Abstract
Purpose
Distinct morphological emphysema phenotypes were assessed by CT to show characteristic perfusion defect patterns.
Material/Methods
Forty-one patients with severe emphysema (GOLD III/IV) underwent three-dimensional high resolution computed tomography (3D-HRCT) and contrast-enhanced magnetic resonance (MR) perfusion. 3D-HRCT data was visually analyzed for emphysema phenotyping and quantification by consensus of three experts in chest-radiology. The predominant phenotype per segment was categorized as normal, centrilobular, panlobular or paraseptal. Segmental lung perfusion was visually analyzed using six patterns of pulmonary perfusion (1-normal; 2-mild homogeneous reduction in perfusion; 3-heterogeneous perfusion without focal defects; 4-heterogeneous perfusion with focal defects; 5-heterogeneous absence of perfusion; 6-homogeneous absence of perfusion), with the extent of the defect given as a percentage.
Results
730 segments were evaluated. CT categorized 566 (78 %) as centrilobular, 159 (22 %) as panlobular and 5 (<1 %) as paraseptal with no normals. Scores with regards to MR perfusion patterns were: 1–0; 2–0; 3–28 (4 %); 4–425 (58 %); 5–169 (23 %); 6–108 (15 %).
The predominant perfusion pattern matched as follows: 70 % centrilobular emphysema - heterogeneous perfusion with focal defects (score 4); 42 % panlobular - homogeneous absence of perfusion (score 5); and 43 % panlobular - heterogeneous absence of perfusion (score 6).
Conclusion
MR pulmonary perfusion patterns correlate with the CT phenotype at a segmental level in patients with severe emphysema.
Key points
• MR perfusion patterns correlate with the CT phenotype in emphysema.
• Reduction of MR perfusion is associated with loss of lung parenchyma on CT
• Centrilobular emphysema shows heterogeneous perfusion reduction while panlobular emphysema shows loss of perfusion.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chronic Obstructive Pulmonary Disease (COPD) remains a major public health problem. It is the fourth leading cause of chronic morbidity and mortality in the United States, and is projected to rank fifth in 2020 in burden of diseases worldwide [1]. It is widely recognized that COPD is a complex syndrome with numerous pulmonary and extra-pulmonary components. Importantly, significant heterogeneity exists with respect to clinical presentation, physiology, imaging, response to therapy, decline in lung function and survival [2]. Due to variability of the disease accurate phenotyping is an important step towards guiding clinical application and research of appropriate therapy in order to improve quality of life and patient survival.
Pulmonary function tests (PFTs), in particular forced expiratory volume in 1 second (FEV1), FEV1/FVC (forced vital capacity) and diffusing capacity for carbon monoxide (DLco), are the main tools used to assess clinical severity of COPD. PFTs provide a global lung assessment but, as a result, do not account for the heterogeneous nature of the disease where there may be significant regional variability. Radiological imaging, in contrast, has the ability to assess the lung in small compartments. Computed tomography (CT) is the gold standard for morphological evaluation of lung tissue in vivo. CT helps categorize COPD into airway-or emphysema-predominant phenotypes [3]. Emphysema subtypes within the emphysema-predominant population can be separated into centrilobular, panlobular and paraseptal predominant disease. Although morphological assessment has shown a correlation with lung function [4–6], whether or not the presence of specific structural lung abnormalities alone predicts meaningful clinical outcome remains unclear [7, 8]. The pathophysiology of COPD raises questions as to phenotyping the disease on a purely morphological basis. The relationship between impaired ventilation as a result of airway obstruction and parenchymal destruction, with a decrease in lung perfusion has been well examined. It is known, however, that the distribution of perfusion does not necessarily match morphological and emphysematous parenchymal changes [9]. Novel therapies, such as volume reduction surgery or endobronchial valve placement, try to correct this mis-match either at an extra-anatomical level or at a lobar or segmental level [10–13]. Additional functional information is therefore important in stratifying these patients prior to therapy. This can be obtained with lung perfusion imaging. This was originally performed with nuclear medicine studies, but much higher spatial resolution has been provided by MR perfusion imaging [14].
