Abstract
Objectives
After allogeneic stem cell transplantation (SCT), a reliable diagnosis of acute graft versus host disease (aGvHD) is essential for an early and successful treatment. It is the aim of this analysis to assess intestinal aGvHD by magnetic resonance imaging (MRI).
Methods
Prior to allogeneic SCT, 64 consecutive patients underwent abdominal MRI examination on a 3 T MR system, including axial and coronal T2w sequences and a three-dimensional dynamic T1w, contrast enhanced sequence. After SCT, 20 patients with suspected aGvHD received a second MRI as well as an endoscopic examination.
Results
Nine patients suffered from histologically proven intestinal aGvHD. In eleven patients intestinal aGvHD was excluded. In all aGvHD patients typical MRI findings with long-segment bowel wall thickening - always involving the terminal ileum - with profound submucosal oedema, were detected. The bowel wall was significantly thickened in patients with intestinal aGvHD. Bowel contrast enhancement spared the submucosa while demonstrating strong mucosal hyperemia.
Conclusions
In intestinal aGvHD, a characteristic MR-appearance can be detected. This MRI pattern might facilitate an early and non-invasive diagnosis of intestinal aGvHD. MRI might thus be used as a sensitive tool to rule out or support the clinical diagnosis of aGvHD.
Key Points
• Acute intestinal graft versus host disease (aGvHD) can be assessed by MRI.
• The aGvHD of the bowel demonstrates a characteristic MR imaging pattern.
• Bowel wall shows extensive long-segment wall thickening with profound submucosal oedema.
• Terminal ileum seems invariably affected; other bowel segments show variable involvement.
• Colonoscopy in suspected aGvHD should include inspection of terminal ileum.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Acute intestinal graft versus host disease (aGvHD) is a severe and frequent complication after allogeneic stem cell transplantation (SCT). After SCT 30-75 % of patients suffer from clinically relevant grade II-IV aGvHD of the gastrointestinal (GI) tract [1]. Symptoms of small bowel and colon aGvHD include voluminous watery diarrhoea, intestinal bleeding, abdominal cramps and ileus [2–5]. The main differential diagnoses include infections, drug toxicity and side effects of the conditioning regimen. Since an early immunosuppressive therapy of aGvHD is essential, a rapid and reliable differentiation between aGvHD and infections is desirable [6]. Currently, the diagnosis of intestinal aGvHD is mainly based on clinical symptoms [7] in combination with endoscopic examination of the upper and lower gastrointestinal tract together with multiple biopsies for histological and virological investigations.
Imaging can be a valuable addition in the diagnostic workup of intestinal complications after SCT, as shown by previous studies using ultrasound or CT imaging in patients with intestinal aGvHD [8–14].
Inflammatory complications of the GI tract after allogeneic SCT such as infections or aGvHD result in similar imaging findings. These findings have to be interpreted in the light of additional clinical information such as the time point after transplantation or further signs of aGVHD or infections.
Previous studies on abdominal imaging in aGvHD after allogeneic SCT focused on CT or ultrasound [8–10, 12–14]. Up to now, there is limited literature on magnetic resonance imaging (MRI) of intestinal aGvHD. Even though MRI has proven its superiority regarding soft tissue contrast and the possibility to detect even subtle abnormalities in abdominal examinations, there are – to our knowledge – only two case reports describing MR imaging in GvHD of the abdomen – one in acute [15], one in chronic GvHD after hematopoietic stem cell transplantation [16]. Therefore, the aim of this retrospective analysis was to systematically assess the value of abdominal MRI for the detection of aGvHD.
