Abstract
Objectives
We sought to evaluate the capability of spectral CT to detect the therapeutic response to 125I interstitial brachytherapy in a pancreatic carcinoma xenograft nude mouse model.
Methods
Twenty mice bearing SWl990 human pancreatic cancer cell xenografts were randomly separated into two groups: experimental (n = 10; 1.0 mCi) and control (n = 10; 0 mCi). After a two-week treatment, spectral CT was performed. Contrast-to-noise ratio (CNR) and iodine concentration (IC) in the lesions were measured and normalized to the muscle tissue, and nIC CD31 immunohistochemistry was used to measure microvessel density (MVD). The relationships between the nIC and MVD of the tumours were analysed.
Results
The nIC of the experimental group was significantly lower than that of the control group during the multiphase examination. A significant difference in the MVD was observed between the two groups (P <0.001). The nIC values of the three-phase scans have a certain positive correlation with MVD (r = 0.57, p < 0.0001; r = 0.48, p = 0.002; r = 0.63, p = 0.0017 in the 10, 25, and 60 s phase, respectively).
Conclusions
Spectral CT can be a useful non-invasive imaging modality in evaluating the therapeutic effect of 125I interstitial brachytherapy to a pancreatic carcinoma.
Key Points
• Spectral CT offers opportunities to assess therapeutic response in pancreatic cancer cases.
• Spectral CT findings correlated with vascular changes associated with 125I seed implantation.
• Spectral CT with monochromatic imaging removed most 125I seed artefacts.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pancreatic cancer has the poorest prognosis among the malignant cancers, with a five-year survival rate of less than 5 %, and is the fourth leading cause of cancer death in the US [1, 2]. At present, only approximately 20 % of pancreatic cancer patients qualify for surgical resection of curative intent. Therefore, nonsurgical methods have attracted increasing attention [2, 3]. In recent years, 125I seed implantation has been widely used to treat prostate cancer and pancreatic carcinoma because of its high precision, minimal trauma, high accumulative dose, and few complications [4, 5]. Wang et al. have applied 125I seed implantation to treat advanced pancreatic cancer and found significant improvement in the clinical symptoms and life quality of patients [4].
Multiphase, multidetector computed tomography (MDCT) of the pancreas is the primary modality for screening, detecting, staging, following up, and evaluating the therapeutic response of pancreatic cancer [6]. Spectral CT has been recently introduced as a new dual energy CT based on the rapid alternation between two peak voltage settings (140 and 80 kVp, i.e., “fast switching”) [6–8]. This imaging mode allows the reconstruction of virtual monochromatic spectral images with energies ranging from 40 to 140 keV, which provides the ability to reduce beam-hardening artefact effects and optimize contrast with selectable monochromatic energy (keV) and accurate material-decomposition (MD) images (e.g.,water-based and iodine-based MD images) for quantitative iodine concentration (IC, in milligrams per millilitre) measurement [8–13]. Spectral CT imaging can be used to reduce beam-hardening artefacts and optimize contrast with selectable monochromatic energy (keV) [7, 10]. Moreover, spectral CT imaging (GSI mode) has clinical applications in cancer differentiation, liver characterization, and contrast resolution enhancement in vascular imaging [14–22]. However, to date, there are few studies dealing with the use of spectral CT for evaluating therapeutic response of 125I interstitial brachytherapy in pancreatic carcinoma.
The current study aims to evaluate the capacity of spectral CT imaging to detect the therapeutic response of 125I interstitial brachytherapy with a xenograft animal pancreatic cancer model.
Materials and methods
Animal model
The animal experiments were reviewed and approved by the Official Committee on Animal Affairs. Twenty BALB/C male nu/nu nude mice weighing 20 g (mean: 20 ± 0.6 g) at 5 weeks of age were purchased from the Chinese Academy of Sciences Shanghai Experimental Animal Center. The human pancreatic carcinoma cell line SW1990 was provided from the American Type Culture Collection (ATCC, Manassas, VA, USA). Human-cultured SW1990 cells (5 × 106 cells in 0.5 mL) were inoculated subcutaneously into the dorsal flank of each nude mouse. After 3 weeks of implantation with tumour cells, the tumour size reached 10 mm to 15 mm. All mice were randomized into the experimental (n = 10) and control (n = 10) groups. 125I seeds (1.0 mCi) were implanted into the experimental group, whereas blank seeds (0 mCi) were implanted into the control group.
