Abstract
Objectives
To compare image quality and radiation dose of pre-transcatheter aortic valve implantation (TAVI) aortoiliofemoral CT angiography (AICTA) provided by standard vs. dual-energy mode with reduced iodine load protocols.
Methods
One hundred and sixty-one patients underwent a two-step CTA protocol before TAVI including cardiac CTA with injection of 65 mL of iodinated contrast agent (ICA), immediately followed by AICTA. From this second acquisition, the following three different patient groups were identified: Group 1: 52 patients with standard AICTA (60 mL ICA, 100 kVp, mA automodulation); Group 2: 48 patients with dual-energy AICTA with 50 % iodine load reduction (30 mL ICA, fast kVp switching, 600 mA); Group 3: 61 patients with an identical protocol to Group 2, but exposed to 375 mA. The qualitative/subjective image quality (13-point score) and quantitative/objective image quality (contrast attenuation and image noise) were evaluated. The radiation dose was recorded.
Results
There was no significant difference in non-diagnostic images between the three protocols. Contrast attenuation, signal-to-noise ratio and contrast-to-noise ratio were significantly higher, whereas noise was significantly lower in the standard protocol (all P < 0.05). The radiation dose was lower in the dual-energy protocol at 375 mA (P < 0.05).
Conclusions
Dual-energy AICTA before TAVI results in a reduction of iodine load while maintaining sufficient diagnostic information despite increased noise.
Key Points
• Dual-energy AICTA before TAVI results in a 50 % reduction of iodine load.
•The reduction of iodine load maintains sufficient image quality despite increased noise.
• Using 375 mA in dual-energy mode results in a reduction of radiation dose.
• A high tube current setting (600 mA) should be used in overweight patients.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Aortic stenosis is the most frequent valvular disease, and severe aortic stenosis is highly prevalent in the elderly [1]. Surgical aortic valve replacement is the gold standard, but transcatheter aortic valve implantation (TAVI) is an alternative in this high-risk population [2–4] because approximately one-third of patients either have contraindications for surgery or decline it [5]. Although many approaches are possible for TAVI, the transfemoral approach is the most frequently used. For this reason, a high resolution and dynamic anatomical review must be performed prior to the procedure, based on echocardiography, computed tomography angiography (CTA) and conventional angiography.
Prior to TAVI, CTA would be helpful in providing information about the aortic root anatomy, cardiac structures [6–10] and aortoiliofemoral anatomy (tortuosity, calcifications/atherosclerosis and diameters) [9–11]. This comprehensive evaluation will determine the feasibility of the procedure, size of the prosthetic valve and the best percutaneous access. As retrospectively gated cardiac CTA is preferable for accurate evaluation of the aortic root, aortoiliofemoral CTA (AICTA) is generally not performed during the same acquisition with limited detector-width scanners (the majority of current equipment); therefore, it is recommended that a two-step examination be performed [9, 10], including a cardiac CTA followed by an AICTA. Reducing the amount of iodinated contrast agents (ICAs) is important in elderly patients with frequently impaired renal function [12, 13]; this step can be achieved with dual-energy CT using virtual monochromatic images [14, 15]. Previous studies focused on chest CT angiography, demonstrated that up to 50 % of the iodine load could using dual-energy CT [16], and stated that optimal vascular analysis was obtained from 60 keV reconstructions [17].
The purpose of this study was to compare the image quality (qualitative and quantitative criteria) and radiation dose of pre-TAVI AICTA provided by standard vs. dual-energy mode with reduced iodine load protocols.
Methods
The institutional review board approved this retrospective study, and informed consent was waived. The equipment is commercially available, and none of the authors received financial or technical support.
Patient population, CT protocols, and image reconstruction
Between August 2011 and February 2013, 191 patients with severe aortic stenosis (clinical and echocardiographic diagnosis) and contraindications for surgery were referred for CTA evaluation prior to TAVI. All examinations were performed using the Discovery 750HD MDCT (General Electric Healthcare, Milwaukee, WI, USA). The same ICA (Iohexol 350 mg/mL) was administered in all patients after placing an 18-gauge peripheral venous access catheter in the antecubital vein.