Recent studies, although generally correlating the degree of parenchymal destruction with loss of perfusion, have shown variable concordance between CT morphology and MR perfusion particularly at a lobar level [15]. In addition, a number of different patterns of morphological disease and lung perfusion have been observed, from homogeneous to markedly heterogeneous. There are no previous studies investigating perfusion patterns in emphysema and our question is whether different morphological subtypes of emphysema are the reason for the varying perfusion patterns with MRI. This leads to our hypothesis that distinct morphological emphysema phenotypes assessed by CT show a characteristic perfusion pattern.
Due to the potential variability of disease even within individual lobes we examined the lung at a segmental level to decrease the potential for overlap of COPD subtypes.
Materials and Methods
We examined 41 consecutive patients (11 female, 30 male; mean age 62 +/−6 years, range 51–76) with a clinical diagnosis of severe COPD. The body Mass Index (BMI) of our study population was 25 ± 4 kg/m2 (range 18–31 kg/m2). Patients had a smoking history of 51 ± 23 pack years (range 10–120 pack years).
The CT examination was performed as part of a standard work-up for evaluation prior to surgical or endobronchial treatment. Inclusion criteria were smoking, severe changes in lung function tests indicative of obstructive disease (GOLD III or IV), and no evidence of an α1-antitrypsin deficiency. Radiological diagnosis of emphysema was based on CT analysis. Patients with the airway-predominant phenotype, lobar atelectasis, a tumour or pneumonia were not included in this analysis.
CT and MRI examinations were performed on the same day one to two hours apart.
Data acquisition
3D-HRCT was performed using a 16-row multilple detector CT (MDCT) scanner (Aquilion 16, Toshiba Medical System Corporation, Toshigi, Japan). The examination was performed during an inspiratory breath-hold in supine position. CT acquisition parameters were: collimation 1 mm; 120 kV; 300 mA; gantry rotation time 0.5 s; pitch 1.43;and large imaging field. All images were reconstructed using a high frequency reconstruction algorithm (standard lung kernel) with a slice thickness of 1 mm and a reconstruction interval of 0.8 mm. No intravenous contrast medium was administered.
All images were transferred to a standalone personal computer (PC) via our picture-archiving and communication system (PACS).
MR examinations were performed in supine position on a clinical 1.5-T whole-body MR system (Magnetom Symphony; Siemens Medical Solutions, Erlangen, Germany) with a maximum gradient strength of 30 mT/m and a slew rate of 125 T/m/s. Two six-channel body phased array coils were placed anterior and posterior of the patients and used for signal detection. A time-resolved contrast-enhanced 3D gradient echo pulse sequence (TREAT) with generalized auto-calibrating partially parallel acquisitions (GRAPPA) was applied using the following imaging parameters: repetition time (TR) 1.9 ms; echo time (TE) 0.8 ms; flip angle 40°; receiver bandwidth 1220 Hz/pixel; acceleration factor 2; reference k-space lines for calibration 20; field of view 480 mm × 360 mm; matrix 256 × 96; slab thickness 160 mm; 44 partitions; and scan time per 3D dataset 1.5 s. A total of, 20 consecutive datasets were acquired during one inspiratory breath-hold in coronal orientation, starting with the beginning of the contrast injection. Gadolinium-DTPA was injected through the antecubital vein using an automatic power injector at a rate of 5 mL/s with a dose of 0.1 mmol/kg body weight followed by a saline flush of 30 mL injected at the same injection rate.
Image analysis
Evaluation was performed by three experienced chest radiologists in a consensus reading session. CT and MR images were evaluated separately and in a random order with the radiologists blinded to the patient data.
