Abstract
Objective
To assess the imaging features of primary hepatic angiosarcoma on multiphasic CT and MR.
Methods
Multi-institutional review identified 35 adults (mean age, 57.1 years; 22M/13F) with pathologically proven hepatic angiosarcoma and pretreatment multiphasic CT (n = 33) and/or MR (n = 7).
Results
Multifocal hepatic involvement was seen in all 35 cases, with at least 10 lesions in 74.3 % (26/35). Mean size of the dominant mass was 8.9 ± 4.7 cm (range, 2.6–20 cm). Individual nodules were typically circumscribed. Arterial-phase foci of hypervascular enhancement without washout were seen in 89.7 % (26/29). Heterogeneously expanding foci of enhancement generally followed blood pool in 88.6 % (31/35). Progressive centripetal (n = 16) or diffuse “flash-fill” (n = 4) enhancement pattern resembling cavernous haemangiomas predominated in 20 cases, whereas a “reverse haemangioma” centrifugal pattern predominated in 11 cases. Rapid interval growth was seen in 24 (96.0 %) of 25 cases with serial imaging. Vascular invasion was not seen in any case. Underlying cirrhotic morphology was seen in 42.3 % (15/35).
Conclusion
Primary hepatic angiosarcomas typically manifest as aggressive multifocal tumors containing small heterogeneous hypervascular foci that progressively expand and follow blood pool. The appearance can mimic cavernous haemangiomas, but distinction is generally possible. In the setting of cirrhosis, lack of tumour washout and vascular invasion argue against multifocal hepatocellular carcinoma.
Key Points
• Hepatic angiosarcoma manifests on CT and MR as rapidly progressive multifocal tumours
• Multiphasic imaging demonstrates hypervascular foci that progressively expand and follow blood pool
• Enhancement pattern can resemble cavernous haemangiomas or show a “reverse” centrifugal pattern
• Lack of tumour washout of hypervascular lesions argues against multifocal hepatocellular carcinoma
• Careful assessment of the cross-sectional imaging findings may suggest the diagnosis
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Primary hepatic angiosarcoma is a rare but aggressive malignancy, with the majority of patients dying less than a year after diagnosis [1–3]. Among the environmental exposures associated with this malignant mesenchymal tumour, Thorotrast (thorium dioxide), vinyl chloride, and arsenic are the most established [4]. However, most current and new diagnoses of hepatic angiosarcoma will not have a relevant associated exposure history. The association of hepatic angiosarcoma with cirrhosis, whether playing a direct causative role or not, has been under-recognized [3].
The cross-sectional imaging features of primary hepatic angiosarcoma are largely anecdotal, since the literature is restricted to either case reports or small case series [1, 2, 5–13]. The only imaging series to date with more than 10 patients was reported by Koyama et al. in 2002 [2], who described cross-sectional imaging features in 13 patients, including three cases with multiphasic CT or MR. Although some published reports have suggested that hepatic angiosarcoma may mimic the imaging appearance of benign cavernous haemangiomas [7, 9, 14], the small patient numbers preclude any firm conclusions or generalizations. Through multi-institutional review of the recent records at four comprehensive cancer centres, we accumulated a substantially larger cohort of biopsy-proven cases with multiphasic cross-sectional imaging. The purpose of this study was to evaluate the imaging features of primary hepatic angiosarcoma, with emphasis on the tumour enhancement pattern at multiphasic CT and MR.
Materials and methods
This retrospective study complied with the Health Insurance and Portability and Accountability Act and was approved by our respective institutional review boards; the need for informed consent was waived. Multi-institutional review at four comprehensive cancer centres over the period from 2002 to 2013 identified 35 adults with pathologically proven primary hepatic angiosarcoma imaged prior to any therapy with contrast-enhanced CT and/or MR. Mean patient age was 57.1 years, with a range of 31 to 87 years. There were 22 men and 13 women. Demographic data are provided in Table 1.
Contrast-enhanced multidetector-row CT studies were available for review in 33 patients and contrast-enhanced MR studies were available in seven patients; five patients had both CT and MR studies. Phases of imaging included pre-contrast (n = 26), arterial (n = 29), portal venous (n = 32), and delayed (n = 20). Given the multicentre nature of the cohort, which spanned over a decade, the specific CT and MR protocols varied considerably, but the phases of post-contrast imaging could be categorized, and emphasis was placed on the phase-specific tumour enhancement characteristics. For CT, all cases were obtained in single-energy mode with 120 kVp. For the MR studies, neither hepatobiliary-specific contrast agents nor diffusion-weighted imaging was employed. Serial examination of 25 patients allowed for assessment of interval change over time.