A two-step examination was performed as follows:
-
First, all patients underwent a retrospectively electrocardiogram (ECG)-gated standard cardiac CTA after pulsed injection of 65 mL of ICA. This part of the examination was not considered in this study.
-
Within 2 min, AICTA was performed with a reinjection of ICA. A bolus tracking was used to determine the optimal timing (region of interest drawn on the proximal abdominal aorta, with a trigger set to begin at 150 HU).
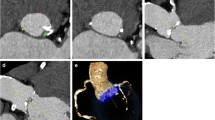
Three successive groups were identified according to the AICTA protocol that evolved over time with scientific knowledge and technological development (Fig. 1):
The TAVI CT planning protocol evolving over time. TAVI CT planning consists of the following two-step exam: a retrospective gated cardiac CTA after injection of ICA and an AICTA after a second injection of ICA. As cardiac CT protocol (blue rectangle) did not differ during this time, the three groups differed based on the aortoiliofemoral CTA protocol (green, yellow, and orange rectangles)
-
1.
Standard Group: During the first period (August 2011 to December 2011), the AICTA was performed with 60 mL of ICA (flow rate =4 mL/s) using a polychromatic standard protocol: 100 kilovolt peak (kVp); milliamperes (mA) automodulation; Rotation time: 0.7 s; Pitch: 0.984; Collimation: 0.625 mm; Reconstruction: 60 % Filtered back projection (FBP)/40 % Adaptative Statistical Iterative Reconstruction (ASIR); Filter: Standard (GE®), slice thickness: 0.625 mm.
-
2.
Spectral 600 (S600) Group: During the second period (December 2011 to August 2012), AICTA was performed after injection of 30 mL of ICA (flow rate = 3 mL/s) using a dual-energy mode at 600 mA (mA were not modifiable at this time) [16]. Fast switching: 80/140 kVp; Rotation time: 0.6 s; Pitch: 0.984; Collimation: 0.625 mm; Reconstruction: 60 % FBP/40 % ASIR; Filter: Standard (GE®), slice thickness: 0.625 mm.
-
3.
Spectral 375 (S375) Group: During the third period (From August 2012 to February 2013), a technical upgrade allowed a decrease of the tube current in the dual-energy mode, thereby reducing radiation dose as recommended by the guidelines [18, 19]. Then, AICTA was performed using the same technical and injection parameters as for S600 group patients, to the exception of the tube current set at 375 mA.
Images from the S600 and S375 groups were reconstructed with dedicated software (Gemstone Spectral Imaging GSI®, GE Healthcare) at a monochromatic energy of 60 keV, as suggested by recent scientific knowledge [17].
Patients were excluded from the analysis if:
-
1.
They did not receive the strict amount of ICA for either cardiac CTA (65 mL in all groups) or AICTA (60 mL in standard group and 30 mL in S600 and S375 groups). Three patients with body mass indices (BMIs) >35 were prescribed greater volumes of ICA. In 14 patients, the ICA volume could not be retrieved from the medical records. Finally, four patients with history of allergy received another ICA.
-
2.
They did not receive ICA due to renal failure (creatinine clearance <30 mL/min: nine patients).
Finally, 161 patients could be included and analysed, including 52 patients in the standard group, 48 in the S600 group, and 61 in the S375 group.
Data analysis
All examinations were retrieved from the Picture Archiving and Communication system (PACS). All data were anonymized, and information that is usually accessible to radiologists was deleted so that the readers could not know which acquisition had been performed. Images were analysed with dedicated vascular reconstruction software (AW 4.4-4.5, Advantage WorkStation, GE Healthcare). Two readers with 4 and 8 years of experience in cardiovascular imaging performed the analyses of all qualitative and quantitative parameters in consensus. A third reader with 15 years of experience in cardiovascular imaging served as a referee in case of discrepancy.
