Abstract
Objectives
The purpose of this study was to determine the optimal cut-off value of lymph node size for diagnosing metastasis in gastric cancer with multidetector-row computed tomography (MDCT) after categorizing perigastric lymph nodes into three regions.
Methods
The study included 90 gastric cancer patients who underwent gastrectomy. The long-axis diameter (LAD) and short-axis diameter (SAD) of all visualized lymph nodes were measured with transverse MDCT images. The locations of lymph nodes were categorized into three regions: lesser curvature, greater curvature, and suprapancreatic. The diagnostic value of lymph node metastasis was assessed with receiver operating characteristic (ROC) analysis.
Results
The area under the curve was larger for SAD than LAD in all groups. The optimal cut-off values of SAD were determined as follows: overall, 9 mm; differentiated type, 9 mm; undifferentiated type, 8 mm; lesser curvature region, 7 mm; greater curvature region, 6 mm; and suprapancreatic region, 9 mm. The diagnostic accuracies for lymph node metastasis using individual cut-off values were 71.1 % based on histological type and 76.6 % based on region of lymph node location.
Conclusions
The diagnostic accuracy of lymph node metastasis in gastric cancer was improved by using individual cut-off values for each lymph node region.
Key points
• Multidetector-row computed tomography is widely used to predict pathological nodal status.
• An optimal cut-off value of lymph node size has not been determined.
• Cut-off values were assessed according to histology and nodal location.
• The optimal cut-off values differed based on histology and nodal location.
• Diagnostic accuracy was improved by using individual cut-off values for each region.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Gastric cancer is a major cause of cancer-related deaths worldwide, and it is the most common cause of cancer-related mortality in eastern Asia [1]. Lymph node metastasis is one of the most important factors affecting the prognosis of gastric cancer [2, 3]. Locally advanced tumours usually require preoperative chemotherapy to improve curative resection rates and long-term survival. In European countries, perioperative chemotherapy using a regimen of epirubicin, cisplatin, and fluorouracil is a standard treatment for localized gastric cancer [4, 5]. The National Comprehensive Cancer Network (NCCN) guidelines state that perioperative chemotherapy or preoperative chemoradiation is the preferred approach for T2 or more advanced gastric cancer [6]. Although accurate staging of lymph node metastasis is desirable for preoperative treatment, the rate of accuracy in detecting lymph node metastasis with conventional diagnostic tools is only around 60 % [7–13]. The use of multidetector-row computed tomography (MDCT) has recently gained wide adoption worldwide, allowing for more detailed imaging with thinner section collimation. However, an optimal cut-off value for lymph node size to diagnose pathological metastasis has not yet been determined. Although some previous studies have used different criteria for long-axis diameter (LAD) or short-axis diameter (SAD), diagnostic accuracies remain around 70 % for T2 or more advanced gastric cancer, even using MDCT [14, 15].
The mean size of benign lymph nodes on MDCT differs according to the specific location of lymph nodes in the abdomen and mediastinum [16–18]. However, no studies have diagnosed lymph node metastasis using different cut-off values based on the location of lymph nodes in gastric cancer patients. Thus, this retrospective study was conducted to assess the diagnostic accuracy of nodal size after categorizing perigastric lymph nodes into three regions.
Materials and methods
Patient population
The present study included 90 gastric cancer patients who underwent gastrectomy between January 2010 and December 2012 at Osaka University Hospital. All tumours were histologically diagnosed as adenocarcinoma of the stomach. Patients who had pathological T1 cancer or who underwent preoperative chemotherapy were excluded. Since patients with T2 or more advanced gastric cancers are candidates for preoperative treatment according to NCCN guidelines [6], we included only gastric cancer of T2 or a more advanced stage in this study. Patients underwent extended lymphadenectomy, either D2 or D2 minus splenic hilum node (station no. 10) dissection, according to the Japanese Gastric Cancer Association treatment guidelines [19]. Pathological tumour depth, nodal status, and surgical curability were classified according to the seventh edition of the International Union Against Cancer (UICC) classification system [20].