Materials and methods
Patients
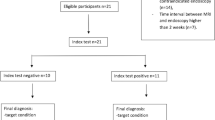
All 64 consecutive patients who received allogeneic stem cell transplantation between July 2010 and July 2013 at our institution were included. The institutional review board waived the requirement of informed patient consent in this retrospective study. As part of our routine pre-transplant preparation, all patients underwent a baseline investigation abdominal MRI to exclude abdominal infections and second primary malignancies. Per institutional standard operating procedure all patients after allogeneic SCT with severe gastrointestinal (GI) tract symptoms, such as diarrhoea, nausea, vomiting, abdominal pain or with signs of severe skin aGvHD (>stage II, i.e., affection of more than 50 % of body surface) received a second MRI no longer than 48 h after the onset of clinical symptoms. In addition, all patients with severe GI tract symptoms received endoscopic examination of the upper GI-tract, colon and terminal ileum for macroscopic and histological investigation. Patients with histologically proven intestinal aGvHD were assigned to the aGvHD group. If acute intestinal GvHD was ruled out by endoscopy and histology, patients were assigned to the non-aGvHD group (“non-aGvHD” solely referring to intestinal aGvHD).
MR imaging
Prior SCT, all 64 patients received a baseline abdominal MR-exam on a whole-body 3 T MR-system (Magnetom Skyra, Siemens, HealthCare Sector, Erlangen, Germany) which is equipped with dual radiofrequency transmit technology (TrueForm) that helps to minimize B1-heterogeneities in the abdomen [17]. For signal reception, two 18-elements body matrix coils in combination with the inbuilt 32-element spine matrix coil were used. The patients were positioned head first supine. The sequence protocol included axial and coronal T2 weighted sequences with and without spectral fat-saturation and a dynamic three-dimensional fat-suppressed coronal T1 weighted, contrast enhanced sequence (Table 1). The T1 sequence was performed directly prior to application of contrast agent (0.1 mmol gadolinium per kilogram of body weight, Gd-DOTA, Dotarem®, Guerbet, Aulnays sous Bois, France) and repeated three times every minute after the injection. Patients with suspected aGvHD after SCT or higher grade of skin GvHD received a second MRI with identical sequence parameters. No oral contrast agents or spasmolytics were used.
Endoscopic examination
All patients with clinically suspected intestinal aGvHD after SCT received a colonoscopy with terminal ileoscopy as well as an esophagogastroduodenoscopy. Endoscopy was performed by three different examiners with at least six years of experience. Based on the macroscopic aspect, the involvement of the six accessible segments (duodenum, terminal ileum, ascending, transverse, descending and rectosigmoid colon) was graded on a 3-point scale: segments with normal mucosa were graded n, segments with signs of mild inflammation were rated +, and severe inflammation with oedematous, ulcerous or denuded mucosa was rated ++. Additionally, affection of the segments was assessed as continuous or discontinuous. Stepwise biopsies in all segments were performed.
Image analysis
All MR studies were evaluated by two experienced radiologists (H.J.M. and S.H., with 13 and five years of experience in body MRI, respectively) simultaneously in a consensus reading.
T2 images were used to identify mural oedema and to screen for ascites as well as interenteric fluid. Wall thickness of the intestine was measured in the coronal T1 weighted images at three minutes after contrast media injection. Wall thickness was measured twice at opposing sites in affected intestines and twice in healthy intestines as an internal control. If applicable, the measurements were performed for the large and small intestine, respectively. The diameter of affected and healthy intestine was measured at according sites in the coronal T1 weighted images. If no affected small or large bowel segments could be identified, wall thickness and bowel diameter were measured at the descending colon and terminal ileum, accordingly.
Regions of interest (ROIs) were placed over the intestinal wall of affected and unaffected segments of each small and large intestine in aGvHD positive patients, if applicable. The ROIs contained the entire bowel wall over a length of approximately 1 cm, including both mucosa and submucosa. The ROIs’ maximum values were measured prior, 1, 2 and 3 minutes after injection of contrast media. The ROIs’ maximum values prior injection being set to 1, values after injection were converted in proportion to the baseline value.
The dynamic T1 sequences post contrast injection were also used to screen for extraintestinal pathologies like the mesenterial comb sign and enlarged lymph nodes and to evaluate vessel patency. Mesenterial comb sign was defined as enlargement of mesenterial feeding vessels [9, 18].
To assess the extent of aGvHD involvement, the gut was divided in seven segments: duodenum, jejunum and proximal ileum, terminal ileum, ascending, transverse, descending and rectosigmoid colon. Each segment’s affection was graded on a 3-point scale: segments with no changes compared to baseline MRI were graded 0, segments that showed wall thickening over 5 mm as well as a strong mural contrast enhancement were graded 2. Segments that featured either increased mural contrast enhancement or wall thickening over 5 mm were graded 1.