Quantitative analysis of image quality
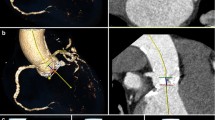
The method is illustrated in Fig. 2. Contrast attenuation (CA) (mean CT numbers in Hounsfield units, HU) and noise (standard deviation of CT numbers in HU) were drawn from a circular region of interest (ROI) in the following seven arterial segments: right and left common femoral arteries, right and left external iliac arteries, right and left common iliac arteries, and the infrarenal aorta. Mean CA and mean noise levels from the seven arterial segments were calculated in all patients. The CA values of the left psoas major muscle and air noise were also measured. The ROI surface varied from 0.35 to 1 cm2 as a function of vessel diameters.
Aortoiliofemoral CTA performed in dual-energy mode (375 mA tube current). Axial views were reconstructed at 60 keV. Measurements were performed using regions of interest (ROIs). ROIs were placed in the right and left common femoral arteries (a), right and left external iliac arteries (b), right and left common iliac arteries (c), infrarenal aorta (d), left major psoas muscle (e), and in the air (f). The results are expressed as ROI surfaces (=mm2), mean CT numbers in Hounsfield units (HU) (=Av) and standard deviations of CT numbers in HU (=Std)
Signal-to-noise ratio (SNR) and contrast-to-noise ratio (CNR) were calculated as follows:
-
SNR = mean arterial CA/mean arterial noise
-
CNR = (mean arterial CA − CA of left psoas major)/mean arterial noise
Qualitative analysis of image quality
The level of valid information for diagnosis was expressed as a score ranging from 0 to 13 (0 = non-interpretable examination to 13 = optimal quality). This score was the sum of four qualitative scales (Figs. 3, 4) including the following:
Quantitative score ranging from 0 to 13 points representing the level of valuable diagnostic information. This score is the sum of the following four qualitative scales: image noise, artefacts on vascular walls, quality of arterial enhancement in each of the seven vascular segments (the mean of the seven segments is used for the final score), and diagnostic confidence
Quantitative scoring in four patients (numbered 1 – 4). Axial views centred on inter renal aorta (a) and iliac bifurcation (b). Acquisition protocol is displayed in the right coloured column. Images from dual-energy protocols were reconstructed at 60 keV. The same level (300 Hounsfield units [HU]) and width (850 HU) were used for all four patients. • Patient 1 (Standard protocol): Minimal noise (4/4), no artefact on vascular walls (3/3), optimal arterial enhancement (3/3). Measurements of aorta (a) and iliac arteries (b) were feasible (diagnostic confidence 3/3). Overall score was 13/13 (optimal examination). • Patient 2 (S600 protocol): slight noise (3/4), minor artefacts on vascular walls (2/3), optimal arterial enhancement (3/3). Measurements and vascular anatomy were fully evaluated (diagnostic confidence 3/3). Overall score was 11/13. • Patient 3 (S375 protocol): Noisy mages remaining sufficient for diagnosis (2/4), minor artefacts on vascular walls (2/3), fair arterial enhancement (2/3), measurements and vascular anatomy fully evaluated (diagnostic confidence 3/3). Overall score 9/13. • Patient 4 (S375 protocol): Noisy images, but diagnosis remained possible at aorta level (1/4), artefacts on vascular walls (2/3), poor arterial enhancement (1/3). Measurements were only possible at aortic level (a) (diagnostic confidence 1/3). Final score was 5/13. This examination was considered non-diagnostic with overall score < 7.
-
1.
Image noise, range 0 – 4.
-
2.
Artefacts on vascular walls, range 0 – 3.
-
3.
Quality of arterial enhancement in the seven arterial segments described above, range 0 – 3. The mean value of this criterion was used for calculating the overall score.
-
4.
Diagnostic confidence range 0 – 3 as follows:
-
0
poor, non diagnostic examination due to excessive noise and/or poor arterial enhancement;
-
1
confident for limited situations such as large calcifications with severe stenosis, but limited for smaller lesions in smaller arterial segments (diameter <10 mm);
-
2
probably confident, measurements and anatomy are fully evaluated, but image is slightly noisy and/or there are minor blurring artefacts on vascular walls and/or arterial segments are underestimated because of metallic artefacts;
-
3
completely confident, arterial measurements and anatomy fully evaluated;
-
0
This score was adapted from previous point scale criteria used by Singh et al. [20] and Loewe et al. [21]. Images were considered available for diagnosis if each of the four criteria was greater than or equal to the median, hence, if the final score was greater than or equal to the median, i.e., ≥ 7. By extension, images were considered nondiagnostic if the final score was lower than the median, i.e., < 7.