All CT and MR studies were retrospectively reviewed by three abdominal radiologists (P.J. Pickhardt, M.G. Lubner, and D. Kitchin, with 19, 10, and 6 years of cross-sectional imaging experience, respectively) for consensus findings. The number, size, location, morphology, and margin (e.g. well-circumscribed) of individual intrahepatic lesions were recorded. Location and size of the largest or dominant mass were noted. Mean CT attenuation numbers were recorded for representative lesions on the unenhanced series, if available, using a standard region of interest (ROI) method.
Lesion enhancement characteristics on the post-contrast series were studied in detail. The presence, degree, and pattern of hypervascular enhancement (i.e. tumoral enhancement on the late arterial phase) were assessed, as were the portal venous and delayed phases. Particular attention was given as to whether lesional enhancement generally followed aortic blood pool attenuation (CT) and signal intensity (MR), or if tumour washout occurred beyond the arterial phase. For cases where progressive tumour enhancement followed blood pool on multiphasic imaging, further categorization was made according to the presence of a “haemangioma-like” pattern (i.e. peripheral enhancement with centripetal progression or complete flash-fill) or a “reverse haemangioma” pattern (i.e. central enhancement with centrifugal progression).
Other CT and MR features evaluated included the presence or absence of vascular invasion, T2 signal intensity characteristics, morphologic changes of underlying cirrhosis, and evidence of extrahepatic metastatic disease. Additional imaging studies such as ultrasound, catheter-based angiography, and PET were available in only a minority of cases and were not the focus of this study. The clinical records of patients were reviewed with regard to clinical presentation, relevant environmental exposure history, and survival after diagnosis.
Results
Patient-specific imaging findings are shown in Table 1. Multifocal intrahepatic tumours were identified in all 35 patients (Figs. 1, 2, 3, 4, 5, 6, and 7), with 10 or more lesions in 74.3 % (26 of 35 cases). Both the left and right hepatic lobes were involved in 91.4 % (32 of 35 cases), with lesions only identified in the left lobe in the remaining three cases. The mean size (±SD) of the dominant hepatic mass was 8.9 ± 4.7 cm, with a range of 2.6 to 20 cm. The dominant mass was located in the right hepatic lobe in 22 cases, the left hepatic lobe in 10 cases, and straddled the two lobes in three cases. There was often a wide intra-patient range in individual lesion size, with associated multifocal tumour nodules measuring 2 cm or smaller in all but three cases. The tumours were typically well circumscribed. Mean non-contrast CT attenuation of representative tumour nodules was 36.0 HU, with a relatively narrow range of 30–45 HU, generally reflecting blood pool attenuation. Some lesions appeared heterogeneous on unenhanced CT, with areas of mixed attenuation suggesting intra-tumoral haemorrhage.
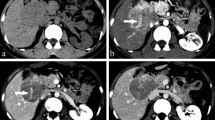
Primary hepatic angiosarcoma with rapid progression in a 54-year-old woman. Fat-suppressed T1-weighted post-contrast images in the arterial (a), portal venous (b), and more delayed (c) phases show a dominant mass in the right hepatic lobe that demonstrates irregular internal enhancement with centrifugal progression that followed blood pool (arrows, “reverse haemangioma” sign). Over 20 additional smaller tumours were present, widely varying in size and rate of enhancement on the different phases. Both centrifugal and centripetal progression patterns matching blood pool were present, but the former predominated. Arterial (d) and portal venous (e) phase contrast-enhanced CT study performed 2 months later shows marked enlargement of dominant mass with similar enhancement pattern as before. The additional lesions show minimal enhancement on arterial phase but fill in on portal venous phase. f The hepatic masses are hyperechoic on ultrasound
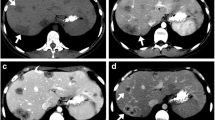
Primary hepatic angiosarcoma in a 71-year-old woman. Contrast-enhanced CT with arterial (a) and portal venous (b) phases in liver windows shows a dominant 18-cm right hepatic mass that demonstrates areas of central (arrows) and peripheral (arrowheads) irregular enhancement that follow blood pool and progresses in both centripetal and centrifugal directions. Over 30 additional hepatic lesions were present. Note also diffuse splenic metastatic disease, which is relatively hypovascular in this case
