Abstract
Purpose
Our aim was to assess the diagnostic performance in determining strangulation in small bowel obstruction (SBO) for five CT findings commonly considered in published small bowel obstruction (SBO) management guidelines.
Materials and methods
Medical databases were searched for “bowel obstruction”, “computed tomography”, “strangulation”, and related terms. Two reviewers independently selected articles for CT findings investigated with surgical or histological reference standards for strangulation. Bivariate random-effects meta-analytical methods were used.
Results
A total of 768 patients, including 205 with strangulation from nine studies, were evaluated. The reduced bowel wall enhancement CT sign had the highest specificity (95 %, CI 75–99), with a positive LR of 11.07 (2.27–53.88) and DOR of 22.86 (4.99–104.61). The mesenteric fluid sign had the highest sensitivity (89 %, CI 75–96) with a negative LR of 0.16 (0.07–0.39) and a DOR of 13.9 (5.73–33.75). The bowel wall thickness had a sensitivity of 48 % (CI 41–54), a specificity of 83 % (CI 74–89), a positive LR of 2.84 (1.83–4.41) and a negative LR of 0.62 (0.53–0.72). The other CT findings had lower diagnostic performance.
Conclusion
Two CT findings should be used in clinical practice: reduced enhanced bowel wall is highly predictive of ischemia, and absence of mesenteric fluid is a reliable finding to rule out strangulation.
Key Points
• Reduced bowel wall enhancement on CT increases the probability of strangulation 11-fold.
• Absence of mesenteric fluid on CT decreases the probability of strangulation 6-fold.
• The clinical reliability of other CT signs is doubtful for predicting strangulation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Over the past 20 years, small bowel obstruction (SBO) management has shifted towards more non-operative treatment in the absence of strangulation signs [1, 2]. Strangulating small bowel obstruction, defined as a small bowel obstruction associated with intestinal ischemia, is estimated to account for about 10 % of all cases of SBO (range 5–42 %), with a mortality rate ranging from 20 to 40 % [3, 4]. Consequently, preoperative diagnosis of strangulation is pivotal. However, past research has failed to identify clinical factors that might enable physicians to reliably predict which patients could safely undergo conservative care by even the most experienced surgeons [3–5]. By contrast, consensus now exists in favour of recommending the use of multidetector computed tomography (CT) in the evaluation of patients with SBO. This technique can provide incremental clinically relevant information as evidence of ischemia warranting a change in management [2]. In most studies, the sensitivity of CT for diagnosing bowel-wall ischemia in patients with SBO, range from 73 % to 100 %, with the specificity ranging from 61 % to 100 % [6–11] . Indeed, numerous CT signs such as reduced or absent enhancement of bowel wall on contrast-enhanced images, mural thickening, unusual course of the mesenteric vasculature, mesenteric vascular engorgement, diffuse mesenteric haziness, a large amount of ascite, presence or absence of faeces sign, parietal pneumatosis, bowel-wall oedema (target sign) and increased bowel wall attenuation on unenhanced images have been reported as findings related to bowel strangulation [6, 12–16]. However, the positive indicators of ischemia in these studies are based on a heterogeneous number and combination of CT findings, i.e., one [6, 10], two or more [14, 15] CT signs, different combinations [7, 8] or even subjective patterns [16, 17], with no standardization and generally small sample sizes. Although the diagnostic performance of these CT findings has been appreciated to different extents in the literature, no meta-analysis has been conducted to date, and recently published surgeons’ guidelines based on systematic reviews of the literature [1, 2] recommend that strangulation detection should be based on five suggestive CT findings (mesenteric fluid, mesenteric venous congestion, free peritoneal fluid, reduced bowel wall enhancement and wall thickening). However, it is not yet clear exactly how to consider CT as predictive of strangulation and how to use these different findings to diagnose or consider unlikely strangulation in patients with SBO.
The aim of our study was thus to assess the diagnostic performance of these five reported CT findings of ischemia in SBO, while compiling the literature data and performing a meta-analysis. We hypothesized that these CT findings had different predictive values for diagnosing strangulation in SBO, and therefore, that only those having the best diagnostic accuracy should be evaluated in future studies in order to come up with a more uniform index to assess the real impact of CT for strangulation diagnosis.
