Abstract
19F MRI is emerging as a new imaging technique for cell tracking. It is particularly attractive because of its potential for direct and precise cell quantification. The most important challenge towards in vivo applications is the sensitivity of the technique, i.e. the detection limit in a reasonable imaging time. Optimal sensitivity can be achieved with dedicated 19F compounds together with specifically adapted hardware and acquisition methods. In this paper we introduce the 19F MRI technique focusing on these key sensitivity issues and review the state-of-the-art of 19F MRI and developments towards its clinical use. We calculate 19F detection limits reported in preclinical cell and clinical 19F drug studies in terms of tissue concentration in a 1 cm3 voxel, as an alternate way to compare detection limits. We estimate that a tissue concentration of a few millimoles per litre (mM) of 19F is required for a human study at a resolution of 1 cm3.
Key Points
• Direct and precise cell quantification can be done by 19 F MRI.
• 19 F MRI sensitivity is the most important parameter towards clinical application.
• A number of (technical) considerations can improve sensitivity significantly.
• A few millimoles per litre (mM) of 19 F per voxel is required for adequate detection.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Clinical imaging modalities such as magnetic resonance imaging (MRI), positron emission tomography (PET) and single photon emission computed tomography (SPECT) allow in vivo monitoring of cells, e.g. in cellular therapy. Among those, MRI has been reported to have the highest resolution and, in some cases, sensitivity for cell tracking [1]. Moreover, unlike PET and SPECT, MRI requires no radioactive material and can acquire both anatomical and functional information.
MRI normally measures signals from protons (1H) in tissue water. This provides high resolution images of soft tissues with the contrast dependent on tissue-specific 1H spin relaxation times and the variety of available imaging protocols. However, despite this high resolution and soft tissue contrast, it is generally not possible to distinguish individual cells. Thus cells of particular interest, such as those used in cellular therapy, must be labelled to distinguish them from the background. Labelling can be carried out in a number of ways such as using conventional contrast agents (CAs) that alter 1H relaxation times, reporter genes that generate contrast by trapping endogenous CAs, or by employing a nucleus other than 1H, e.g. fluorine-19 (19F).
CAs for MRI are typically either paramagnetic, usually gadolinium-based [2, 3], or superparamagnetic [4, 5], usually iron oxide-based. They affect the 1H spin relaxation times locally and thus provide a regional change in contrast. For cellular imaging, superparamagnetic CAs have been widely studied and discussed [6, 7] with a variety of applications such as lymph node imaging [8–10], cancer imaging [11–13] and cell tracking [14, 15]. The main advantage of the superparamagnetic CAs is their relatively high sensitivity; unfortunately, they do not possess high specificity. For instance, in particular disease models where disease progression may alter intrinsic contrast (e.g. by haemorrhage or vascular changes), distinguishing between labelled cells and background signal may become a major problem [16]. A solution to this is to use nuclei other than hydrogen with minimal background in tissues. For this purpose, 19F has attracted much attention [17]. Another important issue is that clinical superparamagnetic CAs for cell tracking have been taken off the market [18] and this brings even more interest to 19F MRI as a high-potential technique.
The 19F nucleus has spin 1/2, 100 % natural abundance, sensitivity close to that of 1H, and owing to the lack of measurable endogenous fluorine, there is no background signal in tissue images. Moreover, contrary to the CAs in MRI that are measured indirectly via their interactions with water molecules, 19F is measured directly and thus results in higher specificity. In order to perform 19F MRI experiments using a conventional MRI system, some modifications to both hardware and software are required. Once these modifications are accomplished, in vivo experiments can be carried out, typically by acquiring an anatomical 1H MR image to be overlaid by a 19F image.
19F MRI is becoming of particular importance in the field of cellular imaging as the number of labelled cells in a region of interest (ROI) is directly proportional to the 19F signal which enables cell quantification [19]. This quantitative technique can be used to assess and optimize new approaches in cellular therapy/diagnosis. However, typical fluorinated compounds, such as those used in MR spectroscopy [20], consist of a relatively low number of fluorine atoms per molecule with long 19F longitudinal relaxation times (T1). This may lead to a relatively low signal to noise ratio per unit scan time (SNR/t), giving rise to low sensitivity and long scan times, which would be a problem in the imaging of humans. Thus, high 19F content, relatively short T1 and high magnetic fields are key ingredients for successful in vivo 19F MRI. These requirements lead to the use of perfluorocarbons (PFCs) for most cell labelling applications.
The overall objective of this work is to comprehensively discuss the technical aspects of 19F MRI focusing on cell tracking as a growing field with high potential for clinical applications. Towards this aim, we first introduce 19F MRI agents for cell labelling and then the 19F MRI technique is presented, with particular attention to its sensitivity and other challenges in clinical translation.
19F agents for cell labelling
In cell tracking, typically cells are labelled either ex vivo, before being transferred into the subject, or in situ. The different cell labels developed for 19F MRI have been reviewed elsewhere [1, 17, 19, 21]. In general, the 19F labels developed thus far consist of PFCs resulting in high 19F density per molecule. PFCs have been proposed as blood substitutes for several decades [22]; and new agents are still in clinical trials (e.g. Oxycyte by Oxygen Biotherapeutics Inc., Morrisville NC USA, is currently in phase II trials in several countries; http://clinicaltrial.gov/ct2/show/NCT00174980?term=perfluorocarbon&rank=5). Furthermore, some PFCs are also approved for medical applications such as contrast-enhanced ultrasound (e.g. SonoVue by Bracco Imaging SpA, Milan, Italy). Thus, overall PFCs are known to be well-tolerated in vivo. For MRI, PFCs with a single 19F resonance (or single dominant resonance) are preferable to avoid imaging artefacts. Preclinical and clinical research involving 19F MRI with PFC agents is ongoing.
