508-z