Materials and methods
This meta-analytic review was compiled with reference to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement [18].
Search strategy
The PubMed search engine, Embase, Web of Science and Cochrane electronic databases were searched for “bowel obstruction”, “computed tomography”, “strangulation”, and related terms in articles published between January 1990 and December 2013, in English, French, Italian, German and Spanish languages. The MeSH terms and keywords used for the search are listed in Table 1.
Study selection
Two reviewers (I.M and A.R) independently selected eligible primary studies, screening titles and abstracts, with disagreements resolved by consensus. The inclusion criteria were as follows: (a) original research; (b) consecutively enrolled patients with clinical symptoms of SBO proved by CT or surgery, regardless of the presence of strangulation and of type of management (surgical or conservative); (c) CT as diagnostic index test; (d) a reference standard: surgery for patients with a diagnosis of ischemia, with a precise definition in the materials and methods section (i.e., describing how the ischemia diagnosis was made by the surgeons: discoloration, reversal of discoloration after soaking the involved loop in normal saline, and/or resection for ischemia confirmed by pathologic findings), or uneventful clinical outcome for those without ischemia; (e) at least one of the following CT findings was screened: reduced or absent bowel wall enhancement, mesenteric congestion (defined as enlargement of small mesenteric veins around the obstruction site), mesenteric fluid (defined as hazy fluid attenuation in the mesentery of the involved intestinal segment), mural thickening, peritoneal fluid regardless of its amount; (f) sufficient data were available to construct 2x2 contingency tables for at least one of the CT signs investigated (i.e., cross tabulation displaying the frequency distribution of strangulated and non-strangulated cases according to the presence or not of each CT sign). Studies with a sample size of less than ten patients were excluded. Reference lists of the retrieved studies were checked manually in order to identify additional relevant studies.
Data extraction and quality assessment
The same two co-authors independently reviewed each selected article for data extraction, and discrepancies were resolved by consensus. The following data were extracted: (a) study design and patient characteristics (i.e. first author, year of publication, country of origin, single or multicenter study, department of the first author, consecutive recruitment, number of patients, age, sex ratio, inclusion criteria); (b) imaging techniques (i.e., CT type, collimation, section width, use of oral contrast agent, use of intravenous contrast agent and its volume, type and outflow); (c) image evaluation (i.e., number of readers, retrospective or prospective CT reading, consensus reading, precise description about the CT sign studied); (d) reference standard (i.e., time between admission and surgery, histopathologic analysis, surgery findings, and duration of the medical follow-up).
From the group of patients included in the study, complete 2x2 contingency tables were extracted or reconstructed from the available raw data from each report and for each CT sign.
The methodological quality and potential sources of bias of the individual studies were assessed using the seven standard items from the second edition of the Quality Assessment of Diagnostic Accuracy Studies (QUADAS 2) [19]. The QUADAS 2 toll consisted of four key domains that discuss patient selection, index test, reference standard and flow of patients through the study and timing of the index tests and reference standard (flow and timing). Each domain was assessed in terms of the risk of bias, and the first three domains were also assessed in terms of concerns about applicability. Each question was assigned a response of  ,
,  , or
, or  , respectively for yes, unclear, or no, as it was recommended [19].
, respectively for yes, unclear, or no, as it was recommended [19].
Data synthesis and statistical analysis
Study level analysis and meta-analytic model
The diagnostic performance of each CT finding (i.e., sensitivity, specificity and corresponding 95 % confidence intervals) was recalculated for each primary study from these data.
To each CT finding, we applied a bivariate random-effect model developed for meta-analysis of diagnostic accuracy studies, as recommended by the Cochrane collaboration [20–22], in which the logit-transforms of the true sensitivity and true specificity in each study are assumed to have a bivariate normal distribution across studies, thereby allowing for the possibility of correlations between them. We used the NLMIXED procedure in the SAS statistical software package to calculate summary sensitivity and specificity values based on the inverse logit transform of the estimated model parameters, while assuming their estimates have a normal distribution. Corresponding positive and negative likelihood ratios (LR), diagnostic log odds ratios (DOR) and their corresponding 95 % confidence intervals were derived as functions of these summary estimates. The following interpretations could be applied to positive LR and negative LR: positive LR greater than 10 and negative LR of less than 0.1 implied large changes; positive LR of 5–10 and negative LR of 0.1–0.2 implied moderate changes; positive LR of 2–5 and negative LR of 0.2–0.5 implied small changes; positive LR of less than 2 and negative LR greater than 0.5 implied tiny changes; and LRs of 1 implied no changes [23].