Visual data analysis was performed on the original axial as well as coronal and sagittal images using the multi-planar reformation mode on a dedicated viewing station. The predominant emphysema type was determined for each lung segment. Categories included normal lung or emphysematous lung with either centrilobular, panlobular or paraseptal subtype. Each segment was also graded for the percentage of emphysematous parenchymal destruction. Ten segments were assigned for the right lung and eight for the left.
The MR data was analysed with the use of dedicated lung perfusion software (MeVIS Pulmo-Software) in original coronal orientation and MPR mode. The images were presented as colour-coded maps of maximum peak enhancement. The source perfusion images were used in conjunction for better visualisation of anatomic landmarks.
Six patterns of pulmonary perfusion were used (Table 1). The predominant pattern of pulmonary perfusion was assessed for each lung segment and the segment attributed a score from 1 to 6. In addition, the extent of the perfusion deficit was graded in percent for each segment.
Additionally, CT data were assessed for the main pulmonary artery to aorta ratio and dilatation of the main pulmonary artery. Measurements were performed by one reader (experienced chest radiologist) using electronic callipers.
Statistical analysis
A chi-square test was used to test for an association between CT morphology and MRI perfusion patterns. Also, chi-square tests were run separately for each CT severity score. However, for up to 60 % of the severity scores there were too few centrilobular emphysematous changes; thus, no statistical analysis could be performed. Chi-square tests were performed for the 61-80 % and 81-100 % severity groups. A kappa test was used for analysis of the agreement between the degree of parenchymal destruction (CT severity %) and degree of perfusion loss (MR severity %).
Results
A total of 730 segments were evaluated (99 %); eight segments had to be excluded from evaluation due to atelectasis. All segments had a degree (>0) of emphysema, with all patients having moderate to severe emphysema. CT phenotyping categorized 566 as centrilobular (78 %), 159 as panlobular (22 %) and 5 as paraseptal (<1 %) with no normal cases (Table 2). Scores with regards to MR perfusion patterns were: 1–0; 2–0; 3–28 (4 %); 4–425 (58 %); 5–169 (23 %); 6–108 (15 %) (Table 3).
The predominant perfusion pattern matched as follows: 70 % of centrilobular emphysema cases demonstrated heterogeneous perfusion with focal defects (score 4); 43 % of the panlobular predominant segments showed inhomogeneous absence of perfusion (score 5); and 42 % of the panlobular segments had homogenous absence of perfusion (score 6) (Table 4).
The overall chi-square test for the association between CT morphology and MRI perfusion patterns showed a p-value less than <0.001, indicating a statistically significant association. The p value for the CT severity group 61-80 % was 0.014 and for the severity group 81-100 % the p-values was <0.001.
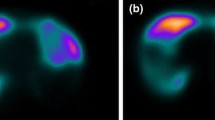
The perfusion pattern in centrilobular emphysema was predominantly heterogeneous with focal perfusion defects (60-82 %; Fig. 1) until the severity reached 81-100 % parenchymal destruction. At this point there is an even distribution of perfusion patterns with heterogeneous and homogeneous loss of perfusion (Fig. 2; Table 5). The overall severity of disease with panlobular emphysema was almost exclusively greater than 60 % destruction of the parenchyma, reflecting the destructive nature of this phenotype. The corresponding perfusion pattern had a strong predominance towards loss of perfusion, from 71 % up to 94 % with increasing parenchymal destruction (Fig. 3; Table 6). The overall agreement between the degree of parenchymal destruction (CT severity %) and degree of perfusion loss (MR severity %) showed a kappa value of 0.37.
Thirty-eight of 41 patients had a normal main pulmonary artery diameter and all patients had a normal main pulmonary artery to aorta ratio. Three patients had enlarged (>30 mm) main pulmonary arteries (3.1 cm, 3.2 cm and 3.6 cm) despite normal pulmonary artery to aorta (PA/Aorta) ratios (0.8, 0.9 and 0.8 respectively) (Table 7).