125I seed
125I seeds were provided by Shanghai GMS Pharmaceutical Company, Limited (XinKe Pharmaceutical Ltd., Shanghai, China). A single seed is 0.8 mm × 4.5 mm (diameter × long) with a radioactive half-life of 59.6 d and a main transmission of 27.4 keV to 31.4 keV X-ray and 35.5 keV γ-ray. The penetration distance in the human tissue is only 1.7 cm, which eliminates potential injury to the physician, staff, and family. The internal irradiation was relatively long-acting, which can last up to 180 d.
Spectral CT imaging protocol
After a two-week treatment, spectral CT was performed. After a 24-hour fasting period, 20 anaesthetized mice were studied as three-phase spectral CT at 10 s, 25 s, and 60 s after intravenous contrast agent administration. The whole mouse was examined by high-definition CT (Discovery CT750HD, GE Healthcare, Wisconsin, USA) with a single tube, fast kilo-voltage switching between 80 kVp and 140 kVp in less than 0.5 msec. The non-ionic contrast media iopamidol (Iopamiro 300; Shanghai BRACCO Sine Pharmaceutical, China) at the dosage of 0.1 ml was injected at a rate of 0.02 ml/s through the tail vein. The other CT parameters included: collimation thickness of 0.625 mm, at an interval of 0.625 mm, tube current of 600 mA, rotation speed (temporal resolution) of 0.5 s, helical pitch of 0.984, 20-mm detector coverage, 20-cm field-of-view (FOV), and a 512 × 512 reconstruction matrix. MD images using water and iodine as basis material pairs were reconstructed with projection-based MD software and a standard reconstruction kernel.
The spectral CT images were analysed with the GSI Viewer software 4.4 (GE Healthcare, Waukesha, Wisconsin), with a standard soft-tissue display window pre-set (WL 40 and WW 400). Two types of images were reconstructed from the single spectral CT acquisition for analysis: a set of water-based and iodine-based MD images, and monochromatic image sets corresponding to photon energies ranging from 40 to 140 keV. From the monochromatic image sets, an operation was first made to obtain an optimal energy level (keV) to provide the best contrast-to-noise ratio (CNR) between the lesion and muscle.
In order to get the optimal keV images, two circular regions-of-interest (ROI, mean number of pixels, 400; range, 310–560) were placed by a radiologist (X.Z.L.) with five years of experience in spectral CT on the lesion and the normal adjacent muscle tissue. The GSI Viewer software package automatically calculated and displayed the CNR values for the 101 sets of monochromatic images in real time. From the CNR plot, the optimal single energy (keV) level for generating the best CNR between the lesion and the normal adjacent muscle tissue could be selected. Colour-scale images were used to help identify the tumour and its necrotic areas.
Quantitative analysis of nIC
The two radiologists (X.Z.L. and K.M.C.) with 16 and 32 years ofexperience in abdominal imaging, respectively, both have five years of experience performing the quantitative measurements for analyzing the monochromatic images and MD images by a spectral imaging viewer in consensus at a workstation (AW4.4; GE Healthcare).
Because quantitative measurement of the iodine concentration (IC) was essential for the quantitative assessment of different lesions, the MD images were used to measure IC in the lesions and adjacent muscle tissues. The ROI was placed on the monochromatic image and copied to the iodine image. The ROI was placed over the area covering the solid tumour, made as large as possible to reduce noise, and positioned away from prominent metal artefacts and necrotic areas, and the copy-paste function was used to make sure the ROIs were at the same places in the three phases. The IC data in ROI were exported into Excel format. To ensure consistency, an average of two to three separate ROIs was obtained. In order to minimize variations between mice, the ICs in lesions were normalized to the IC-adjacent muscle tissues to derive a normalized iodine concentration (nIC = IC lesion/IC muscle tissue).
Histochemical examination
The mice were autopsied after spectral CT. Tumours were excised and fixed in 10 % buffered formalin, sectioned at 5 μm, and then stained with H&E (Sigma Aldrich, St. Louis, Missouri, USA). The blood vessel marker CD31 expression of the tumour tissues was analysed by immunohistochemical staining.
Intratumoral microvessels were detected using a monoclonal antibody against the CD31 antigen (clone JC/70; Dako, Glostrup, Denmark). Microvessel density (MVD) was analysed using CD31 staining [23] under 100× magnification by light microscopy to identify the highest vascular density area (hot spot) within the lesions. The five areas with the highest MVD were selected for counting under 200 × magnification (0.442 mm2/field) to obtain the mean value. Only the CD31 staining in the tumour area was reviewed, and any endothelial cell cluster consisting of two or more cells was considered to be a single, countable microvessel. Some immunopositive leukocytes were excluded on morphological grounds.