Preoperative examination
The MDCT protocol has previously been described in detail [21, 22]. All 90 patients underwent enhanced MDCT after overnight fasting, with an MDCT system (Discovery CT750 HD; GE Healthcare, Milwaukee, WI, USA). Each patient was placed in a prone position on the imaging table to avoid artefacts caused by air in the stomach. Pre-contrast imaging was not performed. A total of 100 mL of non-ionic contrast material (iopromide; Proscope, Tanabe Seiyaku, Osaka, Japan) containing 300 mg of iodine per mL was administered intravenously at 3 mL/second using a power injector (Auto-Enhance A-50; Nemoto Kyorindou, Tokyo, Japan). Imaging was performed 30 seconds and 75 seconds after initiation of contrast material injection, corresponding to the arterial and venous phases. Imaging began at the level of the dome of the right hemidiaphragm and ended at the caudal edge of the stomach, so as to include the entire liver. CT parameters were as follows: 64 detector rows; section thickness, 0.625 mm; pitch, 1.375 mm; reconstruction interval, 0.625 mm; 200 milliamperes; 120 kilovolts; and tube rotation time, 0.4 seconds. Transverse images with a section thickness of 2.5 mm were created using volumetric data obtained during MDCT. A written informed consent for preoperative staging with MDCT was obtained from all patients.
Evaluation
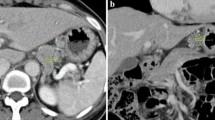
Transverse CT images were reviewed without knowledge of the surgical or histopathological findings of the resected lymph nodes. The mediastinal window settings consisted of a window level (WL) of 60 and a window width (WW) of 300, with standard function. The LAD and SAD of all visualized lymph nodes were measured on MDCT images (Fig. 1). Diameters of less than 5 mm were rounded down to 0 mm in this study. The locations of regional lymph nodes, identified on preoperative MDCT and confirmed at the time of surgery, were recorded based on nodal grouping according to the Japanese Gastric Cancer Association (JGCA) classification system [23]. In addition, this study categorized locations into three regions: the lesser curvature region (Nos. 1, 3, 5, 7), greater curvature region (Nos. 2, 4, 6), and suprapancreatic region (Nos. 8, 9, 10, 11, and 12).
A transverse CT image of a 64-year-old man shows an enlarged lymph node in the lesser curvature region (a). A transverse CT image of a 65-year-old man shows lymph node enlargement in the suprapancreatic region (b). The long-axis diameter (LAD, solid line) and short-axis diameter (SAD, dotted line) of each lymph node were measured, as shown
Statistical analysis
The diagnostic value of lymph node metastasis was assessed by calculating the area under the receiver operating characteristic (ROC) curve, not only for the overall patient population but also for each histological and regional group. The cut-off value was based on the ROC curve with Youden’s index (J), calculated using the equation J = sensitivity + specificity – 1. Statistical analyses were performed using the SPSS statistical package, version 15.0 (SPSS Inc., Chicago, IL, USA).
Results
Overall patient characteristics are shown in Table 1. The median number of dissected lymph nodes was 39, and the pathological node-positive rate was 52 % (47/90). More than half of the patients had differentiated-type tumours.
The mean nodal sizes detected on MDCT were 12 mm for LAD and 8 mm for SAD. Nodes associated with differentiated tumours (mean LAD, 13.6 mm; mean SAD, 8.5 mm) were larger than those associated with undifferentiated tumours (mean LAD, 10.9 mm; mean SAD, 7.4 mm). Regarding lymph node location, the greater curvature region (mean LAD, 6.1 mm; mean SAD, 3.8 mm) had smaller nodes than the region of lesser curvature (mean LAD, 9.6 mm; mean SAD, 6.3 mm) and the suprapancreatic region (mean LAD, 9.4 mm; mean SAD, 5.4 mm).