Discussion
In this study CT-based morphological changes in emphysema patients were matched with the MR perfusion pattern. The key findings were that 70 % of the centrilobular emphysema cases demonstrated heterogeneous perfusion with focal defects; 43 % of the panlobular predominant segments showed heterogeneous absence of perfusion; and 42 % of panlobular segments had homogeneous absence of perfusion. The p value between CT morphology and MRI perfusion pattern was <0.001 indicating a significant relationship between these two variables.
Quantitative assessment of emphysema by CT imaging offers an objective measure of parenchymal disease that correlates well with histopathological findings and is predictive of the degree of expiratory airflow obstruction [4].
Whether or not the presence of specific structural lung abnormalities (including emphysema, airway wall thickening and/or bronchiectasis) predict meaningful clinical outcome is an area of current interest [7, 8]. Increasing emphysema severity as defined by CT has been associated with worse health status [6] and increased mortality [16]. The National Emphysema Treatment Trial provides the most compelling evidence supporting distinct CT-based phenotypes in defining an increased risk of mortality in patients with homogeneous emphysema and impaired FEV1 or diffusing capacity of carbon monoxide undergoing LVRS, whereas upper lobe–predominant emphysema and a low post-rehabilitation exercise capacity identify a group of emphysema patients who experience a mortality and functional benefit from LVRS [17]. These compelling data strongly support that a combination of CT imaging and, physiological (functional) testing can clearly impact therapeutic decision making, thus validating its value for patient phenotyping [2].
Whereas CT provides reliable morphological data, functional lung imaging is the realm of MRI. The use of MRI in phenotyping, however, remains in its early stages. Morphological assessment is limited by a poor signal. In one study, changes in parenchymal signal intensity measured by MRI at inspiration and expiration correlated with FEV1 (r = 0.51) [18], suggesting it may be useful as a predictor of airflow obstruction but limited in emphysema phenotyping.
In a recent study, different MRI sequences were compared to CT for detection of the severity of emphysema and the leading emphysema type on a lobar level. MRI matched the CT in half of the cases: the sensitivity for emphysema severity was 44 %, 48 % and 41 %, and the leading type of emphysema was 68 %, 55 % and 60 % for T2w-HASTE, T1w-VIBE and T1w-ce-VIBE, respectively [19]. Given these relatively poor values, it is clear that non-contrast-enhanced proton MRI is unlikely to be clinically useful for diagnosis or categorization of emphysema.
In regions with reduced ventilation, the physiological response of hypoxic vasoconstriction occurs [20] leading to reduced local pulmonary blood flow (PBF) [21]. The reduction of the pulmonary vascular bed is related to the severity of parenchymal destruction [22]. In patients with severe emphysema a high agreement between emphysematous destruction on CT images and perfusion impairment on MR images was found. However, it has been shown that the distribution of perfusion does not necessarily match parenchymal destruction. In case of discrepancies, CT was more likely to give a more severe score than MRI [15]. In this paper assessment was performed on a lobar level, which may have affected the evaluation of perfusion to parenchymal destruction matching. An alternative or additional hypothesis is that different emphysema phenotypes demonstrate different perfusion patterns.
The perfusion abnormalities in COPD clearly differ from those caused by vascular obstruction. While wedge-shaped perfusion defects occur in embolic vascular obstruction, a low degree of contrast enhancement is generally found in patients with COPD/emphysema [23, 24]. Furthermore, the peak signal intensity is usually reduced. These features allow for relatively easy visual differentiation. In COPD patients the quantitative evaluation of 3D perfusion showed diffusely decreased PBF, mean transit time (MTT) and perfused blood volume (PBV) and the changes were heterogeneous [25]. It was suggested that patients with emphysema exhibit hypoxia as well as destruction of lung parenchyma and fewer alveolar capillaries. This causes increased pulmonary arterial resistance and, secondary to adaptive processes, pulmonary hypertension and right ventricular dysfunction. Ultimately, this results in decreased PBF in addition to heterogeneous perfusion and decreased PBV. MTT is determined by the ratio between PBV and PBF. The results suggested that MTT is significantly decreased, reflecting a larger degree of decrease in PBV compared with PBF, with a concomitant increased heterogeneity of regional PBV. Obviously, accurate quantitative measurements of such regional changes are important for improved understanding of lung pathophysiology in COPD. A recent study compared quantitative emphysema CT analysis with quantitative perfusion MRI evaluated by one 10-mm peripheral region of interest (ROI) for each lobe on each slice [26]. PBF was negatively correlated to emphysema volume by the CT data (r = −0.61).