Statistical analysis
All data were plotted as mean ± standard deviation (SD). All statistical analyses were implemented using SPSS 17.0 software (SPSS Inc., Chicago, IL). Independent t-test and ANOVA were carried out. Statistical significance was considered at p < 0.05 in the two-tailed tests. Linear regression was used to compare nIC with MVD.
Results
Spectral CT metrics/imaging
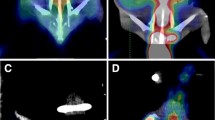
At two weeks after treatment, all mice of the experimental and control groups underwent unenhanced imaging and three spectral CT imaging phases (10, 25, and 60 s). The mean optimal keV for displaying the lesions in our study was 70 keV (Fig. 1). The amount of artefacts was reduced on the monochromatic images compared with the conventional polychromatic images (Fig. 2). The spectral CT with monochromatic imaging improved the tumour visibility in the vicinity of the 125I seeds and successfully removed most of the artefacts from the 125I seeds. A sample set of the images derived from a single spectral CT acquisition in a mouse implanted with 125I seed is shown in Fig. 3.
Selection of the best contrast-to-noise ratio (CNR) for displaying tumour visibility in the vicinity of the 125I seeds with the GSI Viewer analysis tool. a ROI selections for the tumour (arrow) and normal adjacent muscle tissues on a coronal image. b The optimal monochromatic energy of 70 keV achieved the best CNR for the lesion
The set of images showing that there are obvious differences between the conventional polychromatic image and the monochromatic image with blank seed in a pancreatic carcinoma xenograft: (a) the conventional polychromatic image showed the tumour in the vicinity of the 125I seed was not clearly delineated owing to the artefacts from the 125I seed; (b) the monochromatic image improved the tumour visibility in the vicinity of the 125I seed and successfully removed most of the artefacts from the 125I seed
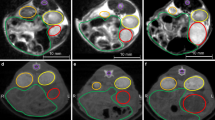
GSI images of a mouse with 125I seed implanted in a pancreatic carcinoma xenograft: (a) the monochromatic image obtained at 70 keV energy level improved tumour visibility and reduced the artefacts of the 125I seed (black arrow). b colour overlay of the iodine-based MD images, (c) water-based MD images, and (d) iodine-based MD images for identifying the solid components of the tumour (red arrow) and its necrotic areas (white arrow)
Each group showed the same enhancement pattern in the spectral CT images. During the 10 s examination, the solid components of the pancreatic carcinoma xenograft initially showed minimal enhancement and a progressive fill-in enhanced pattern during the 25 s and 60 s examinations, whereas the cystic part remained unenhanced. Colour-scale images were used to identify the solid components of the pancreatic carcinoma and necrotic areas. The experimental group implanted with 125I seeds had lesions with prominent necrotic areas (Fig. 4), whereas the control group implanted with blank seeds had small or no necrotic areas.
GSI scatterplot and histogram images of the water and iodine concentration plots for mice implanted with 125I seed in a pancreatic carcinoma xenograft: (a) yellow ROI represent the solid components of the pancreatic carcinoma, whereas red ROI represent the necrotic areas, (b) GSI scatterplot images, (c) GSI histogram images
Quantitative analysis of nIC
The nIC of the experimental group was significantly lower than that of the control group during the three spectral CT phases (Table 1). The nIC values of the experimental and control groups were, respectively, 0.171 ± 0.029 and 0.286 ± 0.037 in the 10 s scan, 0.290 ± 0.051 and 0.471 ± 0.127 in the 25 s examination, and 0.407 ± 0.062 and 0.632 ± 0.179 in the 60 s examination. A significant difference in nIC was observed between the two groups in the 10 s (t = 7.736, p < 0.0001), 25 s (t = 4.182, p = 0.0006), and 60 s examinations (t = 3.756, p = 0.0014).
Histological analysis
Twenty seeds, including blank seeds, were implanted in the tumours of 20 nude mice. All seeds were well located without loss and displacement and removed. As shown in Fig. 5, the histological appearance of the tumours in the control group was different from that in the 125I experimental group. Large necrotic regions were observed around the 125I seeds in the experimental group. The cancer cells adjacent to the necrotic region were loosely arranged, with condensed nuclei (Fig. 5a). Meanwhile, the cancer cells in the control group were densely arranged, with large darkly-stained nuclei and obvious karyokinesis (Fig. 5b). These results indicated that the 125I seed implantation inhibited the growth of cancer cells in the xenografts.