We analysed the detectability of lymph node metastasis on MDCT with ROC curves (Fig. 2). The area under the curve (AUC) was larger for SAD than LAD in all groups. Based on the ROC curves, the optimal cut-off values of SAD were as follows: overall, 9 mm; differentiated type, 9 mm; undifferentiated type, 8 mm; lesser curvature region, 7 mm; greater curvature region, 6 mm; and suprapancreatic region, 9 mm. With these cut-off values, all parameters – including accuracy, sensitivity, and specificity – were higher for the differentiated type than for the undifferentiated type (Table 2). The three regions showed similar accuracy, but the sensitivity in the suprapancreatic region was much lower than in the lesser curvature or greater curvature regions (Table 2).
In the MDCT diagnosis of clinical N status with a single cut-off value (SAD 9 mm), the overall accuracy, sensitivity, and specificity were 70.6 %, 55.3 %, and 86.0 %, respectively. When we used individual cut-off values according to histological type (SAD 9 mm for the differentiated type, SAD 8 mm for the undifferentiated type), the accuracy (71.1 %) was similar to overall values (Table 3). On the other hand, after categorizing lymph node locations into three regions, accuracy could be increased to 76.6 % with individual cut-off values (SAD 7 mm for the lesser curvature region, SAD 6 mm for the greater curvature region, SAD 9 mm for the suprapancreatic region) (Table 3).
Discussion
The present study showed that SAD was superior to LAD as an indicator for diagnosing lymph node metastasis. This result is in accordance with the revised version (ver.1.1) of the Response Evaluation Criteria in Solid Tumours (RECIST), which adopted SAD as a criterion for lymph node metastasis [24]. Compared with the conventional method using a single cut-off value, diagnostic accuracy was improved by using individual cut-off values for each lymph node region. Specificities were high in all categorized regions, but the sensitivity decreased to 42 % in the suprapancreatic region. Indeed, the size of benign lymph nodes located in the suprapancreatic region is usually larger than those in other regions as identified during gastric cancer surgery. Radiologists as well as gastric surgeons should keep in mind that cut-off values for diagnosing nodal metastasis differ according to the region of lymph node location.
Accurate preoperative staging of regional lymph node metastasis in gastric cancer is very important in planning therapeutic strategies, especially for preoperative chemotherapy. Although there are a number of different criteria and methods for assessing nodal status, no solid criteria exist for appropriately diagnosing metastatic lymph nodes. The definition of metastatic lymph nodes differs among studies using MDCT, and various cut-off values have been applied [14, 15, 25–30]. Ahn et al. defined metastatic lymph nodes as having SAD of ≥8 mm [29], while Chen et al. used a definition of ≥8 mm for LAD [30]. Previous studies have reported diagnostic accuracy of lymph node metastasis in gastric cancer that has varied from 54 % to 84 % [14, 15, 25–35]. When comparing accuracy among studies, differences in eligibility criteria must be considered. Most previous studies have included patients with any stage of gastric cancer. Particularly in Japan and Korea, more than half of patients with gastric cancer have T1 stage (mucosal or submucosal) tumours. If the eligibility criteria include such early-stage cancers, the diagnostic accuracy of lymph node metastasis is usually inflated, because these cancers are associated with a low incidence of lymph node metastasis [14, 15]. Furthermore, since NCCN guidelines indicate preoperative treatment for gastric cancer of stage T2 or greater[6], preoperative diagnosis of N status is more relevant for T2-or-higher tumours than for T1 tumours. As such, we included only T2 or higher-stage gastric cancer in this study. The accuracy (76.6 %), sensitivity (89.4 %), and specificity (62.8 %) in our study were similar or superior to those in previous studies also including early-stage cancer.