We delimited the 95 % confidence ellipse around the mean estimate of sensitivity and specificity of each CT finding in a ROC graph. These ellipses clearly showed, by their visual power, differences in diagnostic performance between each CT finding according to whether or not they overlapped.
All statistical analyses were performed with two statistical software packages (SAS, version 9.3, SAS Institute Cary, NC; and R, version 3.0.2, R Foundation for Statistical Computing).
Assessment of heterogeneity
Heterogeneity (between-study variation) of the results between studies was assessed graphically using Forest plots of sensitivity and specificity for each CT sign, and was statistically quantified overall for each CT finding with the squared inconsistency index (I2) test statistic, including 95 % CIs. The I2 was calculated as follows: I2 = 100x[(Q-df)/Q], where Q is the Cochran heterogeneity statistic and df is the degree of freedom [24]. The I2 statistic expresses the percentage of total variation across studies caused by heterogeneity rather than chance. A higher percentage indicates more heterogeneity [24–26].
Publication bias
Publication bias was visually assessed for each CT sign using a scatterplot of the inverse of the square root of the effective sample size (ESS) versus the diagnostic log odds ratios (lnDOR), which should have a symmetric funnel shape when publication bias is absent [27]. Publication bias was formally tested using a regression of ln-DOR against 1/ESS½ and weighted according to the ESS, with p < 0.10 indicating significant asymmetry.
Results
Search strategy and study selection
Figure 1 shows the study selection process in a flow chart. The initial search yielded 330 studies with 56 duplicate titles, resulting in 274 titles and abstracts that were screened for eligibility. For 46 studies, the full text was retrieved. Most of these eligible studies (54 %, 19/35) were excluded as it was impossible to construct 2x2 contingency tables for any of the CT signs studied—these studies exclusively assessed the global performance of CT for diagnosing ischemia in SBO. Finally, nine studies fulfilled the inclusion criteria, for the evaluation of 768 patients overall [5, 6, 12, 13, 28–32].
Included studies
All included studies had been conducted in single centre, except one [28] that was conducted at two institutions. They were initiated by the Radiology (n = 5) or Surgery (n = 4) departments.
The number of patients included in each study ranged from 19 [12] to 233 [31]. All patients had presented with initial symptoms of SBO confirmed by CT (with visualisation of dilated and non dilated small bowel) or surgery. Six of the nine studies (for a total of 351 patients, 45.7 %) had included only patients with surgically proven SBO. The causes of SBO varied between studies, especially as some studies included only specific causes of SBO that constituted a selection criterion for inclusion. The age of included patients ranged from 13 to 100 years old (average 65.2 years). Further details on studies and patient characteristics are summarized in Table 2A.
The CT was single-detector in one study, multi-detectors in four, unknown in four (with CT manufacturers specified in two studies and not specified in the two others) (Table 2B). Oral contrast material was used in four studies, including 158/273 patients (57.8 %), while it was not used in two French studies and this data was not available in three studies. Intravenous contrast material was administered in 677/760 patients (89 %) with an acquisition at a venous phase. This data was not available for eight patients in the Zielinski et al. study [32], because the CTs for these patients had been performed outside the institution and the authors did not report the CT technique used, but all were deemed to be of acceptable quality. Details on the type and volume of the intravenous contrast agent administered are given in Table 2B. The CT readings were always retrospective and the number of the readers varied between studies: two studies had only one reader, five studies had two readers with consensus, and two studies had two readers and another one for consensus. Further details on the CT technique and characteristics are summarized in Table 2B.