Hepatic angiosarcoma mimicking flash-filling cavernous haemangioma in a 78-year-old woman. Contrast-enhanced CT with arterial (a) and portal venous (b) phases shows a 3-cm lesion with homogeneous hypervascular enhancement that matches blood pool on portal venous phase, closely mimicking a flash-filling cavernous haemangioma. Additional smaller lesions with a similar enhancement pattern were present. Ultrasound-guided core biopsy established the diagnosis. c Image through the right lower quadrant from the initial CT show a small subcentimetre mesenteric nodule (arrow), which progressively enlarged on CT scans performed 6 months (d) and 2 years (e) later. Note heterogeneous enhancement of metastasis on e
Primary hepatic angiosarcoma with underlying cirrhosis in a 63-year-old man. Fat-suppressed T1-weighted post-contrast images in the arterial (a) and portal venous (b) phases show a hypervascular lesion (arrows) in the right hepatic lobe, which does not wash out on the portal venous phase but rather follows blood pool, arguing against HCC. Over 10 additional lesions were present. Lesions were hyperintense on T2-weighted imaging (c). The lesions enlarged on subsequent contrast-enhanced CT performed 2 months later (d). Note cirrhotic liver morphology
Hepatic angiosarcoma with hypovascular appearance in a 42-year-old woman. Contrast-enhanced CT (a) shows a dominant hypovascular 10-cm mass. Innumerable (>100) smaller nodules present were also predominately hypovascular. This pattern prevailed in only four cases (11 %). The dominant hypovascular lesion was still hypermetabolic at 18F-FDG PET (b)
Thorotrast-induced hepatic angiosarcoma in a 64-year-old woman 50 years after exposure. Contrast-enhanced arterial (a) and portal venous (b) phase CT shows multiple low attenuation lesions with peripheral nodular and curvilinear enhancement on the arterial phase with slight progression that follows blood pool on portal venous phase. Note the markedly dense, diminutive spleen and dense lymph nodes related to the remote Thorotrast exposure
Hepatic angiosarcoma in a 52-year-old man with pericardial metastasis. Contrast-enhanced CT through the thorax (a) and upper abdomen (b) shows a predominately low attenuation pericardial mass (arrowheads) that demonstrates peripheral nodular enhancement and an enhancing dominant hepatic mass, both of which progressively enhanced and followed blood on all phases (only portal venous phase shown). Another patient in this series (not shown) presented with haemopericardium from pericardial metastatic disease
On the late arterial phase, heterogeneous foci of hypervascular enhancement were seen somewhere within at least one tumour nodule in 89.7 % (26 of 29 cases), typically including the dominant mass. The regions of hypervascular enhancement usually accounted for a relatively small fraction of the total tumour volume. Regions of hypervascular enhancement varied widely in morphology, including multinodular, arciform, rim-like, bizarre branching, and diffuse patterns (Figs. 1, 3, 4, and 6). In many cases with hypervascular tumours, there were additional hepatic nodules that did not demonstrate arterial-phase enhancement, suggesting intra-patient tumour heterogeneity. In three cases, no appreciable hypervascular component was identified on the arterial phase (Table 1).
On the portal venous phase and more delayed post-contrast series, heterogeneous foci of enhancement that initiated on the arterial phase continued to slowly expand and generally followed blood attenuation or signal intensity in 88.6 % (31 of 35) (Figs. 1, 2, 3, 4, 6, and 7). In the remaining four cases (11.4 %), tumour nodules remained hypovascular on all post-contrast series (Fig. 5). There were no instances of delayed washout in areas of hypervascular enhancement, which was a distinguishing feature from multifocal hepatocellular carcinoma (HCC) in the setting of cirrhosis (Figs. 2 and 4). However, even in cases with an obvious enhancing component, many tumour nodules exhibited large hypovascular regions, or were completely hypovascular (Figs. 1, 5, and 6). Of the 31 cases where tumour enhancement generally followed blood pool, 20 cases showed lesions that mostly resembled cavernous haemangiomas to varying degrees, with either progressive centripetal fill-in from the periphery (n = 16) or a diffuse “flash-fill” (n = 4) enhancement pattern (Figs. 1, 2, 3, 4, and 7). In the other 11 cases, a “reverse haemangioma” centrifugal pattern predominated, where central enhancing foci expanded towards the periphery of the lesion (Figs.). In 14 of these 31 cases following blood pool, there was a mix of centripetal and centrifugal patterns, with one pattern predominating according to the split described above (Figs. 1 and 2). Results are also summarized in Table 1.