Some considerations that arise with 19F cell labels are the stability of the agent, its localisation, sensitivity to oxygen tension and number of resonance peaks within the receiver bandwidth. These factors are particularly important in quantitative imaging [19]. Specifically, it is assumed that all 19F signals measured are from the labelled cells (i.e. that no label is lost to other cells or the extracellular space) and that the T1 of the compound is unchanged relative to the reference. Both these assumptions may need to be validated experimentally. For instance, specific cellular localisation of the label is generally validated using a secondary label, typically a fluorescent dye [23], with ex vivo histology. This can also be used to study the fate of the label if the cells divide and the number of cell divisions that may have occurred, when dividing cells are used. In practice, with cells such as dendritic cells and macrophages (the most commonly used cell types in cell tracking studies), cell division is not an issue as these cells do not divide. Even in these cases, label can be lost to the extracellular space over time, especially with cell death. This has been shown to occur with fluorinated polylysine-labelled cells which lost nearly half the label within 7 days [24]. The best way to control for non-specific labelling is histology coupled with a fluorescent dye.
Various types of cellular labels and cell labelling strategies have been developed to optimize 19F MRI [21], although most cell tracking work has focused on PFC-based emulsions [25]. Commonly, these emulsions are added directly to the cell culture medium and taken up by the cells ex vivo. An alternative approach is in vivo labelling of phagotypic cells, mostly macrophages, through intravenous injection of the label (e.g. see [26–28]). In all cases, cell labelling is optimized to maximize the 19F content per cell. The 19F content per cell and the total number of cells transferred to the subject are often the main parameters that can be controlled in a particular in vivo experimental model which directly affect the SNR/t.
19F MRI hardware
The hardware of a conventional MRI system requires some dedicated parts for 19F imaging (see Fig. 1). Here we discuss briefly the key hardware components and their role in optimizing cell tracking effectively using 19F MRI.
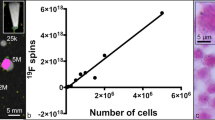
a Schematic of a conventional MR system with required hardware to perform both 1H (black and white) and 19F (red) imaging. A 1H/19F coil is employed to record the response of the excited nuclear spins. Power RF amplifiers are needed to generate sufficient power for the excitation pulses at each nuclear frequency. The interface contains a 1H/19F filter, transmit-receive switches and a pre-amplifier. The final image is obtained by overlaying 19F maps on the structural 1H image. b MR image of mouse legs immediately after injection of perfluoro-[15]-crown-5 ether particles in the footpad. The images shown are 2-mm coronal slices with 1H in grayscale, 19F in false colour and an overlay (adapted with permission from ref. [29])
Magnet
At higher magnetic fields, higher spatial and/or temporal resolutions are accessible because of the increase in SNR, i.e. 19F images with smaller numbers of 19F labelled cells can be obtained in a shorter scan time. When using CAs, increasing the magnetic field may not always be beneficial as their efficiency is field-dependent. In contrast, 19F MRI, in which spin density is relatively low, always benefits from higher field strengths; however, some issues such as B1 and B0 homogeneity as well as prolonged relaxation times should be considered.
A critical issue, particularly at higher fields, is the power deposition in the patient caused by radiofrequency (RF) pulses; this is proportional to B0 2 and defined by the specific absorption rate (SAR). Power deposition in the tissue depends on a number of factors such as tissue characteristics, type of sequence and its parameters. For instance, fast spin echo (SE)-based sequences usually are known as high SAR acquisition techniques since they include several refocusing (180°) pulses. More details on advantages and challenges of high magnetic field, with a specific emphasis on safety issues, have been reviewed elsewhere [30].
Radiofrequency coils
For 19F measurements, a dedicated RF coil setup has to be installed, covering the relevant resonance frequency of 19F. The RF coils, to excite the spin systems, generate a B1 field that ideally should be as homogeneous as possible for precise signal quantification. In practice, this is not always the case and corrections are needed [31–33].
In 19F MRI, low SNR is a serious concern as it may lead to low sensitivity and long scan times which makes the translation to the clinic challenging. Thus, selecting an appropriate RF coil is critical. For instance, surface coils are more efficient for imaging ROIs close to the body surface, e.g. lymph nodes. The RF coils can be designed to operate as receive-only, transmit-only and transmit-receive (transceiver). In clinical practice, commonly, a volume coil (whole-body coil) is present in the magnet to provide the homogeneous transmit field and local coils are used as receive-only coils for high SNR, although this may not be the most appropriate for 19F imaging.
In cell tracking by 19F MRI, 19F and 1H anatomical images are acquired and overlaid to correlate the quantified cell numbers with pathology of interest such as inflamed tissue. To do this, selection of a tunable RF coil with a high bandwidth, to accommodate both 1H and 19F resonance frequencies, could be an option but this may increase the receiver noise figure, a parameter which measures the reduction in SNR due to the losses in the loop coil only. In addition, with tuning and matching from 1H to 19F the sensitivity profiles of the RF coil at the two corresponding frequencies vary [34]. Image registration also needs to be done accurately, especially if different 1H and 19F coils are used.
