Abstract
Objectives
To investigate whether T1-mapping allows assessment of acute kidney injury (AKI) and prediction of chronic kidney disease (CKD) in mice.
Methods
AKI was induced in C57Bl/6N mice by clamping of the right renal pedicle for 35 min (moderate AKI, n = 26) or 45 min (severe AKI, n = 23). Sham animals served as controls (n = 9). Renal histology was assessed in the acute (day 1 + day 7; d1 + d7) and chronic phase (d28) after AKI. Furthermore, longitudinal MRI-examinations (prior to until d28 after surgery) were performed using a 7-Tesla magnet. T1-maps were calculated from a fat-saturated echoplanar inversion recovery sequence, and mean and relative T1-relaxation times were determined.
Results
Renal histology showed severe tubular injury at d1 + d7 in both AKI groups, whereas, at d28, only animals with prolonged 45-min ischemia showed persistent signs of AKI. Following both AKI severities T1-values significantly increased and peaked at d7. T1-times in the contralateral kidney without AKI remained stable. At d7 relative T1-values in the outer stripe of the outer medulla were significantly higher after severe than after moderate AKI (138 ± 2 % vs. 121 ± 3 %, p = 0.001). T1-elevation persisted until d28 only after severe AKI. Already at d7 T1 in the outer stripe of the outer medulla correlated with kidney volume loss indicating CKD (r = 0.83).
Conclusion
T1-mapping non-invasively detects AKI severity in mice and predicts further outcome.
Key Points
• Renal T1-relaxation times are increased after ischemia-induced acute kidney injury.
• Renal T1-values correlate with subsequent kidney volume loss.
• T1-mapping detects the severity of acute kidney injury and predicts further outcome.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute kidney injury (AKI) is common in hospitalized patients and is an independent risk factor for increased morbidity and mortality [1, 2]. Renal ischemia reperfusion injury (IRI) is a major cause of AKI [3, 4]. It occurs, for example, during major surgery, severe trauma and shock, in critically ill patients as well as in the context of kidney transplantation. Reperfusion after the ischemic event leads to formation of reactive oxygen species, up-regulation of pro-inflammatory and pro-apoptotic pathways, and consequently to an inflammatory response in the kidney [5]. In the acute phase, ischemia induced AKI is characterized by damage of endothelial cells resulting in an increased microvascular permeability and development of local edema, acute tubular necrosis, and inflammatory cell infiltration [6, 7]. Depending on the severity of AKI, repair of renal injury may be insufficient, and renal fibrosis and chronic renal insufficiency may occur [2, 8].
In order to be able to improve treatment and ultimately prognosis in patients with AKI, it is important to develop non-invasive diagnostic tests for early diagnosis of AKI, to evaluate and monitor renal pathology, and to determine the prognosis of renal function. So far, besides ultrasound, mostly serum creatinine, urine output, and glomerular filtration rate are used to detect AKI. However, these parameters are insensitive, influenced by many factors other than renal function [9, 10], and are insufficient to estimate the severity and prognosis of AKI. Functional magnetic resonance imaging (MRI) provides promising new techniques such as arterial spin labelling [11], blood oxygen level dependency imaging [12, 13], and diffusion weighted imaging [14–16] to examine non-invasively renal function and pathology.
Furthermore, measurement of the longitudinal and transverse relaxation times, T1 and T2, may be useful to determine renal pathology by quantification of the tissue water content [17–19]. Inflammation during the acute phase after ischemia-induced AKI is associated with accumulation of water in the intracellular and extracellular space, due to cell swelling and interstitial edema, respectively, so that prolongation of T1- and T2-values is expected [20]. Furthermore, interstitial collagen deposition due to renal fibrosis may allow water to accumulate in the enlarged interstitial space [20]. For cardiac imaging T1- and T2-mapping techniques have already been established for clinical use and seem promising to quantify diffuse and focal myocardial edema and fibrosis in patients with myocardial infarction and cardiomyopathy [21–24]. We recently showed that T2-values are increased in the acute phase after AKI depending on its severity and are unchanged in the chronic phase [25]. The ability of T1-mapping to determine the severity of renal damage after AKI and to differentiate acute and chronic renal changes has not been investigated so far.