Five hundred and ninety-three patients (593/768; 77.2 %) were operated and 205 patients (205/768; 26.7 %) showed ischemia at surgery examination, 184 (184/205; 89.7 %) of which underwent intestinal resection for histologically proven transmural ischemia. The prevalence of ischemia varied between studies, ranging from 11 % [32] to 84.2 % [12]. Time intervals were reported between admission and surgery (in three studies) or between CT and surgery (in five studies) and was not reported in one study [30]. The majority of patients had undergone surgery within 48 h (Table 2A). The mortality rate, reported in four studies [5, 29, 30, 32], ranged from 2 % to 9 %. In the three studies that included “control” cases that did not involve surgery but improvement obtained by non-operative management, the clinical follow-up ranged from 1 day to 77 days, with a global mean of around 5–6 days.
Methodological quality of included studies
The quality assessment results for the individual studies are presented in Table 3. Two studies fulfilled almost all of the methodological criteria [6, 31] and were recently published (2010 and 2013). Sixty-six percent of the studies had an inclusion selection criterion that could have introduced bias in the results, as the selection was based on specific or non-exhaustive causes of SBO. There was a risk of bias with the index test in 2/9 studies (22.2 %), in which there was only one reader and depended on the reader’s expertise, so the test performance could have been over or underestimated. For most of the studies (8/9; 88.8 %), it was unclear if the interval between the index test and reference standard introduced bias. Only one study [31] had ideal short timing between admission and surgery, < 24 h in all cases.
Diagnostic performance of CT signs
Each screened CT finding was not assessed in all studies: peritoneal fluid and wall thickening were evaluated in the nine reports, reduced bowel wall findings and mesenteric venous congestion in seven reports, and mesenteric fluid in five reports. Bowel wall thickening was considered positive if it was greater than 5 mm in 2/9 studies [28, 30], 3 mm in 4/9 [5, 13, 29, 31], 2 mm in 1/9 [6], and positive threshold for thickness was not mentioned in 2/9 [12, 32]. The study-specific sensitivity and specificity for each CT sign are presented on the Forest plot in appendix E1 (online). Overall sensitivity, specificity, positive likelihood ratio, negative likelihood ratio, and diagnostic odds ratio values for the diagnostic performance of each CT finding in the nine eligible studies are given in Table 4. The highest specificity (95 %, CI 75–99 %) was achieved with the reduced bowel wall enhancement sign, with a DOR of 22.86 (4.99–104.61), and a high and conclusive positive LR (11.07, CI 2.27–53.88). The highest sensitivity was achieved with the mesenteric fluid sign (89 %, CI 75–96) with a DOR of 13.9 (5.73–33.75), and a negative LR of 0.16 (0.07–0.39). The other CT findings (peritoneal fluid, mesenteric congestion and wall thickening) had lower but equivalent DOR, respectively 3.54 (2.23–5.59), 3.21 (1.39–7.4) and 4.55 (2.6–7.96), with no informative or useful likelihood ratio ranging from 1.61 to 2.84 for positive LR and from 0.50 to 0.62 for negative LR.
The ROC graph (Fig. 2) highlighted two subgroups: 1) sensitive signs (mesenteric fluid, mesenteric congestion and peritoneal fluid) that were mesentery abnormalities, and 2) specific signs (wall thickening and reduced bowel wall) that were intestinal abnormalities.
Heterogeneity
The between-study heterogeneity was low, with no significance for the mesenteric fluid analysis (I2 = 7.2 %, p = 0.36), for the peritoneal fluid analysis (I2 = 0 %, p = 0.82), and for the bowel wall thickening analysis (I2 = 9.6 %, p = 0.35). It was moderate and significant for the reduced bowel wall enhancement analysis (I2 = 49.9 %, p = 0.06) and for the mesenteric congestion analysis (I2 = 57.4 %, p = 0.02).
Publication bias
No significant publication bias was noted, with p values ranging from 0.24 to 0.83 for regression tests of funnel plot asymmetry for each CT finding. However, an examination of each funnel plot (Appendix Z online) revealed that only half of the studies in each subgroup of CT signs were globally symmetrical, with the others being distributed randomly outside the axes of symmetry.