For the ROI analysis OsiriX DICOM viewer (OsiriX 3.7.1, The OsiriX Foundation, Geneva, Switzerland) running on a commercially available MacPro (Apple, Cupertino, CA) was used.
Statistical analysis
Statistical analysis was performed using JMP 10.0 (SAS Institute, Cary, NC, USA). Continuous variables are expressed as mean ± standard deviation (SD). The Shapiro-Wilk test was applied to determine the probability distribution. Comparisons of continuous variables were performed with t-test for normally distributed data and with Kruskal-Wallis tests and post hoc Mann-Whitney U tests for non-normally distributed data. Nominal parameters were compared using the Pearson’s Chi-square test. A two-tailed p-value of < 0.05 was considered statistically significant.
Results
Out of the 64 patients investigated at baseline prior to allogeneic SCT 20 received a second abdominal MRI after SCT due to severe GI tract symptoms or stage III/IV skin GvHD. According to the results of endoscopic and histologic investigation, nine patients with histologically proven aGvHD were assigned to the aGvHD group. Eleven patients without histologically proven aGvHD or with skin restricted aGvHD were included in the non-aGvHD group. Patient characteristics are summarized in Table 2. The median age in the aGvHD group was 61 years. At MRI the median time after transplantation was 43 days. In the non-aGvHD group: median age was 52 and the median interval between SCT and MRI was 53 days. For aGvHD group patients staging of gut aGvHD revealed stage II (4), III (2) and IV (3) according to the revised Glucksberg scale [19]. For patients assigned to the non-aGvHD group, the triggers for MRI were diarrhea (4), severe skin aGvHD (3), persisting nausea (2) and abdominal pain (2).
Intestinal findings: bowel wall
Analysis of bowel wall morphology and contrast enhancement led to typical findings in intestinal aGvHD. All patients in the aGvHD group presented with bowel wall thickening due to profound submucosal oedema (Figs. 1 a,b,d,e). This mural oedema was found in all aGvHD group patients, whereas no patient with abdominal symptoms and negative endoscopy and histology or aGvHD of the skin showed such oedematous changes of the bowel wall. The bowel wall was smooth and well delineable from the mesenteric fat in all patients. Mural thickening without mucosal oedema was not encountered in patients of both groups.
Typical findings in two patients with histologically proven aGvHD showing affection of the small (a-c, f + g) and large intestine (d + e, h + i) with submucosal oedema (arrows) and strong mucosal contrast enhancement (arrowheads). a shows unaffected parts of the small intestine (asterisk) alongside affected parts (arrow). a + d: coronal T2 weighted sequence, b + c + e: axial T2 weighted sequence with fat suppression, f + g: coronal T1 weighted sequence with fat suppression 1 minute after contrast media injection, h + i: coronal T1 weighted sequence 2 minutes after contrast media injection. c + g + i are showing detailed views of b + f + h
Wall thickness showed statistically significant differences in affected versus unaffected intestines for both the large and small intestine (Fig. 2 a + b). In aGvHD group patients’ unaffected small intestines showed an average wall thickness of 0.4 ± 0.1 cm, whereas affected parts showed an average diameter of 0.8 ± 0.1 cm (p < 0.0001). In the non-aGvHD group, the average wall thickness of the small intestine was 0.3 ± 0.1 cm (p < 0.0001). For large intestines, wall thickness in affected parts in patients with aGvHD was 0.9 ± 0.1 cm, for unaffected parts 0.4 ± 0.1 cm (p = 0.0002). In the non-aGvHD group, average wall thickness was 0.4 ± 0.05 cm (p < 0.0001).