Therefore, our purpose was to investigate longitudinal changes of renal T1-relaxation time after AKI and to evaluate whether T1-mapping allows detection of AKI severity and prediction of progression to chronic kidney disease.
Materials and methods
Experimental animals
Male C57Bl/6N mice (Charles River) weighing 25 – 30 g at 10 – 12 weeks of age were used for all experiments. AKI was induced by transient unilateral clamping of the right renal pedicle for either 35 min (35 min IRI, moderate AKI, n = 26) or 45 min (45 min IRI, severe AKI, n = 23), as described previously [25–27]. In brief, mice were anaesthetised with isoflurane. After median laparotomy, a non-traumatic vascular clamp was applied to the renal pedicle. After 35 min or 45 min the clamp was removed and the abdomen was closed. For comparison, n = 9 animals underwent sham operation. These animals were anaesthetised with isoflurane, median laparotomy was performed, and the abdomen was closed after 45 min without clamping of the renal pedicle. After surgery, the mice were returned to their cages and monitored until they were fully awake. Some of the animals were killed on d1 (n = 7 with 35 min IRI, n = 7 with 45 min IRI, n = 5 with sham operation) and d7 (n = 9 with 35 min IRI, n = 9 with 45 min IRI, n = 4 with sham operation) for histology work up. For longitudinal MRI-examinations, n = 10 animals with moderate AKI and n = 7 animals with severe AKI were used. These animals were killed 4 weeks after surgery after the last MRI. Animals received a standard diet with free access to tap water. They were cared for in accordance with our institution's guidelines for experimental animals and the national guidelines of the Society for Animal Science. The local animal protection committee approved the experimental protocol.
Renal histology
Renal histology was evaluated at d1 and d7 as well as after the last MRI-examination 4 weeks after unilateral IRI. For organ retrieval, mice were anaesthetised and perfused with ice-cold saline solution. Kidney tissue was immediately fixed in buffered formalin and embedded in paraffin. Three-micrometer paraffin sections were cut and stained with PAS. Evaluation of AKI was performed by a nephropathologist with >15 years of experience without knowledge of the animal group identity. It was performed using a semi-quantitative grading system: 0 = no AKI, 1 = mild AKI with <25 % of tubuli affected, 2 = moderate AKI with 25 – 50 % of tubuli affected, 3 = severe AKI with 51 – 75 % of tubuli affected, 4 = very severe AKI with >75 % of the tubuli affected. Inflammation was analysed semiquantitatively by scoring the infiltrating cells in 10 randomly chosen, non-overlapping fields of the outer medulla: 0 = no inflammation with <5 infiltrating cells per field, 1 = mild inflammation with 5 – 10 infiltrating cells per field, 2 = moderate inflammation with 11 – 25 infiltrating cells per field, 3 = severe inflammation with 26 – 50 infiltrating cells per field, 4 = very severe inflammation with >50 infiltrating cells per field.
MRI imaging and analysis
Some of the mice underwent repetitive MRI examinations before surgery (d0) and at five time points after surgery (d1, d7, d14, d21, d28) using a 7-Tesla dedicated animal scanner (Bruker, Pharmascan, Ettlingen, Germany) and a one-channel circular polarized volume coil (Bruker, Ettlingen, Germany). Animals were anaesthetised by isoflurane inhalation and their respiration was monitored and kept constant between 30 – 50 breaths/min during the entire examination. Respiratory triggered, fat-saturated T2-weighted turbo spin echo (TSE) sequences were acquired in axial and coronal planes that covered both kidneys. The coronal plane was adjusted to the long axis of both kidneys. Sequence parameters were: TR/TE = 1,500/33 ms, averages = 2, matrix = 256x256, field of view (FOV) = 35x35 mm2, slice thickness = 1 mm. For measurement of T1-relaxation time of renal tissue, a fat-saturated single-shot inversion recovery sequence with multiple inversion times, a non-selective inversion pulse, and an echoplanar (EPI) readout were used. T1-measurements were performed in a central coronal plane with the following sequence parameters: TR/TE = 18,000/16.4 ms, 13 inversion times (30, 100, 200, 300, 500, 700, 1000, 1200, 1500, 2000, 3000, 5000, 8000 ms), matrix = 128x128, FOV = 35x35 mm2, slice thickness = 2 mm, number of slices = 1. Total acquisition time for T1-mapping was approximately 10 – 15 min.