These recent papers have demonstrated correlation of pulmonary perfusion with emphysema volume, but the question remains as to whether the differing pathophysiology between emphysema subtypes leads to variable patterns of perfusion loss, which can be demonstrated with perfusion MRI. A first insight into different perfusion patterns in different COPD phenotypes was recently described [27].
Our results indicate that there is a significant correlation between segmental perfusion patterns and emphysema phenotype (based on the CT severity scores > 61 %), with 85 % of the panlobular emphysema cases demonstrating loss of perfusion rather than heterogeneous perfusion reduction. There is an overlap of perfusion pattern with some of the severe cases of centrilobular emphysema, demonstrating another grey area in phenotyping. It may be possible to further discriminate overall phenotype by examining the results from adjacent segments, or all segments in an individual for those cases where it is not distinguishable. As a limitation, it has to be mentioned that the study group mainly consisted of patients with advanced COPD/emphysema.
The results of our study rely on quantitative perfusion map assessment. There are multiple factors contributing to the changes in perfusion in COPD. We have, to a large degree, accounted for regional changes caused by parenchymal destruction and hypoxic vasoconstriction. Perfusion deficit caused by pulmonary hypertension is more difficult to quantify.
Pulmonary hypertension in patients with COPD is a well recognized comorbidity, potentially defining one of these phenotypes with distinct effects on cardiopulmonary function and mortality [28]. Pulmonary hypertension (PHT) is a hemodynamic diagnosis that requires confirmation by right heart catheterization. The presence of PHT in our patient population is not known (there were no cases with known PHT). There is good evidence for surrogate measures of raised pulmonary artery pressure, however, with the main pulmonary artery (MPA) diameter and the PA/aorta ratio being assessed as with CT [29–32]. These measurements can be clinically helpful as right heart catheterization is invasive and echocardiographic assessment is unreliable in advanced lung disease [33]. Three out of 41 patients (7 %) had a dilated MPA with none having an elevated PA/aorta ratio. Despite these measurements, evidence suggests that a high proportion of patients (50 – 91 %) with severe COPD will have PHT [22, 34]. A significant feature of these studies, however, is the preponderance of mild pulmonary hypertension even in severe COPD. Of the patients with advanced disease studied in the National Emphysema Treatment Trial only 5 % had severe pulmonary hypertension (pulmonary artery pressure > 35 mmHg) [34].
Furthermore, recent studies into the pathogenesis of pulmonary hypertension [35–38] indicate that vascular dysfunction, vascular remodelling and pulmonary hypertension occurred early in COPD, independent of hypoxia. They also suggest that this condition develops early in the course of lung disease, independent of the degree of parenchymal lung damage. Even if the perfusion changes seen in our study had occurred earlier in the disease process, it nonetheless demonstrates that perfusion pattern correlates with morphological phenotype and may indicate that the pattern of vascular dysfunction and, therefore, perfusion defect in these individuals have a use in phenotyping.
The knowledge that vascular changes occur early highlight the need to study lung perfusion in patients with mild forms of the disease, to see if it is possible to identify early those patients who are more likely to develop PHT or a more rapid parenchymal destruction and who may therefore benefit from close monitoring and early therapy.
