Abstract
Objective
To assess the value of contrast-enhanced ultrasound (CEUS) in the absence of hepatic artery signal on Doppler ultrasound (DUS) in the immediate postoperative period after liver transplant.
Methods
This prospective study included 675 consecutive liver transplants. Patients without hepatic artery signal by DUS within 8 days post-transplant were studied with CEUS. If it remained undetectable, a thrombosis was suspected. In patent hepatic artery, a DUS was performed immediately after CEUS; if low resistance flow was detected, an arteriography was indicated. Patients with high resistance waveform underwent DUS+/CEUS follow-up. Arteriography was indicated when abnormal flow persisted for more than 5 days or liver dysfunction appeared.
Results
Thirty-four patients were studied with CEUS. In 11 patients CEUS correctly diagnosed hepatic artery thrombosis. In two out of 23 non-occluded arteries, a low resistance flow lead to a diagnosis of stenosis/proximal thrombosis. Twenty-one patients had absence of diastolic flow, which normalized in the follow-up in 13 patients. In the remaining eight patients, splenic artery steal syndrome (ASS) was diagnosed.
Conclusions
CEUS allows us to avoid invasive tests in the diagnostic work-up shortly after liver transplant. It identifies the hepatic artery thrombosis and points to a diagnosis of ASS.
Key Points
• CEUS is useful in the diagnostic work-up shortly after liver transplant
• CEUS identifies the hepatic artery thrombosis with reliability
• There is little information about DUS and CEUS findings in the ASS
• DUS and CEUS offer functional information useful in the diagnosis of ASS
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Hepatic artery thrombosis is a frequent and major complication after liver transplantation (incidence in adults of 1.8–9 %) [1–3], and is responsible for a large number of liver failures in the early period after grafting [4, 5]. Its prompt diagnosis, before the development of clinical manifestations, improves the prognosis of these patients [6]. Doppler ultrasound (DUS) is an excellent technique for study of the hepatic artery, with a high sensitivity in detecting hepatic artery thrombosis [7]. For these reasons, the use of DUS is widespread in liver transplant programmes, in order to check hepatic artery patency shortly after grafting.
The DUS diagnosis of hepatic artery thrombosis is based on the absence of arterial flow. However, in the first days after liver transplantation the detection of a low arterial flow with high resistance waveform by DUS is quite common and ay be detected in almost half of patients [8]. Occasionally, the arterial flow is minimal and it may be undetectable by DUS, causing a false-positive diagnosis of hepatic artery thrombosis. Moreover, other non-thrombotic complications after liver transplant, such as severe stenosis of the hepatic artery or the splenic arterial steal syndrome (ASS), can cause a significant decrease in blood flow and could also lead to the misdiagnosis of thrombosis in the DUS study [9, 10]. All these facts emphasize the complexity of obtaining an accurate and prompt diagnosis in order to institutethe appropriate treatment. ASS is a condition that is probably underdiagnosed but has been gaining relevance in the last few years. The DUS findings are not specific, there are no established objective ultrasound criteria for the diagnosis of ASS, and, until now, the usefulness of contrast-enhanced ultrasound (CEUS) in the diagnosis of this syndrome has not been evaluated in depth.
Several studies have demonstrated that CEUS improves flow detection in the hepatic artery [11–13], but their clinical value and its role in the management of patients in the immediate post-transplant period have not been defined. Currently there are not any established algorithms including the use of CEUS for the study of hepatic artery in the immediate post-transplant period.
The aim of the study was to prospectively evaluate the usefulness of CEUS in the assessment of arterial flow, and in the diagnosis of the disease of the hepatic artery that causes the arterial hypoperfusion, during the early period after liver transplantation.
Materials and methods
Patients
This prospective study included all liver transplants performed in our hospital from January 2003 to October 2010. During this period, 675 liver transplants in 644 adult patients (males, n = 441, 68.5 %; females, n = 203, 31.5 %) were performed in our institution. The average patient age was 52.9 years (age range, 18–76 years). Forty-five of them were right-liver transplants from living donor, 31 domino liver transplants, three split liver transplants, and the rest were conventional cadaveric liver transplants.
The study received the hospital ethics research board approval and written informed consent was obtained from the patients.