Discussion
Our meta-analysis results demonstrated that current CT findings reported for the diagnosis of ischemia complicating SBO had heterogeneous diagnostic performance. Two CT signs had a useful diagnostic value: reduced bowel wall enhancement was the most specific, with an excellent positive LR, and the mesenteric fluid sign was the most sensitive, with a good negative LR. We showed that CT mesentery abnormalities were quite sensitive signs to detect ischemia, whereas bowel wall abnormalities were quite specific signs.
Reduced bowel wall enhancement was the best predictive CT sign of strangulation, as the pretest probability of ischemia was 11-fold increased if this sign was present (positive LR = 11.07). This CT finding was the result of blockage of the bowel wall arteriovenous microcirculation (by the extent of bowel dilatation or by torsion of the occluded bowel loop vascular pedicle), with bowel wall vessel engorgement, exudation and final mural haemorrhage. This dynamic process leads to alteration of bowel wall infusion. However, this CT finding lacks sensitivity (53 %, 95 CI 39–67 %), which could be explained by two factors. First, as strangulation can result in mural haemorrhage, a lack of enhancement after i.v. contrast cannot be detected because of the spontaneous hyperdensity of the ischemic bowel wall. Geffroy et al. highlighted the diagnostic value of increased unenhanced bowel wall attenuation for the diagnosis of strangulation, reporting a specificity of 100 % and a sensitivity of 56 %, in a highly selected population of surgically treated patients [6]. As there was no reference to unenhanced CT images to identify reduced or absent bowel wall enhancement in the studies included in our meta-analysis, the sensitivity of this sign could have been underestimated. Second, the use of oral contrast could have masked some subtle changes in bowel-wall enhancement as the lowest sensitivities reported in our meta-analysis for decreased bowel wall enhancement were in the two studies where an oral contrast agent was administered [28, 29]. Poor sensitivity for this sign (33 % sensitivity) was also found in a previous study using oral contrast [16]. However, oral contrast is not yet recommended in surgeons’ guidelines [1].
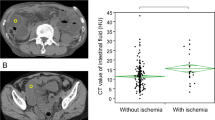
The mesenteric fluid sign, resulting from venous congestion and transudation of fluid across the serosa caused by mesenteric venous outflow obstruction, had the best sensitivity (89 %, 95 CI 75–96 %), with a good negative LR (0.16). Although sensitive, it lacks specificity, probably because its diagnostic value may differ depending on its extension. Indeed, Ha et al. reported a higher specificity for strangulation when mesenteric haziness was diffuse (defined as extending to a wide portion of the mesentery beyond the obstructed site) than when it was focal (defined as confined to the obstructed site) [28]. Moreover, the highest specificity in our meta-analysis was reported in the Jancelewicz article [5], where this sign was considered positive if the fluid was segmental and thus more diffuse. We therefore consider that this finding reflects the dynamic process of venous obstruction, which extends progressively and increasingly far in the mesentery, probably highlighting the severity of strangulation, with a higher risk of irreversible bowel wall ischemia.
The other CT signs that were studied had lower diagnostic performance. In the SBO setting, bowel wall thickening can result from venous engorgement, but could also be the cause of SBO (e.g., in case of inflammatory disease). Although it had been heterogeneously evaluated in the considered studies, the diagnostic performances reported were close between studies regardless of the selected inclusion criterion or bowel thickness cut-off, as indicated by the low between-study heterogeneity reported (I2 = 9.6 %). However, given the lower sensitivity and specificity of this sign compared to reduced bowel wall enhancement, this sign has little diagnostic value, especially if the CT was performed with i.v. contrast. We showed that peritoneal fluid and mesenteric congestion signs had poor predictive values for ischemia diagnosis. Peritoneal fluid is a nonspecific finding, especially when it is present in low abundance since it may occur in many abdominal emergencies, and it has been heterogeneously evaluated in the studies depending on its volume. The mesenteric congestion sign, generally defined as the relative dilatation of mesenteric vessels, is not very reliable due to its subtlety and low reproducibility. Indeed, the interobserver agreement reported in the literature [6, 16] was fair, with a kappa of around 0.35 between readers.