Conclusion
There is an association between the emphysema type and MRI perfusion pattern at a segmental level. As the degree of parenchymal destruction increases there is a shift in emphysema type from centrilobular (predominant and almost exclusive in the low degree groups) to panlobular, corresponding with a shift from heterogeneous perfusion reduction to loss of perfusion.
References
GOLD (2009) Global Initiative for Chronic Obstructive Lung Disease (GOLD). http://www.goldcopd.com/
Han MK, Agusti A, Calverley PM, Celli BR, Criner G, Curtis JL, Fabbri LM, Goldin JG, Jones PW, Macnee W, Make BJ, Rabe KF, Rennard SI, Sciurba FC, Silverman EK, Vestbo J, Washko GR, Wouters EF, Martinez FJ (2010) Chronic obstructive pulmonary disease phenotypes: the future of COPD. Am J Respir Crit Care Med 182:598–604
Lynch DA (2008) Imaging of small airways disease and chronic obstructive pulmonary disease. Clin Chest Med 29:165–179
Kinsella M, Müller N, Abboud R, Morrison N, DyBuncio A (1990) Quantitation of emphysema by computed tomography using a "density mask" program and correlation with pulmonary function tests. Chest 97:315–321
Nakano Y, Muro S, Sakai H, Hirai T, Chin K, Tsukino M, Nishimura K, Itoh H, Pare PD, Hogg JC, Mishima M (2000) Computed tomographic measurements of airway dimensions and emphysema in smokers. Correlation with lung function. Am J Respir Crit Care Med 162:1102–1108
Han MK, Bartholmai B, Liu LX, Murray S, Curtis JL, Sciurba FC, Kazerooni EA, Thompson B, Frederick M, Li D, Schwarz M, Limper A, Freeman C, Landreneau RJ, Wise R, Martinez FJ (2009) Clinical significance of radiologic characterizations in COPD. Copd 6:459–467
Bon JM, Leader JK, Weissfeld JL, Coxson HO, Zheng B, Branch RA, Kondragunta V, Lee JS, Zhang Y, Choi AM, Lokshin AE, Kaminski N, Gur D, Sciurba FC (2009) The influence of radiographic phenotype and smoking status on peripheral blood biomarker patterns in chronic obstructive pulmonary disease. PLoS One 4:e6865
Coxson HO, Mayo J, Lam S, Santyr G, Parraga G, Sin DD (2009) New and Current Clinical Imaging Techniques To Study Chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med 180:588–597
Sandek K, Bratel T, Lagerstrand L, Rosell H (2002) Relationship between lung function, ventilation-perfusion inequality and extent of emphysema as assessed by high-resolution computed tomography. Respir Med 96:934–943
Toma TP, Hopkinson NS, Hillier J, Hansell DM, Morgan C, Goldstraw PG, Polkey MI, Geddes DM (2003) Bronchoscopic volume reduction with valve implants in patients with severe emphysema. Lancet 361:931–933
Yim AP, Hwong TM, Lee TW, Li WW, Lam S, Yeung TK, Hui DS, Ko FW, Sihoe AD, Thung KH, Arifi AA (2004) Early results of endoscopic lung volume reduction for emphysema. J Thorac Cardiovasc Surg 127:1564–1573
Stoller JK, Gildea TR, Ries AL, Meli YM, Karafa MT (2007) Lung volume reduction surgery in patients with emphysema and alpha-1 antitrypsin deficiency. Ann Thorac Surg 83:241–251
Wood DE, McKenna RJ Jr, Yusen RD, Sterman DH, Ost DE, Springmeyer SC, Gonzalez HX, Mulligan MS, Gildea T, Houck WV, Machuzak M, Mehta AC (2007) A multicenter trial of an intrabronchial valve for treatment of severe emphysema. J Thorac Cardiovasc Surg 133:65–73
Molinari F, Fink C, Risse F, Tuengerthal S, Bonomo L, Kauczor HU (2006) Assessment of differential pulmonary blood flow using perfusion magnetic resonance imaging: comparison with radionuclide perfusion scintigraphy. Invest Radiol 41:624–630