Portal or hepatic venous vascular invasion by tumour was not seen in any case. In some cases the hepatic vessels were narrowed or encased by large tumours. Morphologic changes of underlying cirrhosis were present in 42.3 % (15 of 35 cases), which was often not clinically suspected or previously known. In 24 (96.0 %) of the 25 cases with serial cross-sectional imaging after diagnosis, rapid interval tumour growth was observed (Figs. 1, 3, and 4). Extrahepatic metastatic disease was evident on the initial cross-section imaging study in 45.7 % (16 of 35 cases). Splenic metastatic disease was most frequent (n = 6), followed by peritoneal (n = 5), pulmonary (n = 4), and pericardial (n = 2) metastases (Figs. 2, 3, and 7). Ultrasound imaging available for review in 13 patients demonstrated a predominately hyperechoic lesion echotexture, typical of tumours of vascular origin (Fig. 1). Tumours were generally hyperintense relative to liver on T2-weighted MR imaging.
On medical record review, only four patients had a potentially relevant positive environmental exposure history. In one case, a 64-year-old woman with a history of Thorotrast exposure in 1956 presented 50 years later with end-stage liver disease and angiosarcoma (Fig. 6). Two patients had undergone abdominal external beam radiation therapy in the past for unrelated tumours. A fourth patient was HIV-positive. Two patients presented with haemoperitoneum related to capsular rupture from tumour penetration subcapsular haematoma and adjacent mesenteric masses were seen in a third patient. Another patient presented with haemopericardium from pericardial metastatic disease. The majority of patients died within a year of diagnosis. Excluding the two patients lost to follow-up, all patients died of their disease (Table 1), most often within 3 years from the time of initial diagnosis.
Discussion
Hepatic angiosarcoma is a rare tumour but nonetheless represents the most common primary mesenchymal malignancy of the liver [1–3]. The clinical behaviour of this tumour is highly aggressive, with a reported median survival of less than 6 months from the time of diagnosis. Previous exposures to carcinogens such as Thorotrast (thorium dioxide suspension), vinyl chloride, and arsenic have been widely recognized as risk factors [4], but such a history was present in only one patient in our cohort. However, a much more common association that is often overlooked is hepatic fibrosis and cirrhosis, which is reportedly present in 40 % of biopsy specimens at the time of diagnosis [3], closely matching our observation of cirrhotic morphology in 42 % of cases.
Up to now, knowledge regarding the imaging characteristics of primary hepatic angiosarcoma has been limited to case reports and small patient series [1, 2, 5–13]. Previously, the largest case series by Koyama et al. [2] with a total of 13 patients included only three cases with multiphasic CT or MR. Through a combined search of the recent records at four comprehensive cancer centres, we identified 35 patients with biopsy-proven hepatic angiosarcoma, all of whom underwent pretreatment imaging with contrast-enhanced CT and/or MR. All but three of these 35 cases included multiple phases of imaging, allowing for detailed assessment of the temporal enhancement pattern. Although many of our imaging observations have been suggested by prior reports, the substantially larger size of our study cohort allows for better pattern recognition and firmer conclusions.
Common imaging features of primary hepatic angiosarcomas from our series include multiple well-circumscribed nodules or masses that demonstrate a wide range of size and generally involve both hepatic lobes. Tumour enhancement is characterized by small heterogeneous hypervascular foci on the late arterial phase that progressively expand on the portal venous phase and more delayed phases, and typically follow blood pool in terms of CT attenuation or MR T1 signal intensity. Large hypovascular regions are common, and many cases also have smaller hypovascular nodules in addition to enhancing dominant lesions. The wide array of contrast enhancement patterns among lesions suggests substantial intra-patient tumour heterogeneity.