Methods
The ultrasound studies were performed in the ultrasound unit or at the patient’s bedside in the intensive care unit with a Sequoia 512 (Acuson, Mountain View, CA, USA), using a convex or sectorial multifrequency (2-4 MHz) transducer.
According to the hospital liver transplant DUS follow-up protocol, a baseline study was carried out in the first 24 hours after grafting and prior to discharge. DUS was also indicated when the patient had fever, abdominal pain, graft dysfunction, hypertransaminasaemia, cholestasis, or in the follow-up of patients with abnormal baseline arterial Doppler waveform.
DUS examinations included a baseline study of the liver parenchyma and the graft vascular structures. The hepatic artery was explored at intrahepatic (right and left branches) and extrahepatic levels, and the arterial waveforms were analysed semiquantitatively by the Resistive Index (RI = peak systolic velocity – end-diastolic velocity/peak systolic velocity). In cases of low RI (RI < 0.5), the Systolic Acceleration Time (SAT = the time from end diastole to the first systolic peak) was measured.
If no intrahepatic arterial Doppler signal was detected at any of the DUS levels explored, investigation with CEUS was carried out immediately in the same session.
CEUS was performed after the injection of a bolus of 2.4 ml (concentration of 8 μl/ml) of sulphur hexafluoride (SonoVue®, Bracco, Milan, Italy), followed by a flush of 5 ml bolus of saline in the antecubital vein, or in a central venous access. CEUS was carried out with specific contrast software (Pulse inversion; Cadence Pulse Sequence) with low mechanical index (0.17). The liver ultrasound was focused at the anatomic location of the artery where the flow was not detected (main and right hepatic artery or left hepatic artery). CEUS studies were performed continuously and the images were saved as cine loops or still images into the PACS (Picture Archiving and Communication System). All CEUS studies were carried out by five radiologists with more than 9 years of experience in ultrasound, more than 6 years of experience in the use of first-generation ultrasound contrast agents, and 3 years of experience in the use of second-generation ultrasound contrast agents (Sonovue®).
The DUS and CEUS diagnostic algorithm is described in Fig. 1.
Algorithm of the procedure in patients without arterial Doppler signal. Suspected diagnosis on the basis of DUS/CEUS findings appear in italics. *Parvus-tardus: RI < 0.5 and SAT > 0.08 [14]. ** Arteriography is mandatory for the diagnosis of ASS [15, 23]). CEUS contrast-enhanced ultrasound, DUS Doppler ultrasound
CEUS was not performed if DUS was carried out when no radiology staff trained in the use of CEUS were available (non-working days).
Arteriographic studies were performed using the equipment Politron Plus (Siemens) or Axiom Artis (Siemens), through retrograde puncture of the femoral artery and selective catheterization of the coeliac trunk with a standard 5F angiographic catheter. Arterial and venous phases were included in all cases. The angiographic study included the evaluation of the main hepatic artery and intrahepatic arterial branches as well as the splenic artery, and also evaluated the hepatic perfusion and splenoportal blood flow. Several oblique projections and selective study of the hepatic artery were performed if needed to correctly visualize the main hepatic artery and hepatic artery anastomosis.
Patients without hepatic artery Doppler signal investigated with CEUS in the first 8 days following transplant were included in the analysis.
Results
In 48 out of 675 (7.1 %) liver transplants no arterial flow was detected in a DUS study performed in the first 8 days after grafting. Fourteen patients (29 %) were excluded from the study because no radiology staff trained in the use of CEUS were available when the DUS was carried out. Eleven of these 14 patients underwent an urgent arteriographic study, and the diagnosis was: four arterial thrombosis, three ASS (two splenic ASS and one gastroduodenal steal syndrome) and four normal hepatic arteries. Arteriography was not performed in the remaining three liver transplants and the diagnosis was obtained as follows: In one case surgery was performed due to a concomitant haemoperitoneum and the artery was patent. In another case the DUS detection of simultaneous hepatic artery and portal thrombosis was an indication for immediate surgery, having confirmed the thrombosis. Finally, in one asymptomatic patient, a repeated examination (performed by a staff radiologist) 24 hours later identified arterial blood flow.
The study group included 34 liver transplants that fulfilled the criteria of inclusion: no arterial Doppler signal obtained at intra- and/or extrahepatic levels during the first 8 days after liver grafting and with CEUS performed after the DUS study. The DUS signal was absent in the left hepatic artery in one patient, in the right hepatic artery in four patients, and in both hepatic lobes and the main hepatic artery in 29 patients. A total of 121 ultrasound studies were performed in those patients during the first week after transplant and CEUS was included in 40 evaluations.