Radiation dose
For each AICTA, the CT dose index (CTDIvol) and the dose-length product (DLP) were recorded.
Clinical data
Age, sex, body mass index (BMI) and abdominal perimeter were retrieved in medical records.
Patients presenting with either vascular (femoral and/or iliac stenosis, excessive calcifications, arterial dissection, abdominal aorta aneurysm) or extravascular contraindication (cardiac or concurrent pathologies, refusal) were recorded. Also, the number of patients eligible for TAVI after CT planning was recorded in each group. Finally, vascular complications occurring during the procedure (including haematoma or fistulae, femoral and/or iliac dissection or rupture, and systemic ischemia) were recorded.
Statistical analysis
Analyses were performed using dedicated statistical software (IBM SPSS Statistics 20, Armonk, NY, USA)
The primary endpoint was the level of valid information for diagnosis, expressed by the qualitative score ranging from 0 to 13. The significance of the difference in the number of images with scores < 7 between the three groups was then assessed using the Fisher-Freeman-Halton test. Score comparison between the three groups was realized using the Kruskal-Wallis test, and pairwise comparison using Wilcoxon’s test for independent samples. A 95 % confidence interval (CI) for the difference between each time point in the two groups was established using the method of Hodges and Lehmann [22].
Information on patients’ characteristics and quantitative criteria were gathered for all included patients. The Kruskal-Wallis test was used to assess simultaneously whether the three groups differed at AICTA, and pairwise comparisons were performed using Wilcoxon’s test for independent samples. For other clinical data, the Fisher-Freeman-Halton test was employed.
A p value less than 0.05 was considered to be statistically significant.
Results
Patient characteristics (Table 1)
There were no significant differences in sex composition, age, BMI, and abdominal perimeter between the three groups (all p > 0.05). There were no significant differences in vascular and extra-vascular contraindications or in the number of patients eligible for TAVI between the three groups (all p > 0.05). There was no significant difference in the number of patients with vascular complications during TAVI between the three groups (all p > 0.05).
Radiation dose (Table 1)
Radiation dose (CTDIvol and DLP) was significantly different between the three groups (p < 0.001). The S375 group radiation dose was significantly lower, and the S600 radiation dose was significantly higher than in the other groups (all p < 0.001).
Qualitative results (Table 2)
The non-diagnostic examination rates (score <7/13) were similar between the three patient groups (p = 0.334). No examination had a score <7 in the standard and S600 groups, whereas two examinations had a score <7 in the S375 group, involving patients with BMI >30 in both cases. There was a significant difference among the scores of the three groups (p < 0.001). Pairwise comparison revealed the following: (1) a significantly lower score in the S600 group compared to the standard group (p < 0.001) with a median 1- to 1.85-point loss [95 %CI = (1;1.85)]; (2) a significantly lower score in the S375 group compared to the standard group (p < 0.001) with a median 1- to 2-point loss [95 %CI = (1;2)]; and (3) a significantly lower score in the S375 group compared to the S600 group (p = 0.023) with a median 0- to 1-point loss [95 %CI = (0;1)].
The separate comparison of each criterion showed significant differences in noise, vascular wall artefacts and quality of arterial enhancement (all p < 0.05), but no significant difference in diagnostic confidence (p = 0.052) among the three groups.
Quantitative results
Mean vascular CA and noise, air noise, CNR, and SNR were significantly different between the three groups (all p < 0.05). Pairwise comparisons revealed significantly higher mean CA, SNR, and CNR and lower mean noise in the standard group than in the other two groups (all p <0.05). There was no significant difference in air noise between the S600 and standard groups, but air noise was significantly higher in S375 group than in the other two groups (both p < 0.02).
Comparison between the S375 and S600 groups revealed no significant differences in mean CA and noise, SNR, and CNR (all p > 0.05). A subgroup analysis including only overweight and obese patients (BMI >25) (Table 3) revealed significantly higher SNR and CNR, and lower mean noise in the S600 group compared to the S375 group (all p <0.05).