This study also showed that the diagnostic accuracy of lymph node metastasis differed between the differentiated and the undifferentiated types. Noda et al. reported that the mean size of metastatic lymph nodes in differentiated-type tumours was significantly larger than in undifferentiated-type tumours [36]. However, no previous study has investigated the influence of differences in metastatic lymph node size on MDCT findings between histological types. Our results, which showed that the cut-off value for the differentiated type was larger than for the undifferentiated type, were consistent with the report of Noda et al. As undifferentiated tumours grow diffusely, tumour invasion does not directly affect the size of metastatic lymph nodes [37]. This may explain why the mean size of metastatic lymph nodes in undifferentiated tumours is smaller than in differentiated tumours. In our study, all diagnostic parameters – including accuracy, sensitivity, and specificity – were higher in the differentiated type than in the undifferentiated type. This implies that high diagnostic accuracy for metastatic lymph nodes can be expected in the differentiated type, while surgeons and radiologists should consider the difficulty in diagnosing nodal metastasis in the undifferentiated type.
At present, the diagnosis of lymph node abnormalities on MDCT is based primarily on size criteria. In addition to size, other CT features of the lymph node, such as an almost circular shape (longitudinal/transverse diameter ratio <1.5), central necrosis, marked or heterogeneous enhancement (>85 HU in the enhanced scan), and clustered nodes regardless of size, can also be used to differentiate positive from negative lymph nodes [15, 30, 38, 39]. Furthermore, multiplanar reformation images, which enable us to measure the longitudinal diameter of lymph nodes, have been reported as superior to transverse images in assessing lymph node metastasis, although some reports were unable to demonstrate the superiority of these images [15, 26, 30]. Regarding other modalities, Kwee and Kwee reviewed the diagnostic accuracy of preoperative N-staging by comparing endoscopic ultrasound (EUS), MDCT, MRI, and PET-CT [40]. In their study, the accuracy of N status diagnosis ranged from 40 % to 90 % for EUS, 54 % to 80 % for MDCT, 50 % to 65 % for MRI, and 55.1 % for PET-CT. Although EUS showed accuracy similar to MDCT, EUS is not objective, and there is some difficulty in evaluating lymph nodes that are located at a greater distance from the gastric wall. MRI does not involve any radiation exposure, but its diagnostic accuracy is low. Low accuracy was also reported in PET-CT studies due to the low sensitivity in detecting lymph node metastases with FDG-PET [41]. Considering the convenience and the objectivity of MDCT, it seems that this is a useful modality for determining firm MDCT criteria in the diagnosis of lymph node metastasis.
One of the limitations of our study was the small number of patients and thus the lack of validation using other datasets. A large-scale study is needed to verify the clinical usefulness of our findings for preoperative N-staging in gastric cancer. Second, we did not evaluate the reproducibility of lymph node size measurement between reviewers, and therefore future studies of reproducibility are desirable. Third, lymph node metastasis was evaluated in each region, not for each lymph node individually, because it is impossible to match the lymph nodes dissected during surgery with those evaluated on MDCT. We believe that matching the regions of dissected lymph nodes with the regions evaluated preoperatively is the most practical way to evaluate the diagnostic accuracy of lymph node metastasis. Furthermore, the most important point for clinical use is the accurate diagnosis of clinical N status, not individual nodal metastasis. Thus, the improved accuracy of clinical N status observed in our study is beneficial for decision-making regarding preoperative treatment for gastric cancer patients.
In conclusion, the optimal cut-off values of lymph node size for diagnosing metastasis differed with histological type and location. The diagnostic accuracy of lymph node metastasis can be improved by using individual cut-off values based on the regions of lymph node location.