H&E staining of the pancreatic carcinoma xenografts (original magnification, 100×): (a) in the experimental group, large necrotic regions (red arrow) were observed around the 125I seed; (b) In the control group, the pancreatic cancer cells were densely arranged with large, darkly stained nuclei and obvious karyokinesis (black arrow)
The tumours were stained with CD31, an endothelial marker, to measure MVD and thus ascertain whether or not the nIC identified by spectral CT correlated with vascular density. A significant difference in the MVD of the lesion was observed between the two groups (P < 0.001). MVD significantly decreased after the 125I seed implantation, as revealed by the lack of CD31 staining in the experimental group. In the control group, a rich network of vessels was identified throughout the tumour, which was predicted by spectral CT imaging of nIC. Statistical analyses were performed to correlate the nIC with MVD; this showed a certain positive correlation (r = 0.57, p < 0.0001 in the 10 s, r = 0.48, p = 0.002 in the 25 s, r = 0.63, p = 0.0017 in the 60 s).
Discussion
125I seed implantation has been proven to be a safe alternative treatment and a mature technique for advanced pancreatic cancer. In this study, tumour growth was significantly suppressed by 125I seed implantation compared with the control. The results of the present study demonstrate that spectral CT can be a useful non-invasive imaging modality in monitoring and evaluating the therapeutic response of 125I interstitial brachytherapy in a pancreatic carcinoma xenograft.
125I seed implantation showed significant anti-tumour effects. However, this method produces prominent beam-hardening artefacts on conventional CT exactly at the area that needs to be evaluated for residual or recurrent disease of the tumour because of the high density of metal. The reduction of these artefacts would improve tumour visualization and increase the confidence in the interpretation of the examination findings.
Spectral CT imaging obtained with the single tube–rapid dual tube voltage switching technique provides monochromatic images that depict how the imaged object would look if the X-ray source produced only single energy X-ray photons [7, 21, 24, 25]. This process allows for increased contrast resolution, as proven by some reports. Boll indicated that the use of a monochromatic X-ray beam in CT would reduce beam-hardening artefacts and average attenuation effects commonly observed on conventional CT with polychromatic X-ray beams [26]. Matsumoto showed that spectral CT imaging at approximately 70 keV yields lower image noise and higher CNR than the 120 kVp CT for a given radiation dose [7]. Zhao indicated that the image quality and CNR of intrahepatic and extrahepatic portal veins can be improved by spectral CT imaging at 51 keV [14]. In the present study, the highest keV value that can provide the best CNR for the lesions and reduce beam-hardening artefacts was approximately 70 keV. The better image contrast resolution at the optimal energy level provided more accurate detection and evaluation of the therapeutic response.
Spectral CT can provide quantitative information about the elemental and molecular composition of tissue and contrast materials based on their attenuation properties [27]. For medical diagnostic imaging, water and iodine are often selected as the basis pair for MD image presentation because their atomic numbers span the range of atomic numbers for materials generally found in medical imaging and approximate those of soft tissue and iodinated contrast material to result in material attenuation images that are intuitive to interpret [21]. The IC in the tumour is an indication of its blood flow because iodine is the main component of the contrast agent. In addition, the enhancement in the solid components of the tumour depends on the amount of iodine after injection of the contrast agent before CT data acquisition. The quantitative measurements obtained in this study showed that the nIC of the experimental group was significantly reduced compared to the control group. Quantitative analysis using the mean nIC also supported the qualitative observations. Our study showed a prominent correlation between the nIC’s and 125I seed anti-tumour effects. The nIC values for the two groups were statistically different; this may be a useful parameter in detecting and evaluating the viability of the therapeutic response. These data demonstrate that the spectral CT measures of nIC can noninvasively monitor the vascular changes associated with therapy in this xenograft model. Therefore, spectral CT imaging of 125I interstitial brachytherapy in a pancreatic carcinoma xenograft may yield an accurate assessment.
Angiogenesis and lymphangiogenesis, the formation of new blood vessels and lymphatic vessels, respectively, are required for many pathological processes, including tumour growth and metastasis, as well as physiological tissue maintenance. As previously reported, 125I seed implantation successfully suppresses tumour cell proliferation with the induction of apoptotic, cell cycle arrest gene, DNA demethylation, and inhibition of vascular endothelial growth factor. The present study focused on vascular changes with MVD counting. The nIC of the solid components of the pancreatic carcinoma xenograft positively correlated with MVD in the three spectral CT phases. Thus, three-phase spectral CT imaging can be helpful in reflecting the angiogenesis of the pancreatic carcinoma xenograft.