Discussion
In single-source dual-energy CT, two different energy beams are emitted from a fast-switching kilovoltage x-ray tube [80- and 140-kilovolt peak (kVp)], allowing differentiation of various structures within a voxel. This technique generates virtual monochromatic images ranging from 40 to 140 kiloelectron volts (keV). The absorption of iodine, which has an absorption peak (or k edge) of 33 keV, increases with the decrease in beam energy [15].
In the context of TAVI, our results confirmed that single-source dual-energy technology allowed a 50 % reduction of the iodine load for AICTA when compared to the standard protocol.
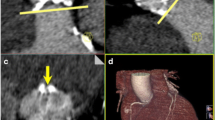
Image quality was qualitatively and quantitatively impaired in dual-energy protocols compared with the standard protocol, mainly due to increased noise. However, image quality remained sufficient for diagnosis, as only two examinations were considered non-diagnostic at 375 mA. Moreover, when decomposing the qualitative score, noise and artefacts were significantly higher and arterial enhancement was significantly lower in dual-energy groups while overall diagnostic confidence was comparable among the three groups. These observations confirmed the results of prior studies [16, 17], but unlike those studies, we noted significantly higher iodine attenuation with the standard protocol compared to dual-energy ones. Yuan et al. [16] used a 50 keV energy, thus increasing the iodine absorption and attenuation. Delesalle et al. [17], with dual-source equipment, did not record any difference in attenuation at 60 keV compared to the standard protocol. The lower amount of iodine administered in our study could explain this difference. We used 30 mL of 350 mg/mL ICA (i.e., 10.5 g of iodine), whereas Delesalle et al. administered 84 ml of 170 mg/mL ICA (Iohexol 240 diluted with 36 mL of saline, i.e., 14.2 g of iodine). The decreased attenuation in our study could have been compensated by a further reduction of the energy of the monochromatic beam, but this choice would have resulted in an increased noise, proving the difficulty of choosing an appropriate energy level (Fig. 5). As previously published [17] and based on our previous experience, we have been using 60 keV to optimize the SNR and performance of vessel analysis software.
Aortoiliofemoral CTA in dual-energy mode (375 mA tube current). An axial view in a monochromatic reconstruction at 60 KeV with ROIs placed in the infrarenal aorta and in the left major psoas muscle (a). Corresponding CT number curves in Hounsfield units (HU) as a function of keV (b). Iodine absorption increases with decreasing keV level, whereas water absorption (left major psoas muscle) varies very slightly with keV. These properties lead to an increase in contrast between the two structures when the keV level is decreased and allow a more accurate diagnosis. Note the increased noise proportional to the decrease in keV represented by standard deviation in vertical bars on either side of the curves
To our knowledge, no prior study has compared CTA results in dual-energy mode according to the tube current. Comparison between S375 and S600 groups showed no significant difference in the number of scores <7/13, mean vessel contrast attenuation, mean vessel noise, air noise, SNR, or CNR. The radiation dose in the S375 group was significantly lower, thereby raising the question of the usefulness of a 600 mA tube current in this clinical setting. However, the quantitative score was significantly lower and air noise was significantly higher in the S375 group. Furthermore, the two examinations considered unacceptable for diagnosis in the S375 group and involved two obese patients (with BMIs of 31 and 33). A subgroup analysis limited to overweight and obese patients (BMI >25) revealed significantly higher qualitative score, SNR and CNR, and lower mean noise in the S600 group compared to the S375 group, proving the necessity of using a higher tube current in obese patients when reducing the iodine load in dual-energy mode . As the radiation dose in the S600 group was significantly higher than in the standard group, the balance between radiation exposure and contrast-induced nephropathy (CIN) prevention must be weighted before using dual-energy mode at 600 mA.