No major adverse reactions related to CEUS were reported.
The results are summarized in Table 1.
CEUS: absence of flow
In 11 out of 34 (32.3 %) patients no arterial flow was detected in the CEUS examination (1–8 days after transplant) (Fig. 2a, b). In ten patients an arteriography was performed on the same day, and confirmed the diagnosis of hepatic artery thrombosis (Fig. 2c). The remaining patient underwent urgent surgery without arteriography because the clinical suspicion of hepatic artery thrombosis was high, and was confirmed at surgery. Treatment of arterial thrombosis was performed during surgery with thrombectomy in ten patients. One patient out of the ten failed and had a new liver transplant. In one case, diagnosed on the eighth day after transplantation, percutaneous intra-arterial thrombolysis was successfully performed.
Hepatic artery thrombosis. (a) Doppler colour ultrasound with a low Pulsed Repetition Frequency (PRF) (0.08) demonstrates permeability of the portal vein and absence of hepatic artery signal. (b) Contrast-enhanced ultrasound (CEUS) 13 seconds after contrast injection shows filling of the portal vein but there is no filling of the hepatic artery. (c) Arteriography confirms a complete acute thrombosis of the main hepatic artery
CEUS: patent hepatic artery
In 23 out of 34 (67.6 %) patients, CEUS showed flow in the hepatic artery.
Two of these patients were diagnosed with hepatic artery stenosis or proximal thrombosis based on finding tardus-parvus intrahepatic arterial waveform (RI < 0.55, SAT > 0.08) [14] with very low flow in the post-CEUS DUS study. A high-velocity Doppler signal at the stenosis was not detected in any case. The angiographic study disclosed an anastomotic stenosis in one patient; in the second patient, who had received a right lobe liver transplantation, thrombosis of segmentary intrahepatic arteries with distal recanalization through collaterals was observed.
In 21 patients with a patent hepatic artery, CEUS showed delayed filling of the hepatic artery, simultaneous with the portal vein (Fig. 3a), and the Doppler waveform after CEUS consisted of small systolic peaks without diastolic phase (Fig. 3b). One of these patients had a severe post-transplant haemoperitoneum requiring urgent surgery. Although the origin of the bleeding was not identified, the hepatic artery was found patent at surgery. The DUS performed 2 days after surgery demonstrated a normal arterial flow.
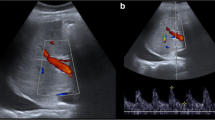
Splenic artery steal syndrome. (a) Contrast-enhanced ultrasound (CEUS) study performed because the Doppler ultrasound was unable to detect hepatic artery flow. The acquisition 25 seconds after contrast injection demonstrates the permeability of the hepatic artery. The filling of the artery is late. (b) Pulsed Doppler ultrasound some minutes after CEUS demonstrates a high resistance waveform in the hepatic artery with absence of diastolic phase
According to our protocol, a daily Doppler study, and CEUS when necessary, was carried out in the remaining 20 patients. In 11 patients, flow returned to normal before the fifth day. In 10 of the 11 patients, the clinical follow-up was uneventful, but one patient had to be re-transplanted for primary graft failure, confirming the permeability of the hepatic artery at surgery. In the remaining nine patients, an arteriography was carried out. In seven of them the alteration of Doppler waveform occurred simultaneously with an elevation of transaminases or worsening of liver function, and in the other two patients the Doppler flow abnormality persisted for more than 5 days. In all nine patients, the angiography showed delayed filling of a patent hepatic artery and parenchymal hypoperfusion of the graft, together with an enlarged splenic artery; in these cases a splenic ASS was suspected. All seven patients with liver dysfunction were treated: five patients by surgical ligation of the splenic artery and two by embolization of the splenic artery using Amplatzer Vascular Plugs. One of the asymptomatic patients was also treated by splenic artery embolization because the Doppler flow was persistently abnormal and the arteriography showed an aneurysm of the splenic artery.
A DUS performed 24–48 hours after treatment showed the normalization of flow in all patients.
The outcome of the non-treated patient was uneventful and Doppler waveform gradually returned to normal (complete normalization was registered 2 weeks after transplantation); therefore the diagnosis of ASS was excluded.