There are several other imaging modalities for screening, detecting, staging, following up, and evaluating the therapeutic response of pancreatic cancer, such as PET–CT and MRI.
The PET–CT system represents an important evolution in technology that helps to bring molecular imaging to the forefront in cancer diagnosis, staging, and therapy monitoring [28]. However, this approach is principally limited by its relatively high cost and low spatial resolution.
MRI provides non-invasive imaging of soft tissue anatomy with high soft tissue contrast and high spatial resolution. However, the presence of metal-related artefacts in MRI imaging can obscure relevant anatomy and disease.
The present study also has some limitations. First, this investigation reflects our preliminary experience with a relatively small number of mice. Although 20 mice were included in our study, the rather small sample size precludes us from drawing broad conclusions. Different animal models may reach different conclusions. Second, spectral CT with GSI mode successfully removed most of the artefacts from the 125I seeds and obviously improved the image quality. However, spectral CT with GSI mode cannot eliminate all of the artefacts from the 125I seeds, which may influence the study results. Third, only MVD was used as an indicator for angiogenesis. Thus, intensive investigation will be carried out in the future.
In conclusion, spectral CT with fast-kVp switching CT with monochromatic images at 70 keV energy level was used in the present study to provide the best CNR for the lesions and reduce artefacts from 125I seeds. Tumour response to 125I seed implantation can potentially be determined by spectral CT, allowing individualized evaluation of the therapeutic effect and thus more favourable therapeutic outcomes. The present study may serve as a basis for the potential clinical use of this new technique.
References
Siegel R, Naishadham D, Jemal A (2013) Cancer statistics, 2013. CA Cancer J Clin 63:11–30
Sharma C, Eltawil KM, Renfrew PD, Walsh MJ, Molinari M (2011) Advances in diagnosis, treatment and palliation of pancreatic carcinoma: 1990–2010. World J Gastroenterol 17:867–897
Michl P, Gress TM (2013) Current concepts and novel targets in advanced pancreatic cancer. Gut 62:317–326
Zhongmin W, Yu L, Fenju L, Kemin C, Gang H (2010) Clinical efficacy of CT-guided iodine-125 seed implantation therapy in patients with advanced pancreatic cancer. Eur Radiol 20:1786–1791
Chiumento C, Montagna A, Clemente S, Cozzolino M, Fusco V (2011) A retrospective analysis after low-dose-rate prostate brachytherapy with permanent (125)I seed implant: clinical and dosimetric results in 70 patients. Tumori 97:335–340
Brook OR, Gourtsoyianni S, Brook A, Siewert B, Kent T, Raptopoulos V (2013) Split-Bolus Spectral Multidetector CT of the Pancreas: Assessment of Radiation Dose and Tumor Conspicuity. Radiology. doi:10.1148/radiol.13121409
Matsumoto K, Jinzaki M, Tanami Y, Ueno A, Yamada M, Kuribayashi S (2011) Virtual monochromatic spectral imaging with fast kilovoltage switching: improved image quality as compared with that obtained with conventional 120-kVp CT. Radiology 259:257–262
Heismann B, Balda M (2009) Quantitative image-based spectral reconstruction for computed tomography. Med Phys 36:4471–4485
Anderson NG, Butler AP, Scott NJ et al (2010) Spectroscopic (multi-energy) CT distinguishes iodine and barium contrast material in MICE. Eur Radiol 20:2126–2134
Brook OR, Gourtsoyianni S, Brook A, Mahadevan A, Wilcox C, Raptopoulos V (2012) Spectral CT with metal artifacts reduction software for improvement of tumor visibility in the vicinity of gold fiducial markers. Radiology 263:696–705
Jung DC, Oh YT, Kim MD, Park M (2012) Usefulness of the virtual monochromatic image in dual-energy spectral CT for decreasing renal cyst pseudoenhancement: a phantom study. AJR Am J Roentgenol 199:1316–1319
Yu L, Leng S, McCollough CH (2012) Dual-energy CT-based monochromatic imaging. AJR Am J Roentgenol 199:S9–S15
Cheng J, Yin Y, Wu H et al (2013) Optimal Monochromatic Energy Levels in Spectral CT Pulmonary Angiography for the Evaluation of Pulmonary Embolism. PLoS One 8:e63140