Kidney volumes were determined separately for the right kidney with AKI and the left kidney without AKI by manual segmentation of axial T2-weighted images. Relative kidney volume loss compared to the same kidney before surgery was calculated.
In order to correct for respiratory motion, the images of the inversion recovery sequence were co-registered using Elastix (open-source software: http://elastix.isi.uu.nl/) and a rigid registration algorithm [28]. T1-relaxation times were calculated on a pixel by pixel basis according to the equation T1 = M 0(1 − 2 exp(−TI/T1)). On T1-maps, regions of interest (ROI) were manually placed into cortex, outer stripe of the outer medulla (OSOM) and inner stripe of the outer medulla (ISOM) by one reader who was blinded to the animal group identity (Fig. 1) and mean T1-values of each layer were determined separately for the right kidney with AKI and the left kidney without AKI. In addition, relative T1-values compared to the values of the same kidney before surgery were determined. As differentiation of cortex and medulla in advanced stages of renal disease (i.e. later time points in the severe AKI group) was nearly abrogated, placement of ROIs in these cases was based on the knowledge of renal morphology at histology.
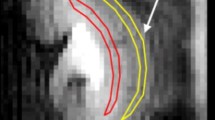
Placement of ROIs on T1-parameter maps. On parameter maps of T1-relaxation time the three anatomical layers of the kidney – cortex (C), outer stripe of the medulla (OSOM), and inner stripe of the outer medulla (ISOM) – were identified (a). ROIs were placed into these layers and covered the entire layer in the imaging plane (b)
Statistical analysis
For statistical analysis, SPSS 21 software (IBM Corporation, United States) was used. Data are presented as mean ± standard error of the mean (SEM). Differences between groups of moderate and severe AKI were assessed using the unpaired t-test. Longitudinal changes of T1-values compared to the values before surgery were determined using analysis of variance for repeated measurements followed by post hoc multiple comparison. Adjustment for multiple comparisons was performed with the Sidak method and adjusted p-values are given. Furthermore, the correlations of T1-relaxation times at different time points with relative kidney volume loss until day 28 were evaluated with linear regression and Pearson’s coefficient of correlation (r). P-values <0.05 were considered statistically significant.
Results
Renal histology after AKI
Renal histology in the contralateral left kidney without ischemia and in both kidneys of sham animals was unremarkable at d1 and d7 with AKI and inflammation scores of 0. In comparison to sham animals, significantly higher AKI and inflammation scores were observed at all time points and with both ischemia times. In the acute phase, at d1 and d7 after surgery, renal histology was characterized by severe tubular injury in the group of 35 min IRI (AKI scores d1 = 4, d7 = 3.89) and 45 min IRI (AKI scores d1 = 4, d7 = 4). The inflammation scores, representing infiltrating leukocytes, were not significantly different between animals with severe and moderate AKI at d1 (2.21 vs. 1.79; not significant, p = 0.183) and d7 (4 vs. 3.67, not significant, p = 0.176). In the chronic phase 28 days after IRI, AKI scores (1.5 vs. 0.7; p < 0.05) and inflammation scores (1.2 vs. 0.4; p < 0.05) were significantly higher in animals with severe compared to moderate AKI. Figures 2 and 3 give an overview over renal histology as well as AKI and inflammation scores in different groups and at different time points after ischemic AKI.