Novel strategies of designing multinuclear coils for in vivo experiments have been reported that allow changes in the resonance frequency using dedicated frequency switches which potentially can be applied to 19F MRI as well [35]. Another strategy is to use a double-tuned RF coil in which both 1H and 19F resonance frequencies are tuned and matched once in the beginning of the experiment [36]. In this way, registration of 1H and 19F images cannot change as a result of the coil and subject displacement; however, one may need to adjust the 90° excitation pulse for each nucleus channel separately. Another reported approach is to use a dual frequency 1H/19F coil able to collect both images simultaneously [34, 37]. It is worth mentioning that parallel imaging using phased array coils [38] can also be beneficial in 19F imaging. Phased array coils increase the SNR and speed up the acquisition time. Moreover, since images of all elements are acquired simultaneously, larger regions of interest can be covered in the same scan time.
Most MR system manufacturers provide spectrometers with multi-nuclei options, including 19F; however, the standard coil package usually does not include 19F coils and these need to be acquired separately. Animal coils are mostly custom-built; although commercial ones are available upon request.
Gradient coils
Gradient coils are another important part of MRI hardware. In quantitative cellular MRI, gradient instability may contribute to inaccurate quantification and experiment reproducibility over time. A slice of interest is selected by employing slice selective gradients. Selecting thicker slices improves the SNR in 19F imaging. Employing fast imaging sequences also improves the SNR/t significantly. In these cases, the slew rate, i.e. how quickly the gradient coils can be switched on or off, is a key parameter.
19F MRI acquisition methods
Spatial mapping of 19F spins has been done by magnetic resonance spectroscopic imaging (MRSI), in which whole 19F spectra are acquired, and by MRI, in which the spectroscopic dimension is discarded. In most cell tracking studies, this dimension is not relevant and thus MRI is more practical.
This section describes the influence of imaging parameters and sequences on the SNR in 19F MRI. In general, the SNR per voxel in two-dimensional (2D) is given by Eq. 1 [39]:
where (FOV)x and (FOV)y are the field of view in the x and y directions. N x and N y are the number of frequency and phase encoding steps, respectively. Δz is the slice thickness, NA is the number of signal averages, and rBW is the readout (receiver) bandwidth. The constant C includes tissue intrinsic parameters (e.g. relaxation times), hardware characteristics (e.g. coil configuration), pulse sequences, imaging parameters etc.
Image acquisition parameters
According to Eq. 1, different imaging parameters such as rBW, FOV, matrix size, slice thickness, repetition time (TR) and echo time (TE) can affect SNR considerably. Some of these parameters are coupled and therefore it is always a compromise between high resolution, high SNR and shorter scan time. Here we will focus on the parameters that play a major role in the optimization of SNR per unit time, as this is the most challenging issue in 19F MRI for cell tracking (see Fig. 2).
a Parameters with the most significant effect on the SNR (arrows show changes in a parameter which lead to an increase in the SNR; upward arrow increase, downward arrow decrease). b Coefficient factor applied to the SNR by doubling some parameters assuming all experimental conditions unchanged. c Schematic of the effect of fluorine concentration and slice thickness on detectability. If one selects a voxel as shown in dashed-dotted line, fluorine signal can be detected, whereas an acquisition with a smaller voxel, e.g. dotted lines, may fail to detect any fluorine because of the lack of enough fluorine spins in that voxel. It is noted that if fluorine atoms are only those existing in the dotted region, then none of the above voxel selections can detect a fluorine signal. As a result of these considerations sensitivity measures are meaningful if reported for a given voxel size. Therefore, the “bench mark” detection limit is proposed in Table 1
For full recovery of magnetization between excitation pulses with maximum SNR, the pulse repetition TR should be more than 5 times T1. However, for 19F compounds, specifically PFCs, usually the T1 is in the order of seconds and this makes the TR (and the scan time) long, leading to a low SNR/t. For instance, a T1 of 1,656 ± 40 ms for biocompatible perfluorooctyl bromide (PFOB), at 7T, has been reported [40]. Therefore, it is desirable to shorten T1, if possible. A reported strategy is to link a fluorinated compound to a lanthanide (III) ion as a paramagnetic centre [41, 42]. For example, chemically linking fluorine to dysprosium (Dy) resulted in a T1 of about 7 ms at 7T [42].
In gradient echo (GRE) sequences, shorter TR and faster imaging with optimum SNR/t is possible if the excitation flip angle is set to the Ernst angle defined as θ E = cos− 1[Exp(−TR/T 1)]. If one employs a 19F compound that shortens T1 by a factor of 2 then, using the same experimental setup, TR can be decreased by the same factor leading to a faster scan and therefore increase in the SNR/t.
The TE is the time between 90° excitation pulse and the signal echo. As mentioned previously, a short T1 value for 19F is desired but this is, usually, accompanied by shortening of the T2 relaxation time with signal loss. To compensate for this, TE is kept as short as possible in order to have a higher SNR.
The rBW is the frequency range in which the signal is collected. By increasing the rBW in MR imaging, shorter TE is possible but it also decreases the SNR. Therefore, an increase in rBW is not recommended unless a compound with more than one resonance peak is employed in which case increasing the rBW will decrease the chemical shift artefact.