Ley-Zaporozhan J, Ley S, Eberhardt R, Weinheimer O, Fink C, Puderbach M, Eichinger M, Herth F, Kauczor HU (2007) Assessment of the relationship between lung parenchymal destruction and impaired pulmonary perfusion on a lobar level in patients with emphysema. Eur J Radiol 63:76–83
Martinez FJ, Foster G, Curtis JL, Criner G, Weinmann G, Fishman A, DeCamp MM, Benditt J, Sciurba F, Make B, Mohsenifar Z, Diaz P, Hoffman E, Wise R (2006) Predictors of mortality in patients with emphysema and severe airflow obstruction. Am J Respir Crit Care Med 173:1326–1334
Fishman A, Martinez F, Naunheim K, Piantadosi S, Wise R, Ries A, Weinmann G, Wood DE (2003) A randomized trial comparing lung-volume-reduction surgery with medical therapy for severe emphysema. N Engl J Med 348:2059–2073
Iwasawa T, Takahashi H, Ogura T, Asakura A, Gotoh T, Kagei S, Nishimura J, Obara M, Inoue T (2007) Correlation of lung parenchymal MR signal intensity with pulmonary function tests and quantitative computed tomography (CT) evaluation: A pilot study. J Magn Reson Imaging 26:1530–1536
Ley-Zaporozhan J, Ley S, Eberhardt R, Kauczor HU, Heussel CP (2010) Visualization of morphological parenchymal changes in emphysema: Comparison of different MRI sequences to 3D-HRCT. Eur J Radiol 73:43–49
Euler U, Liljestrand G (1946) Observations on the pulmonary arterial blood pressure in the cat. Acta Physiol Scand 12:301–320
Cederlund K, Hogberg S, Jorfeldt L, Larsen F, Norman M, Rasmussen E, Tylen U (2003) Lung perfusion scintigraphy prior to lung volume reduction surgery. Acta Radiol 44:246–251
Thabut G, Dauriat G, Stern JB, Logeart D, Levy A, Marrash-Chahla R, Mal H (2005) Pulmonary hemodynamics in advanced COPD candidates for lung volume reduction surgery or lung transplantation. Chest 127:1531–1536
Amundsen T, Torheim G, Kvistad KA, Waage A, Bjermer L, Nordlid KK, Johnsen H, Asberg A, Haraldseth O (2002) Perfusion abnormalities in pulmonary embolism studied with perfusion MRI and ventilation-perfusion scintigraphy: An intra-modality and inter-modality agreement study. J Magn Reson Imaging 15:386–394
Morino S, Toba T, Araki M, Azuma T, Tsutsumi S, Tao H, Nakamura T, Nagayasu T, Tagawa T (2006) Noninvasive assessment of pulmonary emphysema using dynamic contrast-enhanced magnetic resonance imaging. Exp Lung Res 32:55–67
Ohno Y, Hatabu H, Murase K, Higashino T, Kawamitsu H, Watanabe H, Takenaka D, Fujii M, Sugimura K (2004) Quantitative assessment of regional pulmonary perfusion in the entire lung using three-dimensional ultrafast dynamic contrast-enhanced magnetic resonance imaging: Preliminary experience in 40 subjects. J Magn Reson Imaging 20:353–365
Jang YM, Oh YM, Seo JB, Kim N, Chae EJ, Lee YK, Lee SD (2008) Quantitatively assessed dynamic contrast-enhanced magnetic resonance imaging in patients with chronic obstructive pulmonary disease: correlation of perfusion parameters with pulmonary function test and quantitative computed tomography. Invest Radiol 43:403–410
Fan L, Xia Y, Guan Y, Zhang TF, Liu SY (2014) Characteristic features of pulmonary function test, CT volume analysis and MR perfusion imaging in COPD patients with different HRCT phenotypes. Clin Respir J 8:45–54
Orr R, Smith LJ, Cuttica MJ (2012) Pulmonary hypertension in advanced chronic obstructive pulmonary disease. Curr Opin Pulm Med 18:138–143