Discussion
Hepatic artery hypoperfusion is a frequent finding in the early post-transplant period. The complexity of splanchnic haemodynamics after liver transplant and the lack of objective imaging criteria led Saad et al. [15] to propose grouping all hepatic artery hypoperfusion situations after transplant under the title of NOHAH: Nonocclusive Hepatic Artery Hypoperfusion Syndrome. Frequently this is caused by a transient increase of the peripheral resistance in the hepatic arterial bed. In cases of extremely high resistance to flow, the Doppler waveform of the hepatic artery shows an absence of the diastolic phase with a diminution of the systolic phase. The systolic phase of the Doppler waveform decreases in height but also in amplitude, so the low velocity flow is only present for a short period within the cardiac cycle, increasing the difficulty of its detection by Doppler and provoking false-positive diagnoses of hepatic artery thrombosis. In the absence of pathology, it returns spontaneously to normality in a few days, making it unnecessary to carry out other diagnostic or therapeutic actions [8, 16].
The accurate differential diagnosis between undetectable Doppler signal with permeable artery or asymptomatic hepatic thrombosis is of paramount importance. A prompt diagnosis is essential because the treatment of thrombosis should be immediate [6, 17, 18].
The present study, which includes a long series of 675 liver transplantations, demonstrates that CEUS improves the detection of arterial flow in the immediate period after transplant. In our study the use of CEUS confirmed the absence of arterial flow in 11 liver grafts. All cases were confirmed by arteriography/surgery without false-positive diagnosis of thrombosis. In 23 (67.6 %) out of the 34 transplants without Doppler arterial signal, CEUS demonstrated presence of flow, allowing us to select those patients who do not need an immediate arteriography or angio-CT. Our findings endorse those reported previously in other studies that included shorter series [13, 19, 20].
In addition to identification of the low flow settings not associated with an arterial complication, CEUS provides relevant data for the diagnosis of non-thrombotic arterial complications. CEUS offers a real time image of the contrast agent in the hepatic artery and its main branches and also increases the Doppler waveform signal, aspects that can be used to re-explore the artery by DUS. In the present study, 10 out of 34 patients without arterial signal in the DUS had an arterial complication other than thrombosis. Two patients with permeability of the hepatic artery demonstrated by CEUS had a very low flow with tardus-parvus waveform in the Doppler signal, which was only detected after contrast administration, thus suggesting stenosis. Arteriography confirmed a proximal thrombosis but distal permeability at intrahepatic level in one of them, and a severe stenosis in the other. Nevertheless, in this patient the stenosis site was not directly detected by CEUS, a finding that has been recently reported by other authors [21].
In nine patients in whom the post-CEUS Doppler waveform disclosed a low flow constituted by small systolic peaks only, an arterial ASS was suspected because the flow did not normalize in a few days or analytic alterations appeared. The ASS consists of a hepatic hypoperfusion caused by diversion of blood flow into a different artery originating from the same trunk, splenic artery (splenic artery steal syndrome), or gastroduodenal artery (gastroduodenal artery steal syndrome). The mechanism leading to ASS is not fully understood [9, 22, 23]. ASS should probably be considered more frequently than is commonly accepted. Until now, its diagnosis has been based only on arteriographic criteria. Nevertheless, we must take into account that although angiography is considered the ultimate technique, the definitive diagnosis of ASS is established when normal flow is achieved after surgical ligation or embolization of the splenic artery [9, 23]. There is little information about DUS and CEUS findings in this syndrome. A high resistance pattern with decreased flow velocities [9, 22–26] or absence of Doppler arterial flow signal in severe cases [9, 27] has been reported. In our series, which includes only recently transplanted livers without arterial Doppler signal in baseline DUS, all ASS diagnosed by arteriography showed a high resistance pattern made up of only small systolic peaks detected only after CEUS. This Doppler waveform type is similar to the one obtained in the transient high resistance flow frequently present in the immediate postoperative period. Zhu et al. [28] described a delayed enhancement of the hepatic artery with a weak contrast peak and a prompt enhancement of the portal vein. In our experience with CEUS, these findings can also be present in patients with transient arterial hypoperfusion, it being impossible to differentiate the ASS from other causes of hypoperfusion following these criteria.