Abbreviations
- MDCT:
-
multidetector-row computed tomography
- LAD:
-
long-axis diameter
- SAD:
-
short-axis diameter
- ROC:
-
receiver operating characteristics
- AUC:
-
area under the curve
References
Parkin DM (2004) International variation. Oncogene 23:6329–6340
Adachi Y, Kamakura T, Mori M et al (1994) Prognostic significance of the number of positive lymph nodes in gastric carcinoma. Br J Surg 81:414–416
Fukuda N, Sugiyama Y, Midorikawa A, Mushiake H (2009) Prognostic significance of the metastatic lymph node ratio in gastric cancer patients. World J Surg 33:2378–2382
Cunningham D, Allum WH, Stenning SP et al (2006) Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 355:11–20
Ychou M, Boige V, Pignon J-P et al (2011) Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol 29:1715–1721
Ajani JA, Bentrem DJ, Besh S et al (2013) Gastric cancer, version 2.2013: featured updates to the NCCN Guidelines. J Natl Compr Canc Netw 11:531–546
Triller J, Roder R, Stafford A, Schröder R (1986) CT in advanced gastric carcinoma: is exploratory laparotomy avoidable? Eur J Radiol 6:181–186
Sussman SK, Halvorsen RA Jr, Illescas FF et al (1988) Gastric adenocarcinoma: CT versus surgical staging. Radiology 167:335–340
Botet JF, Lightdale CJ, Zauber AG et al (1991) Preoperative staging of gastric cancer: comparison of endoscopic US and dynamic CT. Radiology 181:426–432
Ziegler K, Sanft C, Zimmer T et al (1993) Comparison of computed tomography, endosonography, and intraoperative assessment in TN staging of gastric carcinoma. Gut 34:604–610
Cho JS, Kim JK, Rho SM et al (1994) Preoperative assessment of gastric carcinoma: value of two-phase dynamic CT with mechanical iv. injection of contrast material. Am J Roentgenol 163:69–75
Sohn KM, Lee JM, Lee SY et al (2000) Comparing MR imaging and CT in the staging of gastric carcinoma. Am J Roentgenol 174:1551–1557
Habermann CR, Weiss F, Riecken R et al (2004) Preoperative staging of gastric adenocarcinoma: comparison of helical CT and endoscopic US. Radiology 230:465–471
Yan C, Zhu Z-G, Yan M et al (2009) Value of multidetector-row computed tomography in the preoperative T and N staging of gastric carcinoma: a large-scale Chinese study. J Surg Oncol 100:205–214
Kim YN, Choi D, Kim SH et al (2009) Gastric cancer staging at isotropic MDCT including coronal and sagittal MPR images: endoscopically diagnosed early vs. advanced gastric cancer. Abdom Imaging 34:26–34
Dorfman RE, Alpern MB, Gross BH, Sandler MA (1991) Upper abdominal lymph nodes: criteria for normal size determined with CT. Radiology 180:319–322
Glazer GM, Gross BH, Quint LE et al (1985) Normal mediastinal lymph nodes: number and size according to American Thoracic Society mapping. Am J Roentgenol 144:261–265
Glazer GM, Orringer MB, Gross BH, Quint LE (1984) The mediastinum in non-small cell lung cancer: CT-surgical correlation. Am J Roentgenol 142:1101–1105
Japanese Gastric Cancer Association (2011) Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer 14:113–123
Sobin LH, Gospodarowicz MK, Wittekind, C (eds) (2009) Wiley-Blackwell: TNM Classification of Malignant Tumours, 7th Edition.