Preventing CIN remains a major challenge in TAVI because this procedure involves elderly patients with frequently impaired renal function [12, 23, 24], and TAVI-related procedures generate various examinations requiring intravenous and intra-arterial ICA injections [25]. Thus far, reducing the iodine load during CTA has been studied using two methods that could increase the contrast of iodine. In standard polychromatic CT, reducing the tube voltage to 80 kVp provided acceptable results, but also exhibited limitations in investigating overweight or large abdominal perimeter patient [26–28]. The second possibility is to decrease the virtual monochromatic beam energy in dual-energy mode [16, 17] such as in our study. In dual-energy mode, various monochromatic reconstructions can be obtained allowing the joint study of non-vascular structures. Matsumoto et al. demonstrated significant lower noise and higher CNR in the dual-energy mode with image reconstruction at 70 keV than at 120 kVp with the standard polychromatic protocol [29].
In the context of TAVI planning, several strategies have been described to reduce iodine load. In two recent studies [30, 31], cardiac-specific acquisition was performed during a thoraco-abdomino-pelvic volume acquisition using a high-pitch spiral, allowing a single-step examination with a single injection of ICA. However, this cardiac acquisition was prospectively ECG-gated, thereby compromising the dynamic study of the aortic valve and annulus. Taking benefit of the cardiac bolus injection for imaging abdominal aorta could be an option, but it carries the risk of suboptimal arterial enhancement. Two studies [32, 33] reported direct aortic injection with low ICA volume. These recent publications illustrate the strong need for reducing iodine load in TAVI CT planning. In our study, we have performed AICTA with only 30 mL of ICA obtaining acceptable images for diagnosis. As dual-energy mode will soon be applicable to ECG-gated CT, a further reduction in iodine load should be possible in the near future.
This study had several limitations. First, the study was retrospective and monocentric. Second, the AICTA was performed within 2 min of the first ICA injection for the first cardiac CT, and recirculation phenomenon could increase the attenuation, thus compensating for the reduced iodine load. However, the same first cardiac CT protocol and injection (65 mL, 350 mg/ml) was performed in the three groups to reduce this bias. Third, we did not study the effects of reduced iodine load on extravascular structures. Fourth, we could not evaluate the impact of reduced iodine load on renal function. Fifth, we have not compared the three protocols with a reduced iodine load protocol using a standard polychromatic acquisition at 80 kVp. However, this technique was previously shown as limited in high BMI patients. Considering that our TAVI population included many overweight patients, we found it was not reasonable to test this option. Finally, the two on-site readers could have favourably estimated the images, compared to other radiologists. However, analysis was fully blinded to the acquisition mode, and only the two examinations considered as non-diagnostic in the S375 group had also been estimated as non-interpretable by the radiologist in charge of the initial examination. Furthermore, there was neither significant difference in the number of patients presenting with a vascular contraindication to TAVI, nor in the number of vascular complications during the procedure between the three groups, demonstrating the absence of adverse clinical impact of dual-energy CT on TAVI scheduling and procedure.
Conclusions
Dual-energy AICTA prior to TAVI allows a 50 % reduction of the iodine load, resulting in image quality that remains sufficient for diagnosis, despite image impairment due to increased noise. Using 375 mA in the dual-energy mode for the tube current results in a significant reduction of the radiation dose compared to the standard polychromatic protocol, but these settings should not be used in overweight patients. The radiation dose is significantly higher with a 600 mA tube current compared to the standard protocol, and the balance between radiation exposure and CIN prevention needs to be weighted before opting for this mode.
Abbreviations
- AICTA:
-
Aortoiliofemoral CT angiography.
- BMI:
-
Body Mass Index.
- CA:
-
Contrast Attenuation.
- CNR:
-
Contrast to Noise Ratio.
- CTA:
-
CT Angiography
- CTDIvol :
-
CT Dose Index.
- DLP:
-
Dose Length Product.
- ICA:
-
Iodinated Contrast Agent.
- keV:
-
Kiloelectron Volts.
- kVp:
-
Kilovolt Peak.
- PACS:
-
Picture Archiving and Communication system.
- SNR:
-
Signal to Noise Ratio.
- TAVI:
-
Transcatheter Aortic Valve Implantation.