Our findings suggest that although DUS and CEUS findings cannot establish the diagnosis of ASS, they are useful if this syndrome is suspected. A low arterial flow in the immediate postoperative period made up of low systolic peaks without diastolic phase, sometimes only detectable after CEUS that has not improved in a few days, is suspected as indicatingASS, mainly if associated with abnormal liver function tests. These findings merit an arteriographic study to confirm the ASS and to evaluate the possibility of treatment by occlusion of the splenic artery to improve the flow in the hepatic artery [29]. Further studies are needed to establish the benefit of treating asymptomatic patients to prevent biliary tract damage.
It is interesting to point out that in the group of 14 patients in whom CEUS could not be performed, only 64 % of the arteriographies were abnormal, while 100 % of the arteriographies performed in the patients included in the study were abnormal, indicating a better noninvasive management of the patients. The angio-CT is less invasive than arteriography and could be useful for diagnosing thrombosis, but does not allow the diagnosis of ASS. Moreover, CT uses iodinated contrast and delays the diagnosis compared with CEUS, which establishes the diagnosis in the same session as DUS.
Conclusion
The results of this study support the introduction of CEUS in the diagnostic work-up of the hepatic artery in the immediate period after liver transplantation when DUS is unable to detect arterial flow. In this situation, CEUS should be performed during the same session as DUS, even at the patient’s bedside, avoiding unnecessary invasive techniques and saving time, which is crucial if hepatic artery thrombosis is present. Moreover, DUS performed after CEUS in patent hepatic arteries offers functional information that allows differentiating the hepatic artery stenosis from hepatic artery hypoperfusion syndromes. In the latter case, ASS could be suspected if there is persistence of the low arterial flow or association with abnormal liver function test.
Abbreviations
- CEUS:
-
Contrast-enhanced ultrasound
- DUS:
-
Doppler ultrasound
- ASS:
-
Arterial steal syndrome
- RI:
-
Resistive Index
- SAT:
-
Systolic Acceleration Time
References
Bekker J, Ploem S, de Jong KP (2009) Early hepatic artery thrombosis after liver transplantation: a systematic review of the incidence, outcome and risk factors. Am J Transplant 9:746–757
Silva M, Jambulingam PS, Gunson B et al (2006) Hepatic artery thrombosis following orthotopic liver transplantation: a 10-year experience from a single centre in the United Kingdom. Liver Transpl 12:146–151
Warner P, Fusai G, Glantzounis G et al (2011) Risk factors associated with early hepatic artery thrombosis after orthotopic liver transplantation—univariable and multivariable analysis. Transpl Int 24:401–408
Jain A, Costa G, Marsh W et al (2006) Thrombotic and non-thrombotic hepatic artery complications in adults and children following primary liver transplantation with long-term follow-up in 1000 consecutive patients. Transpl Int 19:27–37
Quiroga J, Colina I, Demetris AJ, Starzl TE, Van Thiel DH (1991) Cause and timing of first allograft failure in orthotopic liver transplantation: a study of 177 consecutive liver patients. Hepatology 14:1054–1062
García-Criado A, Gilabert R, Nicolau C et al (2001) Early detection of hepatic artery thrombosis after liver transplantation by Doppler ultrasonography: prognostic implications. J Ultrasound Med 20:51–58
Flint EW, Sumkin JH, Zajko AB, Bowen A (1988) Duplex sonography of hepatic artery thrombosis after liver transplantation. AJR Am J Roentgenol 151:481–483
García-Criado A, Gilabert R, Salmerón JM et al (2003) High resistive index on hepatic artery Doppler in the immediate postoperative period: significance, contributing factors and prognostic implications in liver transplant recipients. AJR 181:831–838
Uflacker R, Selvy JB, Chavin K, Rogers J, Baliga P (2002) Transcatheter splenic artery occlusion for treatment of splenic artery steal syndrome after orthotopic liver transplantation. Cardiovasc Intervent Radiol 25:300–306
Sevmis S, Boyvat F, Aytekin C et al (2006) Arterial steal syndrome after orthotopic liver transplantation. Transplant Proc 38:3651–3655