Kumano S, Murakami T, Kim T et al (2005) T staging of gastric cancer: role of multi-detector row CT. Radiology 237:961–966
Makino T, Fujiwara Y, Takiguchi S et al (2011) Preoperative T staging of gastric cancer by multi-detector row computed tomography. Surgery 149:672–679
Japanese Gastric Cancer Association (2011) Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer 14:101–112
Eisenhauer EA, Therasse P, Bogaerts J et al (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45:228–247
Kawaguchi T, Komatsu S, Ichikawa D et al (2012) Nodal counts on MDCT as a surrogate marker for surgical curability in gastric cancer. Ann Surg Oncol 19:2465–2470
Kim HJ, Kim AY, Oh ST et al (2005) Gastric cancer staging at multi-detector row CT gastrography: comparison of transverse and volumetric CT scanning. Radiology 236:879–885
Yang DM, Kim HC, Jin W et al (2007) 64 multidetector-row computed tomography for preoperative evaluation of gastric cancer: histological correlation. J Comput Assist Tomogr 31:98–103
Hasegawa S, Yoshikawa T, Shirai J et al (2013) A prospective validation study to diagnose serosal invasion and nodal metastases of gastric cancer by multidetector-row CT. Ann Surg Oncol 20:2016–2022
Ahn HS, Lee H-J, Yoo M-W et al (2009) Diagnostic accuracy of T and N stages with endoscopy, stomach protocol CT, and endoscopic ultrasonography in early gastric cancer. J Surg Oncol 99:20–27
Chen C-Y, Hsu J-S, Wu D-C et al (2007) Gastric cancer: preoperative local staging with 3D multi-detector row CT–correlation with surgical and histopathologic results. Radiology 242:472–482
Chamadol N, Wongwiwatchai J, Bhudhisawasd V, Pairojkul C (2008) Accuracy of spiral CT in preoperative staging of gastric carcinoma: correlation with surgical and pathological findings. J Med Assoc Thai 91:356–363
Yang Q-M, Kawamura T, Itoh H et al (2008) Is PET-CT suitable for predicting lymph node status for gastric cancer? Hepatogastroenterology 55:782–785
Chen B-B, Liang P-C, Liu K-L et al (2007) Preoperative diagnosis of gastric tumors by three-dimensional multidetector row ct and double contrast barium meal study: correlation with surgical and histologic results. J Formos Med Assoc 106:943–952
Bhandari S, Shim CS, Kim JH et al (2004) Usefulness of three-dimensional, multidetector row CT (virtual gastroscopy and multiplanar reconstruction) in the evaluation of gastric cancer: a comparison with conventional endoscopy, EUS, and histopathology. Gastrointest Endosc 59:619–626
Stabile Ianora AA, Pedote P, Scardapane A et al (2003) Preoperative staging of gastric carcinoma with multidetector spiral CT. Radiol Med 106:467–480
Noda N, Sasako M, Yamaguchi N, Nakanishi Y (1998) Ignoring small lymph nodes can be a major cause of staging error in gastric cancer. Br J Surg 85:831–834
Hamada S, Akahoshi K, Chijiiwa Y et al (1997) Relationship between histological type and endosonographic detection of regional lymph node metastases in gastric cancer. Br J Radiol 70:697–702
Fukuya T, Honda H, Hayashi T et al (1995) Lymph-node metastases: efficacy for detection with helical CT in patients with gastric cancer. Radiology 197:705–711
D’Elia F, Zingarelli A, Palli D, Grani M (2000) Hydro-dynamic CT preoperative staging of gastric cancer: correlation with pathological findings. A prospective study of 107 cases. Eur Radiol 10:1877–1885
Kwee RM, Kwee TC (2009) Imaging in assessing lymph node status in gastric cancer. Gastric Cancer 12:6–22
Shimada H, Okazumi S, Koyama M, Murakami K (2011) Japanese Gastric Cancer Association Task Force for Research Promotion: clinical utility of 18 F-fluoro-2-deoxyglucose positron emission tomography in gastric cancer. A systematic review of the literature. Gastric Cancer 14:13–21
Acknowledgements
The scientific guarantor of this publication is Yuichiro Doki. The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article. The authors state that this work has not received any funding. No complex statistical methods were necessary for this paper. Institutional Review Board approval and written informed consent were not required because this is a retrospective diagnostic study.. A written informed consent for preoperative staging with MDCT was obtained from all patients. Methodology: retrospective diagnostic or prognostic study, performed at one institution
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Saito, T., Kurokawa, Y., Takiguchi, S. et al. Accuracy of multidetector-row CT in diagnosing lymph node metastasis in patients with gastric cancer. Eur Radiol 25, 368–374 (2015). https://doi.org/10.1007/s00330-014-3373-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-014-3373-9