The predilection for expanding areas of tumour enhancement that follow blood pool has drawn parallels to cavernous haemangiomas [7, 9, 14], a very common benign hepatic tumour of vascular origin. Indeed, several of the cases in our series were referred from outside hospitals with a history “rapidly expanding cavernous haemangiomas”. However, the precise enhancement pattern seen with hepatic angiosarcomas tends to be much more bizarre and disordered, even when centripetal progression from the tumour periphery is seen. A number of cases demonstrated centrifugal enhancement, which we refer to as the “reverse haemangioma” pattern, or showed a complex mixture of progressive centripetal and centrifugal enhancement. With careful inspection of the enhancement pattern, distinction from benign haemangiomas is usually possible, and has allowed for prospective suspicion of the correct diagnosis in at least some cases in our series. A minority of cases demonstrated complete flash-filling on the arterial phase, with blood pool matching on later phases, which more closely mimicked benign haemangiomas. In the series by Koyama et al. [2], the three cases with multiphase contrast-enhanced CT or MR images showed heterogeneous and progressive enhancement in the dominant lesions. A more recent series by Bruegel et al. [1] consisting of seven patients with multiphasic CT and/or MR described progressive heterogeneous enhancement in the majority. Other case reports and small series have also mentioned progressive enhancement, but with variable discussion as to whether the finding mimics haemangiomas [7, 9] or not [8, 11].
The histopathologic features of hepatic angiosarcoma help to explain the imaging parallels with cavernous haemangiomas [1]. These tumours are composed of poorly organized vessels or cavitary spaces that are lined by malignant endothelial cells, which can also infiltrate or form solid nodules [15]. The variegated enhancement patterns reflect this tumoral heterogeneity, including vascular channels that briskly enhance, and more cavernous spaces that progressively enhance reminiscent of a haemangioma [2].
In the setting of cirrhosis, which appears to be highly associated with this rare malignancy, the more relevant considerations in the differential diagnosis might include multifocal HCC, intrahepatic cholangiocarcinoma, and mixed HCC–cholangiocarcinoma. The lack of tumour washout of hypervascular foci on later phases and the absence of vascular invasion would argue against HCC, but the imaging features may overlap more with cholangiocarcinoma and combined tumours. In the absence of cirrhosis, other considerations in the differential diagnosis should include peliosis hepatis and metastatic disease. There is substantial overlap in the enhancement characteristics of hepatic peliosis and angiosarcoma [16], although the latter will typically be more heterogeneous in nature. Hypervascular metastatic disease could be considered as a possibility in some of the typical cases of angiosarcoma. For the minority of cases showing only hypovascular hepatic masses or nodules (n = 4), the appearance is even more suggestive of metastatic disease (e.g. from colorectal cancer). However, these metastatic lesions may show other features such as flocculated calcification, which were not seen in this series of hepatic angiosarcomas.
Extrahepatic metastatic disease may be seen in up to 60 % of patients at presentation, and was apparent at cross-sectional imaging in 46 % of our series [1–3, 12]. The spleen is a common site of tumour spread and was reported in six of the 13 patients in the series by Koyama et al. [2]. Likewise, hepatic metastases are relatively common in primary splenic angiosarcoma [17, 18]. Given that the spleen is an unusual site for metastatic disease in general, its presence further supports the diagnosis of angiosarcoma. Mesenteric spread was also fairly common in our series but has not been widely appreciated in the medical literature. Intraperitoneal spread made be hastened by capsular rupture and peritoneal haemorrhage, for which hepatic angiosarcoma is at increased risk [19].
We acknowledge several limitations to our study. This was a retrospective review, which has inherent shortcomings but was unavoidable as the rare incidence of this aggressive tumour precludes prospective patient accrual. Given the multicentre nature of this study, as well as the prolonged time period, the specific CT and MR protocols varied considerably. Nonetheless, common sequences of imaging allowed for grouping into the various recognized post-contrast phases. Furthermore, by combining the experience of four large comprehensive cancer centres, we were able to amass a much larger imaging series than previously reported. Finally, the histopathology of these tumours was not specifically re-reviewed for the purpose of this study, but the diagnosis was not in doubt in any of the cases.
In summary, primary hepatic angiosarcoma typically manifests as multifocal masses with small heterogeneous foci of hypervascular enhancement and progressive dynamic enhancement on later phases that tends to follow blood pool. As such, the imaging features may superficially resemble cavernous haemangiomas in some cases. However, careful analysis of the imaging features will generally avoid a benign misdiagnosis. In the setting of underlying cirrhosis, which is often clinically unsuspected, the lack of delayed washout of hypervascular lesions and absence of vascular invasion argue against multifocal HCC.