Hom BK, Shrestha R, Palmer SL et al (2006) Prospective evaluation of vascular complications after liver transplantation: comparison of conventional and microbubble contrast-enhanced US. Radiology 241:267–274
Sidhu PS, Shaw AS, Ellis SM, Karani JB, Ryan SM (2004) Microbubble ultrasound contrast in the assessment of hepatic artery patency following liver transplantation: role in reducing frequency of hepatic artery arteriography. Eur Radiol 14:21–30
Berstad AE, Brabrand K, Foss A (2009) Clinical utility of microbubble contrast-enhanced ultrasound in the diagnosis of hepatic artery occlusion after liver transplantation. Transpl Int 22:654–960
Dodd GD III, Memel DS, Zajko AB, Baron RL, Santaguida LA (1994) Hepatic artery stenosis and thrombosis in transplant recipients: Doppler diagnosis with resistive index and systolic acceleration time. Radiology 192:657–661
Saad W (2012) Nonocclusive hepatic artery hypoperfusion syndrome (splenic steal syndrome) in liver transplant recipients. Semin Interv Radiol 29:140–146
Chen W, Facciuto ME, Rocca JP et al (2006) Doppler ultrasonographic findings on hepatic arterial vasospasm early after liver transplantation. J Ultrasound Med 25:631–638
Sheiner PA, Varma CV, Guarrera JV et al (1997) Selective revascularization of hepatic artery thromboses after liver transplantation improves patient and graft survival. Transplantation 64:1295–1299
Kim HB (2010) Urgent revascularization for hepatic artery thrombosis: maybe good for the few, definitely good for the many. Liver Transpl 16:812–814
Luo Y, Fan YT, Lu Q, Li B, Wen TF, Zhang ZW (2009) CEUS: a new imaging approach for postoperative vascular complications after right-lobe LDLT. World J Gastroenterol 15:3670–3675
Lu Q, Zhong XF, Huang ZX et al (2012) Role of contrast enhanced ultrasound in decision support for diagnosis and treatment of hepatic artery thrombosis after liver transplantation. Eur J Radiol 81:338–343
Zheng R, Mao R, Ren J et al (2010) Contrast-enhanced ultrasound for the evaluation of hepatic artery stenosis after liver transplantation: potential role in changing the clinical algorithm. Liver Transpl 16:729–735
Quintini C, Hirose K, Hashimoto K et al (2008) “Splenic artery steal syndrome” is a misnomer: the cause is portal hyperperfusion, not arterial siphon. Liver Transpl 14:374–379
Nüssler NC, Settmacher U, Haase R, Stange B, Heise M, Neuhaus P (2003) Diagnosis and treatment of arterial steal syndromes in liver transplant recipients. Liver Transpl 9:596–602
Nishida S, Kadono J, DeFaria W et al (2005) Gastroduodenal artery steal syndrome during liver transplantation: intraoperative diagnosis with Doppler ultrasound and management. Traspl Int 18:350–353
Sanyal R, Shah SN (2009) Role of imaging in the management of splenic artery steal syndrome. J Ultrasound Med 28:471–477
Uslu N, Aslan H, Tore HG et al (2012) Doppler ultrasonography findings of splenic arterial steal syndrome after liver transplant. Exp Clin Transplant 10:363–367
García-Criado A, Gilabert R, Bezigotti A, Brú C (2009) Doppler ultrasound findings in the hepatic artery shortly after liver transplantation. AJR 193:128–135
Zhu X, Gao Y, Wang S et al (2012) Contrast-enhanced ultrasound diagnosis of splenic artery steal syndrome after orthotopic liver transplantation. Liver Transpl 18:966–971
Akamatsu N, Sugawara Y, Satou S et al (2014) Hemodynamic changes in the hepatic circulation after the modulation of the splenic circulation in an in vivo human experimental model. Liver Transpl 20:116–121
Acknowledgments
The scientific guarantor of this publication is Ángeles García Criado. The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article. This study has received funding by grant SERAM-INDUSTRIA 2005. No complex statistical methods were necessary for this paper. Institutional Review Board approval was obtained. Written informed consent was obtained from all subjects (patients) in this study. Methodology: prospective, diagnostic or prognostic study, performed at one institution.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
García-Criado, Á., Gilabert, R., Bianchi, L. et al. Impact of contrast-enhanced ultrasound in the study of hepatic artery hypoperfusion shortly after liver transplantation: contribution to the diagnosis of artery steal syndrome. Eur Radiol 25, 196–202 (2015). https://doi.org/10.1007/s00330-014-3377-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-014-3377-5