The major strengths of our meta-analysis, are: pooling results of good quality studies according to the QUADAS assessment; applying stringent inclusion criteria to the included studies, resulting in relatively low between-study heterogeneity; and applying a random-effect meta-analysis, which was useful for pooling the evidence while considering randomly distributed differences between the primary studies [20], as the prevalence of ischemia was quite heterogeneous between studies. Moreover, since the number of strangulated cases per study was relatively small (10–45 patients), the meta-analysis summarized the results in a considerably large number of patients with statistically appropriate confidence limits.
There were some limitations to our meta-analysis. First, given the retrospective nature of the studies included and the resulting lack of data, no comparison could have been done with respect to clinical risk factors or timing between onset of symptoms and imaging. Second, although precise macroscopic correlations could not be evaluated due to time variations between the CT examinations and surgical explorations, the findings reflect the current clinical practice, as the majority of strangulated cases had undergone surgery less than 48 h after admission. Third, although the number of included studies was relatively small, it was sufficient for the meta-analysis, but this could explain the absence of power in testing the publication bias, which remained insignificant for all evaluated CT signs. Fourth, the bowel resection rate was very high in our study, i.e., 184/205 (89.7 %) of strangulated patients, due to the selection criteria, as 5/9 of the selected studies had included only strangulation with surgical resection, but not with surgical reversible ischemia. That is a strength, as the reference standard was perfect with histological proof, but also a major selection bias attesting a population with a more severe disease than expected in clinical practice, which might lead to increased estimates of sensitivity. Bogusevicius et al. [33] reported, in a prospective observational study of 53 patients with SBO of any cause, a 28.3 % rate of strangulation, including 53 % with intestinal necrosis and 47 % with reversible ischemia. In our meta-analysis, the rate of intestinal necrosis was higher, ranging from 57.1 % to 100 % for strangulated cases. Moreover, there might be an overestimation of diagnostic accuracy due to patient selection criteria in most included studies (6/9) where some “difficult to diagnose” patients were excluded (for example inflammatory bowel disease, peritoneal carcinomatosis etc.). Fifth, there was potential classification bias, as only 3/9 studies had an optimal CT reading, with two readers and another one for the consensus. Because of the different levels of reader experience, the image interpretations and the detection of well-known CT signs certainly differed between studies. However, most of the readers were experienced radiologists (as reported in the studies), so we consider that their readings reflected the standard level of knowledge and practice. Sixth, we did not screen for other previously reported CT signs of strangulation in SBO (i.e., bowel wall pneumatosis, increased bowel wall density sign in the unenhanced phase and bowel faeces sign) because they were not evaluated in many studies and there was not sufficient data for meta-analysis. Indeed, bowel wall pneumatosis, which was evaluated in 5/9 included studies, was present in only 8/335 patients (2.3 %) overall, and the faeces sign, which was reported in 3/9 included studies, was present in only 16/255 patients (6.2 %).
Unfortunately, we did not have sufficient data to perform a multivariate meta-analysis, as proposed by Trikalinos et al. [34], in order to find the best combination of CT findings to assess ischemia in bowel obstruction. In fact, current models do not fully utilize all of the available data in meta-analysis, but only the data of studies that evaluate all the index tests investigated together, resulting in a loss of power. Therefore, more general and robust methods are required, as recommended by the Cochrane Collaboration [20]. However, our study showed that our two main CT findings (i.e., reduced bowel wall enhancement and mesenteric fluid) seemed to be independent predictors of strangulation, as their confidence ellipses did not overlap at all on the summary ROC graph. Future prospective studies should test the diagnostic performance of these two CT findings, and in combination, for the diagnosis of strangulation in a population with a prevalence of strangulation representative of the general population of patients with small bowel obstruction.
Finally, two CT findings were found to have a strong clinical impact for strangulation diagnosis: the presence of a reduced enhanced bowel wall is highly predictive of ischemia, with a significant positive LR, and the absence of the mesenteric fluid sign is a strong finding to rule out strangulation, with a good negative LR. The other CT signs did not show sufficient reliability to be used in clinical practice.