References
Iung B, Baron G, Butchart EG et al (2003) A prospective survey of patients with valvular heart disease in Europe: The Euro Heart Survey on Valvular Heart Disease. Eur Heart J 24:1231–1243
Cribier A, Eltchaninoff H, Bash A et al (2002) Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: first human case description. Circulation 106:3006–3008
Cribier A, Eltchaninoff H, Tron C et al (2006) Treatment of calcific aortic stenosis with the percutaneous heart valve: mid-term follow-up from the initial feasibility studies: the French experience. J Am Coll Cardiol 47:1214–1223
Gilard M, Eltchaninoff H, Iung B et al (2012) Registry of transcatheter aortic-valve implantation in high-risk patients. N Engl J Med 366:1705–1715
Iung B, Baron G, Tornos P, Gohlke-Bärwolf C, Butchart EG, Vahanian A (2007) Valvular heart disease in the community: a European experience. Curr Probl Cardiol 32:609–661
Leipsic J, Gurvitch R, Labounty TM et al (2011) Multidetector computed tomography in transcatheter aortic valve implantation. JACC Cardiovasc Imaging 4:416–429
Tops LF, Wood DA, Delgado V et al (2008) Noninvasive evaluation of the aortic root with multislice computed tomography implications for transcatheter aortic valve replacement. JACC Cardiovasc Imaging 1:321–330
Wood DA, Tops LF, Mayo JR et al (2009) Role of multislice computed tomography in transcatheter aortic valve replacement. Am J Cardiol 103:1295–1301
Holmes DR, Mack MJ, Kaul S et al (2012) 2012 ACCF/AATS/SCAI/STS Expert Consensus Document on Transcatheter Aortic Valve Replacement. J Am Coll Cardiol 59:1200–1254
Achenbach S, Delgado V, Hausleiter J, Schoenhagen P, Min JK, Leipsic JA (2012) SCCT expert consensus document on computed tomography imaging before transcatheter aortic valve implantation (TAVI)/transcatheter aortic valve replacement (TAVR). J Cardiovasc Comput Tomogr 6:366–380
Holmes DR Jr, Mack MJ (2011) Transcatheter valve therapy: a professional society overview from the American College of Cardiology Foundation and the Society of Thoracic Surgeons. Ann Thorac Surg 92:380–389
American College of Radiology Manual on Contrast Media Version 9. Available from: http://www.acr.org/~/media/ACR/Documents/PDF/QualitySafety/Resources/Contrast%20Manual/2013_Contrast_Media.pdf. Published 2013
Thomsen HS (2007) European Society of Urogenital Radiology guidelines on contrast media application. Curr Opin Urol 17:70–76
Coursey CA, Nelson RC, Boll DT et al (2010) Dual-Energy Multidetector CT: How Does It Work, What Can It Tell Us, and When Can We Use It in Abdominopelvic Imaging? Radiographics 30:1037–1055
Kalva SP, Sahani DV, Hahn PF, Saini S (2006) Using the K-edge to improve contrast conspicuity and to lower radiation dose with a 16-MDCT: a phantom and human study. J Comput Assist Tomogr 30:391–397
Yuan R, Shuman WP, Earls JP et al (2012) Reduced iodine load at CT pulmonary angiography with dual-energy monochromatic imaging: comparison with standard CT pulmonary angiography–a prospective randomized trial. Radiology 262:290–297
Delesalle M-A, Pontana F, Duhamel A et al (2013) Spectral Optimization of Chest CT Angiography with Reduced Iodine Load: Experience in 80 Patients Evaluated with Dual-Source, Dual-Energy CT. Radiology 267:256–266
The 2007 Recommendations of the International Commission on Radiological Protection. Available from http://nuclear.com/archive/2012/12/04/ML12338A682.pdf
Authors on behalf of the ICRP, Cousins C, Miller DL, Bernardi G et al (2013) ICRP PUBLICATION 120: Radiological Protection in Cardiology. Ann ICRP 42:1–125
Singh S, Kalra MK, Gilman MD et al (2011) Adaptive statistical iterative reconstruction technique for radiation dose reduction in chest CT: a pilot study. Radiology 259:565–573
Loewe C, Becker CR, Berletti R et al (2010) 64-Slice CT angiography of the abdominal aorta and abdominal arteries: comparison of the diagnostic efficacy of iobitridol 350 mgI/ml versus iomeprol 400 mgI/ml in a prospective, randomized, double-blind multi-center trial. Eur Radiol 20:572–583