Histomorphological changes after short (35 min) and prolonged (45 min) renal ischemia reperfusion injury. Shown is the outer medulla after short (35 min, upper row) and prolonged (45 min, middle row) ischemia reperfusion injury (PAS stain, magnification 200-fold) at d1 (first column), d7 (second column), and d28 (last column). For comparison, normal renal morphology after sham surgery is shown in the lower row. AKI and inflammation were detectable at d1 and proceeded until d7. 28 days after surgery renal histology in the 35 min-group was characterised by almost complete tubular regeneration and in the 45 min-group by persistent moderate AKI and inflammation. In addition, tubular atrophy and tubular cast formation occurred in the prolonged ischemia group. By histology no differences between groups were detectable at d1 and d7 after surgery
Semiquantitative assessment of histomorphological changes after short (35 min) and prolonged (45 min) renal ischemia reperfusion injury. AKI scores (a) and inflammation scores (b) at different time points after unilateral renal IRI are shown in the sham group (black), 35 min-group (green), and the 45 min-group (red). Compared to sham animals significant higher AKI and inflammation scores were observed at all time points and with both ischemia times. In the acute phase severe AKI and inflammation was observed with both ischemia times. A significant difference between groups was observed at d28 with significantly higher AKI and inflammation scores in the 45 min-group compared to the 35 min-group (*p < 0.05)
Changes of T1-relaxation times after moderate and severe AKI
In the normal kidney, T1-relaxation times were lowest in the OSOM with 1208 ± 14 ms and highest in the ISOM with 1594 ± 19 ms before surgery. After moderate (35 min IRI) and severe AKI (45 min IRI), T1-values increased in all anatomical layers with most pronounced changes in the OSOM (Table 1). T1-values peaked at d7 after AKI. Examples of T1-maps of one animal with moderate and one with severe AKI are shown in Fig. 4, examples of inversion recovery EPI-images at different inversion times are given in Suppl. Fig. 1. After moderate AKI, relative T1-relaxation times at d7 were significantly increased to 118 ± 3 % in the renal cortex (p < 0.01), to 121 ± 3 % in the OSOM (p < 0.01) and to 113 ± 3 % in the ISOM (p < 0.05) compared to values before surgery. Until the end of the observation period, after short ischemia of 35 min, T1-relaxation times nearly reached baseline and no significant difference compared to preoperative T1-values was detectable (Fig. 5). After severe AKI, relative T1-relaxation times at d7 were increased to 122 ± 2 % in the cortex (p < 0.01), to 138 ± 2 % in the OSOM (p < 0.001) and to 123 ± 1 % in the ISOM (p < 0.001) compared to values before surgery. In the group of severe AKI, a significant T1-elevation in cortex and OSOM persisted until d28. In contrast to renal histology, already at d7 after surgery T1-values in the severe AKI group were significantly higher than in the moderate AKI group (1659 ± 28 ms vs. 1458 ± 36 ms, p = 0.001; Table 1). T1-values remained significantly higher in the severe AKI group at d14-28 compared to the moderate AKI group (p < 0.05). In the contralateral left kidney without AKI T1-values were unchanged throughout the entire observation period (Fig. 5).
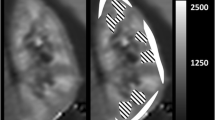
Example of T1-maps after moderate and severe AKI. Depicted are parameter maps of T1-relaxation time after ischemia induced AKI before surgery (d0) and at different time points after surgery (d7, d14, d28). Exemplarily, one animal with a short ischemia time of 35 min (moderate AKI; upper row) and one animal with prolonged ischemia of 45 min (severe AKI; lower row) are shown. Window level and width as well as image size are similar for all parameter maps. In the normal kidney before surgery, T1-relaxation time was lowest in the OSOM and highest in the ISOM. After AKI T1 increased in all anatomical layers. After moderate AKI T1-values nearly returned to baseline until d28 and renal anatomy was preserved. In contrast, after severe AKI, increase of T1 persisted until d28, differentiation between anatomical layers was abrogated, and marked kidney volume loss was observed
T1-relaxation times of renal tissue after AKI. Depicted are changes of relative T1-relaxation times compared to values before surgery after moderate (green curves) and severe AKI (red curves) as well as in the contralateral kidney without AKI (black curves). Values in the cortex (a), the OSOM (b), and the ISOM (c) are shown. Significant differences of relative T1-values compared to the values before surgery are given. *p < 0.05, **p < 0.01, ***p < 0.001
Correlation of T1-relaxation times with kidney volume loss
Relative kidney volume loss until d28 after AKI compared to values before surgery was significantly greater after severe (40 ± 5 % volume loss) than after moderate AKI (16 ± 6 %, p < 0.05), indicating more severe chronic irreversible kidney damage in the severe AKI group. Until d7, no significant kidney volume loss was observed in either group. T1-relaxation times at d7 – 28 significantly correlated with relative kidney volume loss until d28 after AKI (Table 2). The significant correlations of T1-values in the OSOM at d7 (r = 0.83, p < 0.001) and d14 (r = 0.93, p < 0.001) with kidney volume loss until day 28 are shown in Fig. 6.