The FOV, voxel volume and matrix size are strongly interconnected. Matrix size determines the pixel size in a given FOV. The smaller the pixel size the higher the image resolution. The slice thickness determines the third dimension of a pixel to make a voxel. It is obvious that bigger voxels contain more 19F-labeled cells and thus provide more signal intensity and SNR, at the cost of lower resolution. In 19F MRI, high resolution images are not typically required; therefore, an increase in voxel size by selecting a thick slice and/or large pixel size is often an easy way to improve the SNR. For instance, with dendritic cell tracking, keeping the slice thickness constant, reducing in-plane resolution by a factor of 4 and increasing number of averages by a factor of 64, compared to that of the 1H MRI acquisition, a reasonably good fluorine SNR in vivo has been achieved [43].
Imaging sequences
As with 1H MRI, GRE- or SE-based imaging techniques are frequently used for cell tracking with 19F MRI. GRE sequences result in an echo generation with a single excitation pulse and gradient reversal while in the SE imaging the 90° excitation pulse is followed by 180° refocusing pulses. Therefore a GRE sequence, with a low flip angle and consequently a shorter TR, provides faster imaging as well as less RF power deposition. However, it is more sensitive to B0 heterogeneity and susceptibility effects with a faster T2 * decay. SE imaging, on the other hand, has a higher SNR (at the cost of longer scan time) but results in less susceptibility artefacts. It is worth mentioning that relatively fast versions of both GRE and SE sequences are widely available and used in the clinical setting, known as FLASH (fast low-angle shot) and turbo SE, respectively.
The selection of an imaging sequence and its optimization to get the highest SNR/t depend on the characteristics of the 19F agent used. The highest SNR/t, for a given compound, is achieved when the T2 */T1 ratio is close to 1. The generation of compounds with short relaxation times should go together with imaging sequence optimization. For instance, by adding paramagnetic lanthanide tags to fluorine compounds relaxation times in the range of 1–5 ms and a high T2 */T1 ratio could be achieved [44]. However, to fully exploit this condition and recover the full signal before it has decayed, the use of a short TE is required. Using a zero echo time (ZTE) sequence resulted in a 27-fold increase in the SNR, whereas this gain was 11-fold when using a GRE sequence (see Fig. 3).
Diagram illustrating how T2 */T1 can be employed efficiently. Red area is the acquisition window. a A fluorinated compound with long relaxation times, acquired by a conventional sequence. Signal amplitude after excitation is low, as a result of the long T1. b A fluorinated compound with high T2 */T1, acquired by a conventional sequence. Initial signal is high, but it decays rapidly and is inefficiently sampled by a conventional sequence. c The same as b but acquired by a ZTE sequence. As TE is nearly zero, the initially high signal can be acquired. Reprinted, with permission, from ref. [44]
Some fluorinated compounds have multiple resonance peaks and a broad range of frequencies, leading to chemical shift artefacts which can make localisation and quantification ambiguous [45]. For these compounds different scenarios can be applied. For instance, PFOB is such a compound that a fluorine ultrafast turbo spectroscopic imaging (F-uTSI) technique has been proposed to overcome its chemical shift artefact [45]. Using a short sampling time and taking advantage of relatively long T2 of PFOB (ca. 100 ms) allowed a high number of echoes per excitation to be acquired. In this way, SNR was increased up to 75 % with a 40 % shorter scan time, when using back projection [46]. In a 31P and 13C MRI study, images were obtained of multiple metabolites simultaneously by selectively exciting different metabolite spins and exploiting their chemical shift dispersion [47]. By selecting proper bandwidth, imaging matrix size and FOV it was possible to obtain different images associated with different resonance peaks and even discard undesirable images corresponding to undesirable metabolites. This strategy would also be valuable in 19F cell tracking, for instance, to simultaneously image different cell types labelled with different 19F compounds [48].
In addition to the imaging sequence, specific data acquisition and reconstruction techniques can be used to increase SNR/t or reduce the measurement time. 3D compressed sensing, for example, has been shown to decrease the acquisition time by a factor of 8 when imaging cells using 19F MRI [49]. Another study has reported a two-fold increase in the detection sensitivity while using a balanced steady-state free precession (SSFP) sequence with an ultrashort echo time (UTE) and 3D radial readout [50].
Quantification and sensitivity in 19F MRI
In 19F MRI for cell tracking, cell quantification requires prior knowledge of the number of 19F atoms per cell. This can be determined by in vitro NMR experiments, on a known number of labelled cells, before the cell implantation in vivo [51]. This is done by comparison of the peak integral of the 19F signal from the label with that from a reference with a known concentration, within the same experiment. The same strategy can be applied for in vivo MRI experiments, i.e. a reference with a known concentration of 19F is placed adjacent to the subject to image both simultaneously. The number of 19F atoms in the reference is proportional to its signal [52]. Comparison of the reference signal and the signal obtained from the subject allows calculation of the number of 19F atoms in the ROI. From this, the number of cells per voxel can then be calculated. It has to be taken into account that the actual value may be different owing to biological and/or physicochemical phenomena such as cell division, cell death, loss of label, as well as undesirable changes in compound T1 and T2 after being administered into the tissue [19]. These issues can only be addressed through additional ex vivo experiments, such as histology to study label localisation or flow cytometry to study cell division; nevertheless both techniques require additional agents such as a fluorescent dye to be included with the 19F label. However, since most studies using 19F MRI for cell tracking thus far deal with non-dividing cells, label loss due to cell division is not a major issue. However, nonspecific labelling of resident macrophages can still influence the signal detected.