Zhao LQ, He W, Li JY, Chen JH, Wang KY, Tan L (2012) Improving image quality in portal venography with spectral CT imaging. Eur J Radiol 81:1677–1681
Yu Y, Lin X, Chen K et al (2013) Hepatocellular carcinoma and focal nodular hyperplasia of the liver: differentiation with CT spectral imaging. Eur Radiol 23:1660–1668
Wu HW, Cheng JJ, Li JY, Yin Y, Hua J, Xu JR (2012) Pulmonary embolism detection and characterization through quantitative iodine-based material decomposition images with spectral computed tomography imaging. Invest Radiol 47:85–91
Silva AC, Morse BG, Hara AK, Paden RG, Hongo N, Pavlicek W (2011) Dual-energy (spectral) CT: applications in abdominal imaging. Radiographics 31:1031–1046, discussion 1047–1050
Qian LJ, Zhu J, Zhuang ZG et al (2012) Differentiation of neoplastic from bland macroscopic portal vein thrombi using dual-energy spectral CT imaging: a pilot study. Eur Radiol 22:2178–2185
Pang LF, Zhang H, Lu W et al (2013) Spectral CT imaging of myocardial infarction: preliminary animal experience. Eur Radiol 23:133–138
Pan Z, Pang L, Ding B et al (2013) Gastric cancer staging with dual energy spectral CT imaging. PLoS One 8:e53651
Lv P, Lin XZ, Li J, Li W, Chen K (2011) Differentiation of small hepatic hemangioma from small hepatocellular carcinoma: recently introduced spectral CT method. Radiology 259:720–729
Yamada Y, Jinzaki M, Tanami Y, Abe T, Kuribayashi S (2012) Virtual monochromatic spectral imaging for the evaluation of hypovascular hepatic metastases: the optimal monochromatic level with fast kilovoltage switching dual-energy computed tomography. Invest Radiol 47:292–298
Guimaraes AR, Rakhlin E, Weissleder R, Thayer SP (2008) Magnetic resonance imaging monitors physiological changes with antihedgehog therapy in pancreatic adenocarcinoma xenograft model. Pancreas 37:440–444
Lv P, Lin XZ, Chen K, Gao J (2012) Spectral CT in patients with small HCC: investigation of image quality and diagnostic accuracy. Eur Radiol 22:2117–2124
Leng S, Yu L, Wang J, Fletcher JG, Mistretta CA, McCollough CH (2011) Noise reduction in spectral CT: reducing dose and breaking the trade-off between image noise and energy bin selection. Med Phys 38:4946–4957
Boll DT, Patil NA, Paulson EK et al (2010) Focal cystic high-attenuation lesions: characterization in renal phantom by using photon-counting spectral CT–improved differentiation of lesion composition. Radiology 254:270–276
Feuerlein S, Heye TJ, Bashir MR, Boll DT (2012) Iodine quantification using dual-energy multidetector computed tomography imaging: phantom study assessing the impact of iterative reconstruction schemes and patient habitus on accuracy. Invest Radiol 47:656–661
Jian L, Zhongmin W, Kemin C, Yunfeng Z, Gang H (2013) MicroPET-CT evaluation of interstitial brachytherapy in pancreatic carcinoma xenografts. Acta Radiol 54:800–804
Acknowledgments
The authors wish to thank Dr. Danjun Yuan and Dr. Wenjie Wei for their technical support in editing the manuscript. We especially thank Yixing Yu, Duanmin Hu, and Rongbiao Tang for their contributions.
The scientific guarantor of this publication is Ph.D, M.D Linxiao Zhu. Our authors of this manuscript declare no relationships with any companies. This study was supported in part by a grant-in-aid for scientific research from the Science and Technology Commission of Shanghai Municipality (Project No.11JC1407400,10JC1410900 and 10411953000), the National Natural Science Foundation of China (Project No. 81071281 and 81271682), and the Technology Plan of Zhenjiang (Project No. SH2013083). No complex statistical methods were necessary for this paper. Approval from the institutional animal care committee was obtained. The animal experiments were reviewed and approved by the Official Committee on Animal Affairs of Shanghai Jiao Tong University. Methodology: retrospective, randomised controlled trial/experimental, multicentre study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hu, S., Huang, W., Chen, Y. et al. Spectral CT evaluation of interstitial brachytherapy in pancreatic carcinoma xenografts: preliminary animal experience. Eur Radiol 24, 2167–2173 (2014). https://doi.org/10.1007/s00330-014-3257-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-014-3257-z