Correlation of T1-relaxation times in the OSOM with relative kidney volume loss until d28. Depicted are the significant correlations of T1-relaxation time in the OSOM at day 7 (a) and day 14 (b) with relative kidney volume loss until d28. Data from animals with an ischemia time of 35 min (moderate AKI) are marked in green, data from animals with an ischemia time of 45 min (severe AKI) are marked in red
Discussion
We showed that T1-mapping allows non-invasive detection of AKI severity after ischemia reperfusion injury (IRI) in mice. T1-relaxation time already at d7 significantly correlated with kidney volume loss as a measure of chronic irreversible kidney damage, and thus predicted further renal outcome. Importantly, at this early time point after IRI, renal histology did not yet allow us to distinguish between the two AKI severities.
The model of unilateral IRI is a well-established mouse model of AKI. As renal function of the contralateral kidney is not compromised in this model, survival of the animals is normal and overall renal function is only mildly impaired [29]. We chose ischemia times of 35 min and 45 min, since we found in previous studies that an ischemia time of 35 min resulted in renal regeneration, whereas prolonged ischemia of 45 min caused chronic kidney disease with loss of renal function and tubulo-interstital fibrosis [25, 30]. We already established and validated a variety of functional MRI-parameters, such as renal perfusion [30], apparent diffusion coefficient (ADC), and T2-relaxation time [25] to evaluate AKI in mice. In this work, we compared the novel parameter T1-relaxation time in more detail with renal histology at various time points and recognized that T1-mapping detects AKI severity and prognosis of renal function at earlier time points than renal histology. Furthermore, there are several reasons why T1-mapping may be an important additional technique for assessment of renal pathology. First, T1-mapping provides an additional MRI parameter to further characterise acute as well as chronic renal pathology. For comparison, changes in T2-relaxation time, which are also based on the tissue water content, were only observed in the acute phase after AKI [25]. Second, quantification of T1-relaxation time proved robust in our study and variability between animals of the same group was low. Third, T1-mapping and renal perfusion imaging with arterial spin labelling may be combined without additional acquisition time by using the images with non-selective inversion to calculate T1-maps [30].