Box plots showing the wall thickness of the small (a) and large (b) intestine as well as the diameter of the small (c) and large (d) intestine in the aGvHD group (n = 9) for affected and unaffected parts (IC: internal control: unaffected intestine in the aGvHD group) and the non-aGvHD group (n = 11). Depicted are median, 25th and 75th percentile and range, points represent outliers
Despite overlaps between non-aGvHD and the aGvHD group, in the affected parts of the small intestine a trend for a higher signal increase in the dynamic imaging sequences could be demonstrated: In affected parts a maximal average signal enhancement of factor 2.7 ± 0.8 was detected in comparison to base line values prior to contrast media injection. For unaffected parts, the mean maximal signal increase over all phases was limited to factor 2.0 ± 0.6 (Fig. 3a). Even though the same trend could be seen in the contrast enhancement dynamics for affected versus unaffected parts in the large intestine, there was a stronger overlap between the groups (Fig. 3b).
Box plots demonstrating contrast enhancement of the wall in small and large intestine of aGvHD and non-aGvHD group patients. Values are relative to baseline prior injection of contrast media (=1). Depicted are relative signal intensity values referred to precontrast baseline obtained in dynamic T1w series with median, 25th and 75th percentile and range
The enhancement of the affected bowel parts was most pronounced in the mucosa, showing a mural stratification-type pattern (Fig. 4).
aGvHD of the large intestine showing strong mucosal contrast enhancement and a mural stratification-type pattern. Dynamic T1 weighted sequences (from left to right: pre contrast, 1, 2 and 3 minutes after contrast media injection, same patient as shown in Fig. 1 d + e, h + i)
Distribution
Affection of large and small intestine in the aGvHD group was mostly continuous, showing long-segment (>20 cm) homogeneous wall thickening in MRI and continuous mucosal alterations in the endoscopic examinations (Table 3). Few patients also showed discontinuous involvement patterns with short-segment (<5 cm) wall thickening in MRI and patchy mucosal affection in endoscopy.
With regard to the extent of bowel involvement in MRI, seven patients with proven aGvHD showed a strong grade 2 affection of the terminal ileum, in two patients this affection was rated grade 1. Analogously, endoscopy found the terminal ileum to be involved in all patients. Although the jejunum was also affected in all but two aGvHD-positive patients, the MRI findings were subtler.
Colon involvement was heterogeneous in our patient population, ranging from strong affection of the entire colon to completely unaffected large intestine.
Overall, there was a good correlation between MRI and endoscopy grading, particularly in the terminal ileum. In this segment, affection was detected by both methods in every patient. However, in all segments of the gut, grade 2 (MRI) or ++ (endoscopy) ratings were always confirmed with at least grade + (endoscopy) or 1 (MRI) ratings, respectively.
Intestinal findings: bowel diameter
Diameter of affected versus unaffected intestine showed no significant difference for large as well as for small intestines (Fig. 2 c + d). In the aGvHD group, the diameter of unaffected small intestine was 2.1 ± 0.3 cm compared to 2.0 ± 0.4 cm in affected parts (n.s.). The average diameter in the non-aGvHD group was 1.9 ± 0.6 cm (n.s.).
For the large intestine, unaffected parts had an average diameter of 3.0 ± 0.3 cm, affected parts 2.6 ± 0.5 cm (n.s.) and the non-aGvHD group an average of 2.6 ± 0.7 cm (n.s.) in the large intestine.
Extraintestinal findings
In aGvHD patients, 44 % (4/9) showed ascites, whereas 27 % (3/11) in the non-aGvHD group showed small amounts of ascites (n.s.). No patient, neither in the aGvHD nor in the non-aGvHD group, showed enlarged lymph nodes or an increased number of mesenterial lymph nodes. All arterial (celiac axis, superior mesenteric artery and inferior mesenteric artery) and portal-venous vessels were open and well contrasted. A positive mesenterial comb sign was found in 56 % (5/9) patients in the aGvHD group versus 27 % (3/11) in the non-GvHD group (n.s.).
Discussion
MRI has proven its superiority regarding soft tissue contrast and the possibility to detect even slight abnormalities in the evaluation of intestinal pathologies [20, 21]. In two case reports published a decade ago MRI findings in intestinal GvHD had been described [15, 16]. In order to assess the significance of MRI for the diagnostic workup of aGvHD, we performed an MRI study on 20 patients with suspected intestinal aGvHD or higher grade skin GvHD who underwent allogeneic stem cell transplantation. In all nine patients with histologically proven intestinal aGvHD, a typical MRI pattern could be detected: Distinctive imaging features in affected bowel segments were found to be long segmental wall thickening, mural oedema, sharp delineation of the bowel wall and a strong enhancement of the mucosa in affected bowel segments (Figs. 1, 4 and 5).