Haimovici JB, Trotman-Dickenson B, Halpern EF, Dec GW, Ginns LC, Shepard JA, McLoud TC (1997) Relationship between pulmonary artery diameter at computed tomography and pulmonary artery pressures at right-sided heart catheterization. Massachusetts General Hospital Lung Transplantation Program. Acad Radiol 4:327–334
Kuriyama K, Gamsu G, Stern RG, Cann CE, Herfkens RJ, Brundage BH (1984) CT-determined pulmonary artery diameters in predicting pulmonary hypertension. Invest Radiol 19:16–22
Ng CS, Wells AU, Padley SP (1999) A CT sign of chronic pulmonary arterial hypertension: the ratio of main pulmonary artery to aortic diameter. J Thorac Imaging 14:270–278
Schmidt HC, Kauczor HU, Schild HH, Renner C, Kirchhoff E, Lang P, Iversen S, Thelen M (1996) Pulmonary hypertension in patients with chronic pulmonary thromboembolism: chest radiograph and CT evaluation before and after surgery. Eur Radiol 6:817–825
Arcasoy SM, Christie JD, Ferrari VA, Sutton MS, Zisman DA, Blumenthal NP, Pochettino A, Kotloff RM (2003) Echocardiographic assessment of pulmonary hypertension in patients with advanced lung disease. Am J Respir Crit Care Med 167:735–740
Scharf SM, Iqbal M, Keller C, Criner G, Lee S, Fessler HE (2002) Hemodynamic characterization of patients with severe emphysema. Am J Respir Crit Care Med 166:314–322
Wright JL, Petty T, Thurlbeck WM (1992) Analysis of the structure of the muscular pulmonary arteries in patients with pulmonary hypertension and COPD: National Institutes of Health nocturnal oxygen therapy trial. Lung 170:109–124
Magee F, Wright JL, Wiggs BR, Pare PD, Hogg JC (1988) Pulmonary vascular structure and function in chronic obstructive pulmonary disease. Thorax 43:183–189
Santos S, Peinado VI, Ramirez J, Melgosa T, Roca J, Rodriguez-Roisin R, Barbera JA (2002) Characterization of pulmonary vascular remodelling in smokers and patients with mild COPD. Eur Respir J 19:632–638
Seimetz M, Parajuli N, Pichl A, Veit F, Kwapiszewska G, Weisel FC, Milger K, Egemnazarov B, Turowska A, Fuchs B, Nikam S, Roth M, Sydykov A, Medebach T, Klepetko W, Jaksch P, Dumitrascu R, Garn H, Voswinckel R, Kostin S, Seeger W, Schermuly RT, Grimminger F, Ghofrani HA, Weissmann N (2011) Inducible NOS inhibition reverses tobacco-smoke-induced emphysema and pulmonary hypertension in mice. Cell 147:293–305
Acknowledgments
The scientific guarantor of this publication is Prof. Hans-Ulrich Kauczor. The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article. This work has been supported by the Competence Network on Asthma/COPD (ASCONET) through a grant and by the German Center for Lung Research (DZL) through grants from the Bundesministerium für Bildung und Forschung (BMBF) of the federal government of Germany (82DZL00401, 82DZL00402, 82DZL00404). Ravi Menezes kindly provided statistical advice for this manuscript. Institutional Review Board approval was obtained. Written informed consent was obtained from all subjects (patients) in this study. Methodology: retrospective, case–control study, performed at one institution.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bryant, M., Ley, S., Eberhardt, R. et al. Assessment of the relationship between morphological emphysema phenotype and corresponding pulmonary perfusion pattern on a segmental level. Eur Radiol 25, 72–80 (2015). https://doi.org/10.1007/s00330-014-3385-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-014-3385-5