References
Bruegel M, Muenzel D, Waldt S, Specht K, Rummeny EJ (2013) Hepatic angiosarcoma: cross-sectional imaging findings in seven patients with emphasis on dynamic contrast-enhanced and diffusion-weighted MRI. Abdom Imaging 38:745–754
Koyama T, Fletcher JG, Johnson CD, Kuo MS, Notohara K, Burgart LJ (2002) Primary hepatic angiosarcoma: findings at CT and MR imaging. Radiology 222:667–673
Locker GY, Doroshow JH, Zwelling LA, Chabner BA (1979) Clinical features of hepatic angiosarcoma - report of 4 cases and a review of the English literature. Medicine 58:48–64
Popper H, Thomas LB, Telles NC, Falk H, Selikoff IJ (1978) Development of hepatic angiosarcoma in man induced by vinly chloride, thorotrast, and arsenic - comparison with cases of unknown etiology. Am J Pathol 92:349–376
Chiu O, Frank JD, Dow CA (2005) Hepatic angiosarcoma: detection with computed tomography. Australas Radiol 49:163–165
Heo SH, Jeong YY, Shin SS, Chung TW, Kang HK (2007) Solitary small hepatic angiosarcoma: initial and follow-up imaging findings. Korean J Radiol 8:180–183
Itai Y, Teraoka T (1989) Angiosarcoma of the liver mimicking cavernous hemangioma on dynamic CT. J Comput Assist Tomogr 13:910–912
Ohmoto K, Hirokawa M, Takesue M, Yamamoto S (2000) Hepatic angiosarcoma with early central enhancement and arterioportal shunt on dynamic CT. Hepatogastroenterology 47:1717–1718
Okano A, Sonoyama H, Masano Y et al (2012) The natural history of a hepatic angiosarcoma that was difficult to differentiate from cavernous hemangioma. Intern Med 51:2899–2904
Park YS, Kim JH, Kim KW et al (2009) Primary hepatic angiosarcoma: imaging findings and palliative treatment with transcatheter arterial chemoembolization or embolization. Clin Radiol 64:779–785
Peterson M, Baron RL, Rankin SC (2000) Hepatic angiosarcoma: findings on multiphasic contrast-enhanced helical CT do not mimic hepatic hemangioma. Am J Roentgenol 175:165–170
Rademaker J, Widjaja A, Galanski M (2000) Hepatic hemangiosarcoma: imaging findings and differential diagnosis. Eur Radiol 10:129–133
Yu RS, Zhang SZ, Hua JM (2003) Hepatic angiosarcoma: CT findings. Chin Med J 116:318–320
Ginsberg F, Slavin JD, Spencer RP (1986) Hepatic angiosarcoma - mimicking of angioma on 3-phase Tc-99 m red-blood-cell scintigraphy. J Nucl Med 27:1861–1863
Buetow PC, Buck JL, Ros PR, Goodman ZD (1994) Malignant vascular tumors of the liver: radiologic-pathological correlation. Radiographics 14:153–166
Iannaccone R, Federle MP, Brancatelli G et al (2006) Peliosis hepatis: spectrum of imaging findings. AJR Am J Roentgenol 187:W43–W52
Falk S, Krishnan J, Meis JM (1993) Primary angiosarcoma of the spleen - a clinicopathological study of 40 cases. Am J Surg Pathol 17:959–970
Thompson WM, Levy AD, Aguilera NS, Gorospe L, Abbott RM (2005) Angiosarcoma of the spleen: imaging characteristics in 12 patients. Radiology 235:106–115
Mahony B, Jeffrey RB, Federle MP (1982) Spontaneous rupture of hepatic and splenic angiosarcoma demonstrated by CT. Am J Roentgenol 138:965–966
Acknowledgements
The scientific guarantor of this publication is Perry J. Pickhardt, MD. The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article. The authors state that this work has not received any funding. No complex statistical methods were necessary for this paper. Institutional review board approval was obtained. Written informed consent was waived by the institutional review board. Methodology: retrospective, observational, multicentre study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pickhardt, P.J., Kitchin, D., Lubner, M.G. et al. Primary hepatic angiosarcoma: multi-institutional comprehensive cancer centre review of multiphasic CT and MR imaging in 35 patients. Eur Radiol 25, 315–322 (2015). https://doi.org/10.1007/s00330-014-3442-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-014-3442-0