Sensitivity in cell tracking is defined as the minimum number of detectable cells in a given scan time. As most sensitivity studies with 19F-labelled cells to date have been performed in vitro or with animals, it is difficult to extrapolate the sensitivity values of these studies to the conditions for human experiments. Moreover, it is also difficult to compare the results of different preclinical studies as this value depends on a large number of parameters, in particular the number of detectable 19F atoms in the label used, cell loading, coil parameters (type and size), voxel size, field strength, acquisition and reconstruction method, and scan time.
An alternate way to evaluate sensitivity and detection limits is by comparing tissue concentrations of 19F atoms. In Table 1, values for the relevant parameters are listed for some in vitro and in vivo studies together with 19F tissue concentrations. Variations in the detection limit indeed reflect differences in values for the above parameters, but also in the T2 */T1 ratio of the 19F spins. As voxel volume clearly is an important determinant of the detection limit, we also calculated and compared this limit as 19F tissue concentrations in a 1 cm3 voxel size. This bench mark resolution is selected as it represents a typical lower limit value in human 1H MRS at 3 T (1H has a similar intrinsic sensitivity as 19F), which can be measured in a clinically acceptable time and is estimated to be at sufficient resolution for the detection of 19F-labelled cells in humans. This resolution can be used to extrapolate values from preclinical studies to values required for human studies.
Previously, our group reported for an in vitro experiment a detection limit (at an SNR of 3) of 2,000 cells with about 2 × 1013 19F atoms per cell at 7T [43]. This, at the resolution of the experiment (2 mm3), translates to a tissue concentration limit of 33 mM corresponding to 0.066 mM in 1 cm3. If we extrapolate this to 3T, the detection limit would be 9,000 cells, which translates to a tissue concentration limit of about 0.3 mM 19F at a spatial resolution of 1 cm3 (see Table 1). So we would roughly estimate that a few millimoles per litre 19F would be required for a successful human imaging study, dependant on the coil size and type. Interestingly, using a head coil and a clinical 3T system, a detection limit of 4 mM has been anticipated [57].
A realistic assessment of the feasibility of clinical applications can be obtained from quantitative 19F magnetic resonance spectroscopy (MRS) studies in humans [58, 58], which indeed have shown that a few millimoles per litre 19F is required for a human study at 3T, at a spatial resolution of 1 cm3 (Table 1).
Challenges towards clinical applications
19F MRI has been widely used for cellular imaging in different pathologies such as stem cell-based therapy [20, 23], immunotherapeutic cell tracking [43, 51], cell migration in diabetes model [58], inflammation [26, 57, 58–62] as well as graft rejection assessment [25, 63]. Translation of preclinical cell tracking procedures, using 19F MRI, into humans is possible if sufficient sensitivity is achieved. However, most of the 19F MRI reports in the field of cellular imaging have been performed on animals, using custom-built RF coils, and mainly at high and ultra-high magnetic fields. Indeed this improves sensitivity, but the SAR and acquisition times that are not limitations for preclinical studies become important issues in human imaging. Therefore, careful consideration of system hardware and imaging sequences and parameters is required. SAR limits should be carefully considered for the translation of fast imaging sequences and RF coils into clinics.
As discussed previously, the 19F agent used directly affects imaging sensitivity. The generation of new agents certainly also requires the assessment of compound safety, toxicity and clearance before being applied to human imaging. In this regard, PFCs have the advantage of prior clinical use, e.g. as oxygen carriers. A study in rats investigated the biodistribution of a mixed fluorocarbon–hydrocarbon over 24 h [64]. The authors found 70 % of the injected dose in the liver, 17 % in the spleen, 4 % in the lungs, 2 % in the kidneys and 2 % in the blood, with no evidence of metabolism. The half-time retention of that particular compound (silicone-coated polychlorotrifluoroethylene capsules filled with perfluoro-[65]-crown-5 ether) in the liver was estimated to be 25 ± 5 days. Recently, a superfluorinated molecular probe has been synthesized for cell tracking [66]. This probe provides an intense single resonance peak owing to its 36 equivalent 19F atoms and possesses cellular compatibility.
Summary
19F MRI is emerging as a new imaging technique with particular potential for cell tracking. The most important issue of 19F MRI in this field is sensitivity, i.e. the detection limit in a reasonable measurement time. Faster scans clearly lead to a reduced cost but 19F MRI can also decrease the therapeutic costs. For example, a major issue with the optimization of cellular therapeutics is the dearth of knowledge about the fate of the cells in vivo [63]. For instance, dendritic cell therapy, as a novel cancer therapeutic approach, is very costly and currently has a low success rate. This failure is partly due to the misinjection of dendritic cells, even when ultrasound-guided [65]. 19F MRI can potentially improve such a treatment by tracking the injected cells and therefore optimizing the injection route. Importantly, subjects who do not show appropriate cell homing responses can be excluded from such studies at early time points, thereby greatly reducing costs.
To optimize the sensitivity, the generation of dedicated 19F compounds together with improved hardware and acquisition methods is desirable. Compounds with many fluorine atoms, a single resonance frequency and a T2 */T1 close to 1 will be ideal for a good SNR. Furthermore, 19F content per cell (cell loading) also directly affects detection sensitivity. Hardware plays a key role in achieving higher sensitivity, in particular selecting an appropriate RF coil (right type and size) is very important. An optimized imaging sequence is also essential. For example, using a ZTE sequence has been recommended for compounds with T2 */T1 close to 1 [44], whereas a sequence to selectively excite the peaks of interest has been proposed for compounds with multi-resonance peaks [47]. All optimizations must be accompanied by safety assessments before being applied in humans.