References
Catena F, Di Saverio S, Kelly MD, Biffl WL, Ansaloni L, Mandalà V et al (2011) Bologna guidelines for diagnosis and management of adhesive small bowel obstruction (ASBO): 2010 evidence-based guidelines of the world society of emergency surgery. World J Emerg Surg 6(5):21
Maung AA, Johnson DC, Piper GL, Barbosa RR, Rowell SE, Bokhari F et al (2012) Evaluation and management of small-bowel obstruction: an Eastern Association for the Surgery of Trauma practice management guideline. J Trauma Acute Care Surg 73(5 Suppl 4):S362–S369
Sarr MG, Bulkley GB, Zuidema GD (1983) Preoperative recognition of intestinal strangulation obstruction. Prospective evaluation of diagnostic capability. Am J Surg 145(1):176–182
Silen W, Hein MF, Goldman L (1962) Strangulation obstruction of the small intestine. Arch Surg Chic Ill 1960 85:121–129
Jancelewicz T, Vu LT, Shawo AE, Yeh B, Gasper WJ, Harris HW (2009) Predicting strangulated small bowel obstruction: an old problem revisited. J Gastrointest Surg Off J Soc Surg Aliment Tract 13(1):93–99
Geffroy Y, Boulay-Coletta I, Jullès M-C, Nakache S, Taourel P, Zins M (2013) Increased Unenhanced Bowel-Wall Attenuation at Multidetector CT Is Highly Specific of Ischemia Complicating Small-Bowel Obstruction. Radiology
Taourel PG, Fabre JM, Pradel JA, Seneterre EJ, Megibow AJ, Bruel JM (1995) Value of CT in the diagnosis and management of patients with suspected acute small-bowel obstruction. AJR Am J Roentgenol 165(5):1187–1192
Zalcman M, Sy M, Donckier V, Closset J, Gansbeke DV (2000) Helical CT signs in the diagnosis of intestinal ischemia in small-bowel obstruction. AJR Am J Roentgenol 175(6):1601–1607
Frager D, Medwid SW, Baer JW, Mollinelli B, Friedman M (1994) CT of small-bowel obstruction: value in establishing the diagnosis and determining the degree and cause. AJR Am J Roentgenol 162(1):37–41
Balthazar EJ, Liebeskind ME, Macari M (1997) Intestinal ischemia in patients in whom small bowel obstruction is suspected: evaluation of accuracy, limitations, and clinical implications of CT in diagnosis. Radiology 205(2):519–522
Jang KM, Min K, Kim MJ, Koh SH, Jeon EY, Kim I-G et al (2010) Diagnostic performance of CT in the detection of intestinal ischemia associated with small-bowel obstruction using maximal attenuation of region of interest. Am J Roentgenol 194(4):957–963
Balthazar EJ, Birnbaum BA, Megibow AJ, Gordon RB, Whelan CA, Hulnick DH (1992) Closed-loop and strangulating intestinal obstruction: CT signs. Radiology 185(3):769–775
Catel L, Lefèvre F, Lauren V, Canard L, Bresler L, Guillemin F et al (2003) Small bowel obstruction from adhesions: which CT severity criteria to research? J Radiol 84(1):27–31
Frager D, Baer JW, Medwid SW, Rothpearl A, Bossart P (1996) Detection of intestinal ischemia in patients with acute small-bowel obstruction due to adhesions or hernia: efficacy of CT. AJR Am J Roentgenol 166(1):67–71
Kim JH, Ha HK, Kim JK, Eun HW, Park KB, Kim BS et al (2004) Usefulness of known computed tomography and clinical criteria for diagnosing strangulation in small-bowel obstruction: analysis of true and false interpretation groups in computed tomography. World J Surg 28(1):63–68
Sheedy SP, Earnest F 4th, Fletcher JG, Fidler JL, Hoskin TL (2006) CT of small-bowel ischemia associated with obstruction in emergency department patients: diagnostic performance evaluation. Radiology 241(3):729–736
Obuz F, Terzi C, Sökmen S, Yilmaz E, Yildiz D, Füzün M (2003) The efficacy of helical CT in the diagnosis of small bowel obstruction. Eur J Radiol 48(3):299–304
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 62(10):1006–1012
Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB et al (2011) QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 155(8):529–536
Leeflang MM, Deeks JJ, Takwoingi Y, Macaskill P (2013) Cochrane diagnostic test accuracy reviews. Syst Rev 2(1):1–6