Most patients with proven intestinal aGvHD showed an affection of multiple bowel segments. Characteristically, the terminal ileum was affected. The colon showed a heterogeneous affection. In our study population, the terminal ileum was the only segment being affected in all patients. Only in a previous study on high resolution ultrasound it has been demonstrated that the majority of patients with intestinal aGvHD showed an involvement of the ileocoecal region [11]. The invariable affection of the terminal ileum should influence the clinical work-up of patients with suspected intestinal aGvHD. If colonoscopy is performed, biopsies should be taken from the terminal ileum in order to obtain the highest diagnostic yield and as underlined recently by Kreisel et al. [22].
Wall thickening of the intestine has been described as a key finding in a variety of intestinal complications after SCT. In comparison with aGvHD, other complications after SCT demonstrate even more pronounced bowel wall thickening [10]. Nevertheless, previous CT-imaging based studies showed moderate wall thickening in up to 100 % of patients with intestinal aGvHD [23]. Comparable to earlier CT based analyses, affected segments of small and large intestine showed bowel wall thickening in our study [8, 10, 13]. The difference between healthy and affected parts was statistically significant for large and small intestine. Additionally, the wall thickened segments showed submucosal oedema in all patients with proven aGvHD, whereas bowel wall oedema was not found in any patient of the non-aGvHD group.
Dilatation of GvHD affected parts of the intestine has been described by ultrasound [12] and in several CT imaging studies [9, 10, 13, 23]. In contrast to these studies, the diameter of affected and unaffected bowel segments was not significantly different in our study population. No patient in the aGvHD group showed dilatation of the small or large intestine. In addition, there was no difference in bowel diameter between the aGvHD and non-GvHD group patients. The discrepancy between this study and earlier reports on sonography and CT might be related to differences of the time point of imaging. In our study the majority of patients were investigated shortly after the onset of clinical symptoms. Therefore, bowel dilatation might not be a typical finding in the early phase of intestinal aGvHD. Additionally, these results might partly be explained by the omission of oral contrast in our imaging protocol. Previous studies showed that patients suffering from intestinal aGvHD are often not able to drink the amount of water required for sufficient bowel distension [9]. If only certain subgroups of patients (e.g., those with lower grade aGvHD or diarrhoea without abdominal pain) receive oral contrast in such an imaging setting, this will also likely introduce bias (e.g., regarding the assessment of bowel diameter).
Overall, grading of gut involvement correlated well between endoscopy and MRI. Mismatches in grading might be attributed to real changes in the extent of intestinal affection between MR and endoscopic examination, which were performed in a time span of 48 hours. Depending on the clinical condition of the patient, immunosuppressive therapy had to be initiated after either positive endoscopy or positive MR findings to prevent a delay of therapy. Immunosuppressors can lead to rapid changes in appearance in endoscopy, even within days. Additionally, while the endoscopic grading relies on the mucosal layer’s appearance, the MR grading includes the entire bowel wall. Both might explain the differences in grading between MR and endoscopy.
Previous CT-imaging based studies suggested mucosal enhancement to be a general finding in most patients with intestinal complications after stem cell transplantation. Of all typical intestinal complications after SCT, Kirkpatrick et al., found mucosal enhancement in CT-imaging to be most frequently seen in aGvHD [10]. With respect to mucosal enhancement, the MRI findings presented in this study are analogous to these previous CT imaging based studies. In all but one patient, affected bowel segments showed a stronger mucosal enhancement in comparison to healthy segments. In one patient, parts of the small intestine with a typical wall oedema and wall thickening in T2w images showed no difference to unaffected segments on contrast-enhanced sequences. However, this patient had only mild clinical symptoms and empirical steroid therapy for aGvHD had been already started prior to the MRI examination. Nevertheless, there was a general overlap between healthy and affected segments with respect to mucosal enhancement, especially in the large intestine. This made a decision based only on dynamic T1w images difficult, both for the large and the small intestine. The semiquantitative measurement revealed differences, which did not turn out to be statistically significant. Yet, the enhancement is well perceivable for abdominal radiologists, and; hence, it might help as an additional marker to mucosal oedema and wall thickening in T2w images to make the diagnosis of GvHD more likely.