In this study T1-values increased after AKI in the renal cortex and medulla with largest changes in the OSOM, which is similar to T2-changes after AKI [25] and is possibly attributed to the fact that, in particular, the OSOM is highly susceptible to hypoperfusion and hypoxemia [31, 32]. Corresponding to the histomorphological changes of renal tissue that were characterized by highest AKI and inflammation scores at d7 after surgery, maximum T1-values were found at d7 in both groups of AKI severity. T1-increase at this time point may be explained by the increase of tissue water content due to an acute inflammatory response in the early phase after ischemia induced AKI with cell swelling, capillary leakage, and interstitial edema formation [18, 19]. Consequently, T1-increase in the early phase was paralleled by an increase of T2-relaxation times [33]. Our results are consistent with early in vitro and in vivo renal MRI-studies demonstrating a prolonged T1-relaxation time in the early phase after ligation of the renal artery or vein, which was well-correlated with tissue water content [34, 35]. In animals with severe AKI T1-increase in cortex and OSOM persisted until d28, whereas after moderate AKI, T1-values reached baseline at d28. Accordingly, renal histology at d28 was characterized by almost complete tubular regeneration in the group of short 35 min IRI, whereas persistent signs of AKI and inflammation were observed in the group of prolonged 45 min IRI. The persisting T1-elevation in the chronic phase after severe AKI may at least partly be related to renal fibrosis, which only occurs in animals with severe AKI due to inadequate regeneration of renal tissue and incomplete tubular repair. The work by de Miguel et al. supports this hypothesis [20]. They examined rabbits with antiglomerular basement membrane disease by repetitive measurement of T1-relaxation time. Although spatial resolution was poor compared to our study due to use of a 0.5 T whole-body system, the observed changes of T1-values over an observation period of 4 weeks in exemplarily examined animals were similar to our study. Furthermore, they found a good correlation of T1-relaxation time 4 weeks after disease induction with renal fibrosis at histology. They verified by T1-measurement in an animal with fibrosis, but without increased tissue water content that persistent prolongation of T1 is indeed due to renal fibrosis and not just to tissue edema [20]. Thus, alterations of T1-relaxation time of renal tissue presumably reflect acute tissue edema due to capillary leakage and cell swelling as well as chronic renal pathology such as renal fibrosis associated with AKI. Notably, T2-values in both groups of AKI severity were not significantly changed from normal at d28, indicating that T2-mapping does not allow detection of chronic changes of renal tissue [25]. For future studies, the combination of T1- and T2-measurement could be useful to differentiate acute from chronic changes of renal tissue.
Differentiation between groups of AKI severity was possible by T1-mapping already at d7. Furthermore, T1-times in the OSOM at d7 – 28 correlated very well with the kidney volume loss until d28 after AKI as a measure of chronic irreversible kidney damage. Importantly, renal histology and simple scoring of the amount of infiltrating cells at this early time point (d7) did not allow distinction of AKI severities and prediction of kidney volume loss. We hypothesize that the discrepancy between T1-mapping and simple renal histology is because activation of infiltrating inflammatory cells and production of pro-inflammatory cytokines can differ, which is not detectable by simply evaluating the amount of infiltrating cells at histology. An increased production of pro-inflammatory cytokines by activated inflammatory cells in the severe AKI group may lead to more pronounced tissue oedema and cell swelling, which is associated with higher T1-relaxation times at this early time point. These findings indicate that T1-mapping may be very valuable for experimental studies and testing of experimental drugs due to excellent correlations with AKI severity at an early time point and its prognostic value for further outcome of renal function. In particular, the combination of T1-mapping and renal perfusion imaging seems promising as both parameters may be obtained non-invasively within the same acquisition and provide complementary longitudinal information on renal pathology and function. Translation of T1-mapping into clinical practice is feasible and the use of T1-relaxation time as a non-invasive biomarker to determine presence, severity and most important prognosis of AKI in patients has to be examined.
Limitation of the study is the relatively small number of animals for longitudinal MRI. Nonetheless, we were able to show significant differences between groups and time points, as variability of T1-values within groups of different severities of AKI was low. Furthermore, we did not examine the correlation of T1-relaxation time with tissue water content and renal fibrosis, as this has already been demonstrated previously [19, 20].
In conclusion, T1-mapping through assessment of tissue edema and fibrosis allows non-invasive evaluation of AKI severity in mice. Alterations of T1-relaxation times at an early time point after AKI significantly correlate with subsequent kidney volume loss and were predictive for further renal outcome. Thus, T1-mapping may help along with other functional MRI techniques to determine acute and chronic changes of renal tissue after AKI, which may be very valuable for experimental studies and clinical practice.