Harbord RM, Deeks JJ, Egger M, Whiting P, Sterne JA (2007) A unification of models for meta-analysis of diagnostic accuracy studies. Biostatistics 8(2):239–251
Harbord RM, Whiting P, Sterne JA, Egger M, Deeks JJ, Shang A et al (2008) An empirical comparison of methods for meta-analysis of diagnostic accuracy showed hierarchical models are necessary. J Clin Epidemiol 61(11):1095–1103
Deeks JJ, Altman DG (2004) Diagnostic tests 4: likelihood ratios. BMJ 329(7458):168–169
Higgins JPT, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327(7414):557–560
Lijmer JG, Bossuyt PMM, Heisterkamp SH (2002) Exploring sources of heterogeneity in systematic reviews of diagnostic tests. Stat Med 21(11):1525–1537
Dinnes J, Deeks J, Kirby J, Roderick P (2005) A methodological review of how heterogeneity has been examined in systematic reviews of diagnostic test accuracy. Health Technol Assess Winch Engl 9(12):1–113
Deeks JJ, Macaskill P, Irwig L (2005) The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J Clin Epidemiol 58(9):882–893
Ha HK, Kim JS, Lee MS, Lee HJ, Jeong YK et al (1997) Differentiation of simple and strangulated small-bowel obstructions: usefulness of known CT criteria. Radiology 204(2):507–512
Yen C-H, Chen J-D, Tui C-M, Chou Y-H, Lee C-H, Chang C-Y et al (2005) Internal hernia: computed tomography diagnosis and differentiation from adhesive small bowel obstruction. J Chin Med Assoc JCMA 68(1):21–28
Huszty G, Mogami K, Sawada T, Seki H, Sakusabe M, Ohuchi S et al (2007) Preoperative evaluation of irreversible bowel ischemia in obturator hernia. Hepatogastroenterology 54(75):775–779
Schwenter F, Poletti PA, Platon A, Perneger T, Morel P, Gervaz P (2010) Clinicoradiological score for predicting the risk of strangulated small bowel obstruction. Br J Surg 97(7):1119–1125
Zielinski MD, Eiken PW, Bannon MP, Heller SF, Lohse CM, Huebner M et al (2010) Small bowel obstruction-who needs an operation? A multivariate prediction model. World J Surg 34(5):910–919
Bogusevicius A, Grinkevicius A, Maleckas A, Pundzius J (2007) The role of D-dimer in the diagnosis of strangulated small-bowel obstruction. Med Kaunas Lith 43(11):850–854
Trikalinos TA, Hoaglin DC, Small KM, Schmid CH. Evaluating Practices and Developing Tools for Comparative Effectiveness Reviews of Diagnostic Test Accuracy. 2013 Jan. Available from: http://www.ncbi.nlm.nih.gov/books/NBK148804/
Acknowledgements
The scientific guarantor of this publication is Patrice Taourel. The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article. The authors state that this work has not received any funding. One of the authors has significant statistical expertise. Institutional Review Board approval was not required because it was a meta-analytic study with evaluation of retrospective published studies. Written informed consent was waived by the Institutional Review Board. Methodology: retrospective, diagnostic or prognostic study, multicenter study.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work has never been presented before submission.
Appendices
Appendix E1 Forest plot of sensitivity and specificity according to each CT finding. Numbers in brackets are 95 % CIs
A. Decreased enhanced wall

B. Wall thickness

C. Mesenteric congestion

D. Mesenteric fluid

E. Peritoneal fluid

Appendix Z Funnel plot for publication bias for each CT finding
A. Decreased enhanced wall

B. B- Wall thickness

C. Mesenteric congestion

D. Mesenteric fluid

E. Peritoneal fluid

Rights and permissions
About this article
Cite this article
Millet, I., Taourel, P., Ruyer, A. et al. Value of CT findings to predict surgical ischemia in small bowel obstruction: A systematic review and meta-analysis. Eur Radiol 25, 1823–1835 (2015). https://doi.org/10.1007/s00330-014-3440-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-014-3440-2