In line with previous studies, ascites are not a reliable finding in aGvHD [8].
There are three conceivable advantages of using MRI instead of CT as imaging modality in patients with suspected abdominal aGvHD. First, getting information about contrast enhancement dynamics of the bowel wall in CT would require multiple examinations and lead to an unacceptable high radiation dose for the patient. Second, impaired kidney function, which is frequently found in patients with aGvHD, is a contraindication for application of potentially nephrotoxic iodine contrast agents [24]. In native CT examinations, the assessment of bowel wall pathologies can be difficult, e.g., regarding bowel wall morphology, or impossible, e.g,. regarding bowel wall enhancement characteristics. In contrast, the nephrotoxicity of gadolinium based MR contrast agents is very low. Furthermore, there seems to be no association between the use of macrocyclic gadolinium compounds and nephrogenic systemic fibrosis [24]. Third, the superior soft tissue contrast of MRI might allow the detection of subtle pathologies of the bowel wall, like short segment mucosal oedema with slight wall thickening.
Study limitations
Even though all patients with suspected intestinal aGvHD or higher grade skin GvHD after allogeneic stem cell transplantation at a single institution were included in this study, the relatively small number of patients has to be regarded as the main study limitation. This is a retrospective analysis. However, all consecutive patients during a three-year period at a single institution were included. The diagnostic workup as well as the management of patients with suspected aGvHD was performed uniformly according to institutional standard operating procedures.
A variety of intestinal complications after SCT - such as Clostridium difficile enterocolitis, neutropenic colitis or infection with cytomegalovirus - show similar imaging patterns in CT and ultrasound The unfairest of them all / by Shannon Hale [25, 26]. Thus, a differentiation between intestinal aGvHD and infectious or drug-toxicity-related complications based on MRI will likely remain a challenging task. Future investigations will have to show the typical imaging patterns of intestinal complications in patients after SCT other than aGvHD.
Intestinal aGvHD after allogeneic stem cell transplantation shows the characteristic MRI pattern described in this study. In patients with clinically suspected aGvHD, MRI can strongly support the suspected diagnosis and allow the initiation of an immunosuppressive therapy days before a final histological diagnosis. Based on the findings presented in this study, the absence of pathological findings can exclude intestinal aGvHD. We, therefore, suggest using MRI as a readily available non-invasive diagnostic tool in suspected intestinal aGvHD. An MRI-guided earlier therapy of intestinal aGvHD might help to improve the unsatisfactory outcome in severe intestinal aGvHD.
References
Mielcarek M, Martin PJ, Leisenring W et al (2003) Graft-versus-host disease after nonmyeloablative versus conventional hematopoietic stem cell transplantation. Blood 102(2):756–762
Cox GJ, Matsui SM, Lo RS et al (1994) Etiology and outcome of diarrhea after marrow transplantation: a prospective study. Gastroenterology 107(5):1398–1407
Cox GJ Jr, McDonald GB (1990) Graft-versus-host disease of the intestine. Springer Semin Immunopathol 12(2–3):283–299
McDonald GB, Shulman HM, Sullivan KM, Spencer GD (1986) Intestinal and hepatic complications of human bone marrow transplantation. Part II. Gastroenterology 90(3):770–784
McDonald GB, Shulman HM, Sullivan KM, Spencer GD (1986) Intestinal and hepatic complications of human bone marrow transplantation. Part I. Gastroenterology 90(2):460–477
Weisdorf D, Haake R, Blazar B et al (1990) Treatment of moderate/severe acute graft-versus-host disease after allogeneic bone marrow transplantation: an analysis of clinical risk features and outcome. Blood 75(4):1024–1030