Abbreviations
- AKI:
-
acute kidney injury
- CKD:
-
chronic kidney disease
- IRI:
-
ischemia reperfusion injury
- ISOM:
-
inner stripe of the outer renal medulla
- MRI:
-
magnetic resonance imaging
- OSOM:
-
outer stripe of the outer renal medulla
References
Wald R, Quinn RR, Luo J et al (2009) Chronic dialysis and death among survivors of acute kidney injury requiring dialysis. JAMA 302:1179–1185
Leung KC, Tonelli M, James MT (2013) Chronic kidney disease following acute kidney injury-risk and outcomes. Nat Rev Nephrol 9:77–85
Thadhani R, Pascual M, Bonventre JV (1996) Acute renal failure. N Engl J Med 334:1448–1460
Srisawat N, Kellum JA (2011) Acute kidney injury: definition, epidemiology, and outcome. Curr Opin Crit Care 17:548–555
Li X, Hassoun HT, Santora R, Rabb H (2009) Organ crosstalk: the role of the kidney. Curr Opin Crit Care 15:481–487
Basile DP (2007) The endothelial cell in ischemic acute kidney injury: implications for acute and chronic function. Kidney Int 72:151–156
Sutton TA, Molitoris BA (1998) Mechanisms of cellular injury in ischemic acute renal failure. Semin Nephrol 18:490–497
Bonventre JV, Yang L (2011) Cellular pathophysiology of ischemic acute kidney injury. J Clin Invest 121:4210–4221
McCullough PA, Shaw AD, Haase M et al (2013) Diagnosis of acute kidney injury using functional and injury biomarkers: workgroup statements from the tenth Acute Dialysis Quality Initiative Consensus Conference. Contrib Nephrol 182:13–29
Earley A, Miskulin D, Lamb EJ, Levey AS, Uhlig K (2012) Estimating equations for glomerular filtration rate in the era of creatinine standardization: a systematic review. Ann Intern Med 156:785–795, W-270, W-271, W-272, W-273, W-274, W-275, W-276, W-277, W-278
Lanzman RS, Wittsack HJ, Martirosian P et al (2010) Quantification of renal allograft perfusion using arterial spin labeling MRI: initial results. Eur Radiol 20:1485–1491
Prasad PV (2006) Functional MRI of the kidney: tools for translational studies of pathophysiology of renal disease. Am J Physiol Ren Physiol 290:F958–974
Pohlmann A, Hentschel J, Fechner M et al (2013) High temporal resolution parametric MRI monitoring of the initial ischemia/reperfusion phase in experimental acute kidney injury. PLoS One 8:e57411
Lanzman RS, Ljimani A, Pentang G et al (2013) Kidney transplant: functional assessment with diffusion-tensor MR imaging at 3T. Radiology 266:218–225
Thoeny HC, De Keyzer F (2011) Diffusion-weighted MR imaging of native and transplanted kidneys. Radiology 259:25–38
Hueper K, Gutberlet M, Rodt T et al (2011) Diffusion tensor imaging and tractography for assessment of renal allograft dysfunction-initial results. Eur Radiol 21:2427–2433
Shah B, Anderson SW, Scalera J, Jara H, Soto JA (2011) Quantitative MR imaging: physical principles and sequence design in abdominal imaging. Radiographics 31:867–880
Kiricuta IC Jr, Simplaceanu V (1975) Tissue water content and nuclear magnetic resonance in normal and tumor tissues. Cancer Res 35:1164–1167
Kundel HL, Schlakman B, Joseph PM, Fishman JE, Summers R (1986) Water content and NMR relaxation time gradients in the rabbit kidney. Investig Radiol 21:12–17
de Miguel MH, Yeung HN, Goyal M et al (1994) Evaluation of quantitative magnetic resonance imaging as a noninvasive technique for measuring renal scarring in a rabbit model of antiglomerular basement membrane disease. J Am Soc Nephrol 4:1861–1868
Puntmann VO, Voigt T, Chen Z et al (2013) Native T1 mapping in differentiation of normal myocardium from diffuse disease in hypertrophic and dilated cardiomyopathy. JACC Cardiovasc Imaging 6:475–484
Messroghli DR, Walters K, Plein S et al (2007) Myocardial T1 mapping: application to patients with acute and chronic myocardial infarction. Magn Reson Med 58:34–40
Won S, Davies-Venn C, Liu S, Bluemke DA (2013) Noninvasive imaging of myocardial extracellular matrix for assessment of fibrosis. Curr Opin Cardiol 28:282–289