From clinical 19F drug MR reports it follows that a 19F tissue concentration of a few millimoles per litre at a resolution of about 1 cm3 is required for human 19F MRI, in a reasonable measurement time. An extrapolation of the results of preclinical cell tracking studies and human examinations at 3T show the great potential of 19F MRI for cell tracking in humans. Indeed, a recent abstract reported the first successful dendritic cell tracking in patients with colorectal cancer [67]. To facilitate the use of published cell detection limits, as well as having consistent parameters to assess clinical translation, we recommend reporting 19F tissue concentrations in cell tracking studies.
Abbreviations
- CA:
-
Contrast agent
- 2D:
-
Two-dimensional
- Dy:
-
Dysprosium
- FDA:
-
Food and drug administration
- FOV:
-
Field of view
- F-uTSI:
-
Fluorine ultrafast turbo spectroscopic imaging
- GRE:
-
Gradient echo
- MRI:
-
Magnetic resonance imaging
- MRSI:
-
Magnetic resonance spectroscopic imaging
- PFC:
-
Perfluorocarbon
- PFOB:
-
Perfluorooctyl bromide
- RF:
-
Radiofrequency
- ROI:
-
Region of interest
- SAR:
-
Specific absorption rate
- SE:
-
Spin echo
- SNR/t:
-
Signal to noise ratio per unit scan time
- SSFP:
-
Steady-state free precession
- TE:
-
Echo time
- TR:
-
Repetition time
- UTE:
-
Ultrashort echo time
- ZTE:
-
Zero echo time
References
Ahrens ET, Bulte JW (2013) Tracking immune cells in vivo using magnetic resonance imaging. Nat Rev Immunol 13:755–763
Laniado M, Weinmann HJ, Schorner W, Felix R, Speck U (1984) First use of GdDTPA/dimeglumine in man. Physiol Chem Phys Med NMR 16:157–165
Weinmann HJ, Brasch RC, Press WR, Wesbey GE (1984) Characteristics of gadolinium-DTPA complex: a potential NMR contrast agent. Ajr 142:619–624
Amiri H, Bustamante R, Millan A et al (2011) Magnetic and relaxation properties of multifunctional polymer-based nanostructured bioferrofluids as MRI contrast agents. Magn Reson Med 66:1715–1721
Amiri H, Mahmoudi M, Lascialfari A (2011) Superparamagnetic colloidal nanocrystal clusters coated with polyethylene glycol fumarate: a possible novel theranostic agent. Nanoscale 3:1022–1030
Economopoulos V, Chen Y, McFadden C, Foster PJ (2013) MRI detection of nonproliferative tumor cells in lymph node metastases using iron oxide particles in a mouse model of breast cancer. Transl Oncol 6:347–354
Odintsov B, Chun JL, Berry SE (2013) Whole body MRI and fluorescent microscopy for detection of stem cells labeled with superparamagnetic iron oxide (SPIO) nanoparticles and DiI following intramuscular and systemic delivery. Methods Mol Biol 1052:1–17
Lind K, Kresse M, Debus NP, Muller RH (2002) A novel formulation for superparamagnetic iron oxide (SPIO) particles enhancing MR lymphography: comparison of physicochemical properties and the in vivo behaviour. J Drug Target 10:221–230
Mack MG, Balzer JO, Straub R, Eichler K, Vogl TJ (2002) Superparamagnetic iron oxide-enhanced MR imaging of head and neck lymph nodes. Radiology 222:239–244
Weissleder R, Elizondo G, Wittenberg J, Lee AS, Josephson L, Brady TJ (1990) Ultrasmall superparamagnetic iron oxide: an intravenous contrast agent for assessing lymph nodes with MR imaging. Radiology 175:494–498
Larsen EK, Nielsen T, Wittenborn T et al (2012) Accumulation of magnetic iron oxide nanoparticles coated with variably sized polyethylene glycol in murine tumors. Nanoscale 4:2352–2361
Poselt E, Schmidtke C, Fischer S et al (2012) Tailor-made quantum dot and iron oxide based contrast agents for in vitro and in vivo tumor imaging. ACS Nano 6:3346–3355
Zimmer C, Weissleder R, Poss K, Bogdanova A, Wright SC Jr, Enochs WS (1995) MR imaging of phagocytosis in experimental gliomas. Radiology 197:533–538
Boulland JL, Leung DS, Thuen M et al (2012) Evaluation of intracellular labeling with micron-sized particles of iron oxide (MPIOs) as a general tool for in vitro and in vivo tracking of human stem and progenitor cells. Cell Transplant 21:1743–1759
Frank JA, Miller BR, Arbab AS et al (2003) Clinically applicable labeling of mammalian and stem cells by combining superparamagnetic iron oxides and transfection agents. Radiology 228:480–487
Himmelreich U, Dresselaers T (2009) Cell labeling and tracking for experimental models using magnetic resonance imaging. Methods (San Diego, Calif) 48:112–124
Ahrens ET, Zhong J (2013) In vivo MRI cell tracking using perfluorocarbon probes and fluorine-19 detection. NMR Biomed 26:860–871
Bulte JW, Walczak P, Gleich B et al (2011) MPI cell tracking: what can we learn from MRI? Proc Soc Photo-Optical Instrum Eng 7965:79650z