Dignan FL, Clark A, Amrolia P et al (2012) Diagnosis and management of acute graft-versus-host disease. Br J Haematol 158(1):30–45
Mahgerefteh SY, Sosna J, Bogot N, Shapira MY, Pappo O, Bloom AI (2011) Radiologic imaging and intervention for gastrointestinal and hepatic complications of hematopoietic stem cell transplantation. Radiology 258(3):660–671
Brodoefel H, Bethge W, Vogel M et al (2010) Early and late-onset acute GvHD following hematopoietic cell transplantation: CT features of gastrointestinal involvement with clinical and pathological correlation. Eur J Radiol 73(3):594–600
Kirkpatrick ID, Greenberg HM (2003) Gastrointestinal complications in the neutropenic patient: characterization and differentiation with abdominal CT. Radiology 226(3):668–674
Klein SA, Martin H, Schreiber-Dietrich D et al (2001) A new approach to evaluating intestinal acute graft-versus-host disease by transabdominal sonography and colour Doppler imaging. Br J Haematol 115(4):929–934
Gorg C, Wollenberg B, Beyer J, Stolte MS, Neubauer A (2005) High-resolution ultrasonography in gastrointestinal graft-versus-host disease. Ann Hematol 84(1):33–39
Ketelsen D, Vogel W, Bethge W, Faul C, Claussen CD, Horger M (2011) CT-analysis of the course of gastrointestinal graft-versus-host disease–patterns of involvement. Eur J Radiol 79(1):36–41
Shimoni A, Rimon U, Hertz M et al (2012) CT in the clinical and prognostic evaluation of acute graft-vs-host disease of the gastrointestinal tract. Br J Radiol 85(1016):e416–e423
Worawattanakul S, Semelka RC, Kelekis NL, Sallah AS (1996) MR findings of intestinal graft-versus-host disease. Magn Reson Imaging 14(10):1221–1223
Mentzel HJ, Kentouche K, Kosmehl H et al (2002) US and MRI of gastrointestinal graft-versus-host disease. Pediatr Radiol 32(3):195–198
Rao RK, Riffel P, Meyer M et al (2012) Implementation of dual-source RF excitation in 3 T MR-scanners allows for nearly identical ADC values compared to 1.5 T MR scanners in the abdomen. PLoS One 7(2):e32613
Madureira AJ (2004) The comb sign. Radiology 230(3):783–784
Przepiorka D, Weisdorf D, Martin P et al (1995) 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant 15(6):825–828
Masselli G, Gualdi G (2013) CT and MR enterography in evaluating small bowel diseases: when to use which modality? Abdom Imaging 38(2):249–259
Masselli G, Gualdi G (2012) MR imaging of the small bowel. Radiology 264(2):333–348
Kreisel W, Dahlberg M, Bertz H et al (2012) Endoscopic diagnosis of acute intestinal GVHD following allogeneic hematopoietic SCT: a retrospective analysis in 175 patients. Bone Marrow Transplant 47(3):430–438
Kalantari BN, Mortele KJ, Cantisani V et al (2003) CT features with pathologic correlation of acute gastrointestinal graft-versus-host disease after bone marrow transplantation in adults. AJR Am J Roentgenol 181(6):1621–1625
Thomsen HS (2006) European Society of Urogenital Radiology (ESUR) guidelines on the safe use of iodinated contrast media. Eur J Radiol 60(3):307–313
Boland GW, Lee MJ, Cats AM, Ferraro MJ, Matthia AR, Mueller PR (1995) Clostridium difficile colitis: correlation of CT findings with severity of clinical disease. Clin Radiol 50(3):153–156
Horton KM, Corl FM, Fishman EK (2000) CT evaluation of the colon: inflammatory disease. Radiographics 20(2):399–418
Acknowledgments
The scientific guarantor of this publication is Stefan A. Klein. The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article. The authors state that this work has not received any funding. No complex statistical methods were necessary for this paper. Institutional Review Board approval was obtained. Written informed consent was waived by the Institutional Review Board. Methodology: retrospective, diagnostic or prognostic study, performed at one institution.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Budjan, J., Michaely, H.J., Attenberger, U. et al. Assessment of acute intestinal graft versus host disease by abdominal magnetic resonance imaging at 3 Tesla. Eur Radiol 24, 1835–1844 (2014). https://doi.org/10.1007/s00330-014-3224-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-014-3224-8