Lehmann EL, D'Abrera HJ (1975) Nonparametrics: Statistical methods based on ranks. Holden-Day, Oxford
Bruce RJ, Djamali A, Shinki K, Michel SJ, Fine JP, Pozniak MA (2009) Background fluctuation of kidney function versus contrast-induced nephrotoxicity. AJR Am J Roentgenol 192:711–718
Mack MJ, Brennan JM, Brindis R et al (2013) Outcomes following transcatheter aortic valve replacement in the United States. JAMA J Am Med Assoc 310:2069–2077
Bagur R, Webb JG, Nietlispach F et al (2009) Acute kidney injury following transcatheter aortic valve implantation: predictive factors, prognostic value, and comparison with surgical aortic valve replacement. Eur Heart J 31:865–874
Tang K, Wang L, Li R, Lin J, Zheng X, Cao G (2012) Effect of low tube voltage on image quality, radiation dose, and low-contrast detectability at abdominal multidetector CT: phantom study. J Biomed Biotechnol 2012:130–169
Sigal-Cinqualbre AB, Hennequin R, Abada HT, Chen X, Paul J-F (2004) Low-Kilovoltage Multi-Detector Row Chest CT in Adults: Feasibility and Effect on Image Quality and Iodine Dose. Radiology 231:169–174
Nakaura T, Awai K, Oda S et al (2011) Low-kilovoltage, high-tube-current MDCT of liver in thin adults: pilot study evaluating radiation dose, image quality, and display settings. AJR Am J Roentgenol 196:1332–1338
Matsumoto K, Jinzaki M, Tanami Y, Ueno A, Yamada M, Kuribayashi S (2011) Virtual monochromatic spectral imaging with fast kilovoltage switching: improved image quality as compared with that obtained with conventional 120-kVp CT. Radiology 259:257–262
Wuest W, Anders K, Schuhbaeck A et al (2012) Dual source multidetector CT-angiography before Transcatheter Aortic Valve Implantation (TAVI) using a high-pitch spiral acquisition mode. Eur Radiol 22:51–58
Plank F, Friedrich G, Bartel T et al (2012) Benefits of high-pitch 128-slice dual-source computed tomography for planning of transcatheter aortic valve implantation. Ann Thorac Surg 94:1961–1966
Joshi SB, Mendoza DD, Steinberg DH et al (2009) Ultra-low-dose intra-arterial contrast injection for iliofemoral computed tomographic angiography. JACC Cardiovasc Imaging 2:1404–1411
Nietlispach F, Leipsic J, Al-Bugami S, Masson J-B, Carere RG, Webb JG (2009) CT of the ilio-femoral arteries using direct aortic contrast injection: proof of feasibility in patients screened towards percutaneous aortic valve replacement. Swiss Med Wkly 139:458
Acknowledgement
The authors thank Annick Troniou for her invaluable assistance.
The scientific guarantor of this publication is Pr. Jean-Nicolas Dacher, MD, PhD. The authors of this manuscript declare relationships with the following companies: Pr. Jean-Nicolas Dacher is consultant for GE HealthCare. No other conflict of interest is to disclose. The authors state that this work has not received any funding. Dr. Michael Bubenheim kindly provided statistical advice for this manuscript. One of the authors (Michael Bubenheim) has significant statistical expertise. Institutional Review Board approval was obtained. Written informed consent was waived by the Institutional Review Board. Methodology: retrospective, diagnostic or prognostic study.
This work was presented at the Annual Meeting of the European Society of Cardiac Radiology in London (October 2013). It has been selected for presentation within the EPOS highlighted poster presentations session and has received a Certificate of Merit Award.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dubourg, B., Caudron, J., Lestrat, JP. et al. Single-source dual-energy CT angiography with reduced iodine load in patients referred for aortoiliofemoral evaluation before transcatheter aortic valve implantation: impact on image quality and radiation dose. Eur Radiol 24, 2659–2668 (2014). https://doi.org/10.1007/s00330-014-3263-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-014-3263-1