Salerno M, Kramer CM (2013) Advances in parametric mapping with CMR imaging. JACC Cardiovasc Imaging 6:806–822
Hueper K, Rong S, Gutberlet M et al (2013) T2 relaxation time and apparent diffusion coefficient for noninvasive assessment of renal pathology after acute kidney injury in mice: comparison with histopathology. Invest Radiol 48:834–842
Liu AS, Xie JX (2003) Functional evaluation of normothermic ischemia and reperfusion injury in dog kidney by combining MR diffusion-weighted imaging and Gd-DTPA enhanced first-pass perfusion. J Magn Reson Imaging 17:683–693
Gueler F, Rong S, Mengel M et al (2008) Renal urokinase-type plasminogen activator (uPA) receptor but not uPA deficiency strongly attenuates ischemia reperfusion injury and acute kidney allograft rejection. J immunol (Baltimore, Md : 1950) 181:1179-1189
Klein S, Staring M, Murphy K, Viergever MA, Pluim JP (2010) elastix: a toolbox for intensity-based medical image registration. IEEE Trans Med Imaging 29:196–205
Lu X, Li N, Shushakova N et al (2012) C57BL/6 and 129/Sv mice: genetic difference to renal ischemia-reperfusion. J Nephrol 25:738–743
Hueper K, Gutberlet M, Rong S et al (2014) Acute Kidney Injury: Arterial Spin Labeling to Monitor Renal Perfusion Impairment in Mice--Comparison with Histopathologic Results and Renal Function. Radiology 270:117–124
Karlberg L, Norlen BJ, Ojteg G, Wolgast M (1983) Impaired medullary circulation in postischemic acute renal failure. Acta Physiol Scand 118:11–17
Haase VH (2013) Mechanisms of Hypoxia Responses in Renal Tissue. J Am Soc Nephrol. doi:10.1681/ASN.2012080855
Hricak H, Terrier F, Demas BE (1986) Renal allografts: evaluation by MR imaging. Radiology 159:435–441
Yuasa Y, Kundel HL (1985) Magnetic resonance imaging following unilateral occlusion of the renal circulation in rabbits. Radiology 154:151–156
London DA, Davis PL, Williams RD, Crooks LE, Sheldon PE, Gooding CA (1983) Nuclear magnetic resonance imaging of induced renal lesions. Radiology 148:167–172
Acknowledgements
The scientific guarantor of this publication is Prof. Dr. Faikah Gueler. The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article. This study has received funding by REBIRTH Cluster of Excellence of Hannover Medical School (Germany). No complex statistical methods were necessary for this paper. Institutional Review Board approval was not required because it was an experimental study without the use of human data. Approval from the institutional animal care committee was obtained. Some study subjects or cohorts have been previously reported in: Hueper K, Rong S, Gutberlet M, et al. (2013) T2 relaxation time and apparent diffusion coefficient for noninvasive assessment of renal pathology after acute kidney injury in mice: comparison with histopathology. Invest Radiol, 48(12):834-842.
Hueper K, Gutberlet M, Rong S, et al. (2014) Acute Kidney Injury: Arterial Spin Labeling to Monitor Renal Perfusion Impairment in Mice--Comparison with Histopathologic Results and Renal Function. Radiology, 270(1):117-124
Methodology: prospective, experimental, performed at one institution.
Katja Hueper and Matti Peperhove contributed equally as first authors.
Dagmar Hartung and Faikah Gueler contributed equally as last authors.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Suppl. Fig. 1
Examples of inversion recovery EPI-images at different inversion times Shown are inversion recovery EPI-images at different inversion times (TI = 30, 700, 1200, 1500, 8000 ms). In the upper row EPI-images at baseline (d0) and in the lower row images of the same kidney at d7 after severe AKI are shown. (JPEG 1129 kb)
Rights and permissions
About this article
Cite this article
Hueper, K., Peperhove, M., Rong, S. et al. T1-mapping for assessment of ischemia-induced acute kidney injury and prediction of chronic kidney disease in mice. Eur Radiol 24, 2252–2260 (2014). https://doi.org/10.1007/s00330-014-3250-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-014-3250-6