Srinivas M, Heerschap A, Ahrens ET, Figdor CG, de Vries IJ (2010) (19)F MRI for quantitative in vivo cell tracking. Trends Biotechnol 28:363–370
Ruiz-Cabello J, Walczak P, Kedziorek DA et al (2008) In vivo “hot spot” MR imaging of neural stem cells using fluorinated nanoparticles. Magn Reson Med 60:1506–1511
Srinivas M, Boehm-Sturm P, Figdor CG, de Vries IJ, Hoehn M (2012) Labeling cells for in vivo tracking using (19)F MRI. Biomaterials 33:8830–8840
Waters EA, Chen J, Allen JS, Zhang H, Lanza GM, Wickline SA (2008) Detection and quantification of angiogenesis in experimental valve disease with integrin-targeted nanoparticles and 19-fluorine MRI/MRS. J Cardiovasc Magn Reson 10:43
Boehm-Sturm P, Mengler L, Wecker S, Hoehn M, Kallur T (2011) In vivo tracking of human neural stem cells with 19F magnetic resonance imaging. PLoS One 6:e29040
Maki J, Masuda C, Morikawa S et al (2007) The MR tracking of transplanted ATDC5 cells using fluorinated poly-L-lysine-CF3. Biomaterials 28:434–440
Hitchens TK, Ye Q, Eytan DF, Janjic JM, Ahrens ET, Ho C (2011) 19F MRI detection of acute allograft rejection with in vivo perfluorocarbon labeling of immune cells. Magn Reson Med 65:1144–1153
Flogel U, Ding Z, Hardung H et al (2008) In vivo monitoring of inflammation after cardiac and cerebral ischemia by fluorine magnetic resonance imaging. Circulation 118:140–148
Jacoby C, Temme S, Mayenfels F et al (2014) Probing different perfluorocarbons for in vivo inflammation imaging by 19F MRI: image reconstruction, biological half-lives and sensitivity. NMR Biomed 27:261–271
Majcher K, Tomanek B, Jasinski A et al (2006) Simultaneous functional magnetic resonance imaging in the rat spinal cord and brain. Exp Neurol 197:458–464
Srinivas M, Cruz LJ, Bonetto F, Heerschap A, Figdor CG, de Vries IJ (2010) Customizable, multi-functional fluorocarbon nanoparticles for quantitative in vivo imaging using 19F MRI and optical imaging. Biomaterials 31:7070–7077
Moser E, Stahlberg F, Ladd ME, Trattnig S (2012) 7-T MR–from research to clinical applications? NMR Biomed 25:695–716
Chen W, Takahashi A, Han E (2011) Quantitative T(1)(rho) imaging using phase cycling for B0 and B1 field inhomogeneity compensation. Magn Reson Imaging 29:608–619
Tannus A, Garwood M (1997) Adiabatic pulses. NMR Biomed 10:423–434
Watanabe H, Takaya N, Mitsumori F (2011) Non-uniformity correction of human brain imaging at high field by RF field mapping of B1+ and B1. J Magn Reson 212:426–430
Hockett FD, Wallace KD, Schmieder AH et al (2011) Simultaneous dual frequency 1H and 19F open coil imaging of arthritic rabbit knee at 3T. IEEE Trans Med Imaging 30:22–27
Wang C, Li Y, Wu B et al (2012) A practical multinuclear transceiver volume coil for in vivo MRI/MRS at 7 T. Magn Reson Imaging 30:78–84
Waiczies H, Lepore S, Drechsler S et al (2013) Visualizing brain inflammation with a shingled-leg radio-frequency head probe for (19)f/(1)h MRI. Sci Rep 3:1280
Hu L, Hockett FD, Chen J et al (2011) A generalized strategy for designing (19)F/(1)H dual-frequency MRI coil for small animal imaging at 4.7 Tesla. J Magn Reson Imaging 34:245–252
Roemer PB, Edelstein WA, Hayes CE, Souza SP, Mueller OM (1990) The NMR phased array. Magn Reson Med 16:192–225
McRobbie DW, Moore EA, Graves MJ, Prince MR (2007) MRI from picture to proton. Press, Cambridge University
Giraudeau C, Flament J, Marty B et al (2010) A new paradigm for high-sensitivity 19F magnetic resonance imaging of perfluorooctylbromide. Magn Reson Med 63:1119–1124
Chalmers KH, De Luca E, Hogg NH et al (2010) Design principles and theory of paramagnetic fluorine-labelled lanthanide complexes as probes for (19)F magnetic resonance: a proof-of-concept study. Chemistry (Weinheim an der Bergstrasse, Germany) 16:134–148
Chalmers KH, Kenwright AM, Parker D, Blamire AM (2011) 19F-lanthanide complexes with increased sensitivity for 19F-MRI: optimization of the MR acquisition. Magn Reson Med 66:931–936
Bonetto F, Srinivas M, Heerschap A et al (2011) A novel (19)F agent for detection and quantification of human dendritic cells using magnetic resonance imaging. Int J Cancer 129:365–373
Schmid F, Holtke C, Parker D, Faber C (2012) Boosting (19) F MRI-SNR efficient detection of paramagnetic contrast agents using ultrafast sequences. Magn Reson Med
Yildirim M, Keupp J, Lamerichs R (2007) Chemical shift independent imaging of 19F contrast agents using ultrafast MRSI (F-uTSI). Proc Int Soc Magn Reson Med 15:1249
Yildirim M, Díaz-López R, Nicolay K, Rolf L (2013) In vivo 3D spectroscopic imaging of 19F compounds using backprojection. Proc Int Soc Magn Reson Med 21:4009
Hertlein T, Sturm V, Kircher S et al (2011) Visualization of abscess formation in a murine thigh infection model of Staphylococcus aureus by 19F-magnetic resonance imaging (MRI). PLoS One 6:e18246
Srinivas M, Bonetto F, Tel J, et al (2012) Simultaneous and quantitative tracking of distinct cell populations using 19F MRI(ed)^(eds) ISMRM. Intl Soc Mag Reson Med, Melbourne, Australia pp 4370
Zhong J, Mills PH, Hitchens TK, Ahrens ET (2013) Accelerated fluorine-19 MRI cell tracking using compressed sensing. Magn Reson Med 69:1683–1690
Goette MJ, Keupp J, Rahmer J, Lanza GM, Wickline SA, Caruthers SD (2014) Balanced UTE-SSFP for F MR imaging of complex spectra. Magn Reson Med
Ahrens ET, Flores R, Xu H, Morel PA (2005) In vivo imaging platform for tracking immunotherapeutic cells. Nat Biotechnol 23:983–987
Srinivas M, Morel PA, Ernst LA, Laidlaw DH, Ahrens ET (2007) Fluorine-19 MRI for visualization and quantification of cell migration in a diabetes model. Magn Reson Med 58:725–734
Kok MB, de Vries A, Abdurrachim D et al (2011) Quantitative (1)H MRI, (19)F MRI, and (19)F MRS of cell-internalized perfluorocarbon paramagnetic nanoparticles. Contrast Media Mol Imaging 6:19–27
van Heeswijk RB, De Blois J, Kania G et al (2013) Selective in vivo visualization of immune-cell infiltration in a mouse model of autoimmune myocarditis by fluorine-19 cardiac magnetic resonance. Circulation 6:277–284
Klomp D, van Laarhoven H, Scheenen T, Kamm Y, Heerschap A (2007) Quantitative 19F MR spectroscopy at 3 T to detect heterogeneous capecitabine metabolism in human liver. NMR Biomed 20:485–492
Lee CP, Payne GS, Oregioni A et al (2009) A phase I study of the nitroimidazole hypoxia marker SR4554 using 19F magnetic resonance spectroscopy. Br J Cancer 101:1860–1868
Kadayakkara DK, Ranganathan S, Young WB, Ahrens ET (2012) Assaying macrophage activity in a murine model of inflammatory bowel disease using fluorine-19 MRI. Lab Investig J Tech Methods Pathol 92:636–645
Srinivas M, Turner MS, Janjic JM, Morel PA, Laidlaw DH, Ahrens ET (2009) In vivo cytometry of antigen-specific t cells using 19F MRI. Magn Reson Med 62:747–753
Ahrens ET, Young WB, Xu H, Pusateri LK (2011) Rapid quantification of inflammation in tissue samples using perfluorocarbon emulsion and fluorine-19 nuclear magnetic resonance. BioTechniques 50:229–234
Stoll G, Basse-Lusebrink T, Weise G, Jakob P (2012) Visualization of inflammation using (19) F-magnetic resonance imaging and perfluorocarbons. Wiley Interdiscip Rev 4:438–447
Temme S, Bonner F, Schrader J, Flogel U (2012) 19F magnetic resonance imaging of endogenous macrophages in inflammation. Wiley Interdiscip Rev 4:329–343
Weise G, Basse-Luesebrink TC, Wessig C, Jakob PM, Stoll G (2011) In vivo imaging of inflammation in the peripheral nervous system by (19)F MRI. Exp Neurol 229:494–501
Flogel U, Su S, Kreideweiss I et al (2011) Noninvasive detection of graft rejection by in vivo (19) F MRI in the early stage. Am J Transplant 11:235–244
Zarif L, Postel M, Trevino L, Riess JG, Valla A, Follana R (1994) Biodistribution and excretion of a mixed fluorocarbon-hydrocarbon “dowel” emulsion as determined by 19F NMR. Artif Cells Blood Substit Immobil Biotechnol 22:1193–1198
de Vries IJ, Lesterhuis WJ, Barentsz JO et al (2005) Magnetic resonance tracking of dendritic cells in melanoma patients for monitoring of cellular therapy. Nat Biotechnol 23:1407–1413
Tirotta I, Mastropietro A, Cordiglieri C et al (2014) A superfluorinated molecular probe for highly sensitive in vivo (19)F-MRI. J Am Chem Soc 136:8524–8527
Ahrens ET, Balducci A, Helfer B, et al (2014) First clinical experience using fluorine-19 MRI to track immunotherapeutic dendritic cells in colorectal cancer patients(ed)^(eds) ISMRM. Intl Soc Mag Reson Med, Milan, Italy, pp 0474
Acknowledgements
The scientific guarantor of this publication is Prof. Arend Heerschap. The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article. This work was financially supported by the European Union EU-FP7 ENCITE (HEALTH-F5-2008-201842) grant and Netherlands Institute for Regenerative Medicine (NIRM) FES0908. MS is supported by the Netherlands Organization for Scientific Research (NWO) VENI 700.10.409 and the European Research Council (ERC) ERC-2014-StG-336454-CoNQUeST and JdV by NWO-VIDI 917.76.363. Methodology: performed at one institution.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Amiri, H., Srinivas, M., Veltien, A. et al. Cell tracking using 19F magnetic resonance imaging: Technical aspects and challenges towards clinical applications. Eur Radiol 25, 726–735 (2015). https://doi.org/10.1007/s00330-014-3474-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-014-3474-5