Abstract
Objective
To assess the value of the liver and spleen viscoelastic parameters at multifrequency MR elastography to determine the degree of portal hypertension and presence of high-risk oesophageal varices in patients with cirrhosis.
Methods
From January to September 2012, 36 consecutive patients with cirrhosis evaluated for transplantation were prospectively included. All patients underwent hepatic venous pressure gradient (HVPG) measurements and endoscopy to assess oesophageal varices. Multifrequency MR elastography was performed within the liver and spleen. The shear, storage and loss moduli were calculated and compared to the HVPG with Spearman coefficients and multiple regressions. Patients with and without severe portal hypertension and high-risk varices were compared with Mann–Whitney tests, logistic regression and ROC analysis.
Results
The liver storage and loss moduli and the spleen shear, storage and loss moduli correlated with the HVPG. At multiple regression, only the liver and the spleen loss modulus correlated with the HVPG (r = 0.44, p = 0.017, and r = 0.57, p = 0.002, respectively). The spleen loss modulus was the best parameter for identifying patients with severe portal hypertension (p = 0.019, AUROC = 0.81) or high-risk varices (p = 0.042, AUROC = 0.93).
Conclusions
The spleen loss modulus appears to be the best parameter for identifying patients with severe portal hypertension or high-risk varices.
Key points
1. Noninvasive HVPG assessment can be performed with liver and spleen MR elastography
2. The spleen loss modulus enables the detection of high-risk oesophageal varices
3. The spleen loss modulus enables the detection of severe portal hypertension
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Portal hypertension is a frequent complication of cirrhosis, contributing to the development of ascites, oesophageal varices and hepatic encephalopathy. The reference method for assessing portal hypertension is the measurement of the hepatic venous pressure gradient (HVPG) through invasive hepatic vein catheterisation [1]. HVPG ≥ 12 mmHg defines severe portal hypertension and predicts the occurrence of rupture of oesophageal varices, but also of ascites and encephalopathy [2, 3]. HVPG measurement is minimally invasive but carries patient discomfort, increases the burden for medical teams and the cost of medical care. Furthermore, this measurement is limited to highly specialised centres and requires extensive expertise [4]. The risk of variceal bleeding can also be assessed with oesophageal endoscopy. The risk of bleeding not only concerns patients with medium and large size varices, but also patients with small varices and red wale marks or Child C class [3]. Guidelines recommend yearly screening endoscopy in these patients with high-risk varices [3]. Noninvasive methods for diagnosing severe portal hypertension are thus needed.
Several methods have been described for this purpose, including the assessment of platelet count [5–7], spleen size [8] or their combination (platelet to spleen ratio) [9], presence of collateral veins and portal vein dilatation on ultrasound [10, 11], several serum markers [12, 13] and increase in Child-Pugh score [6, 7, 14]. However, none of these methods has been shown to be accurate enough for use in clinical practice and to replace HVPG measurement or endoscopy screening.
More recently, measurements of liver and spleen stiffness with ultrasound using either transient elastography or acoustic radiation force impulse imaging have shown promising results in detecting portal hypertension and estimating the presence of oesophageal varices [15–19]. Both techniques are subjected to biases mainly caused by lack of operator training and tissue heterogeneity [20].
Relative to the current ultrasound elastographic methods, MR elastography has advantages that may improve the diagnostic performance [21]. Very few studies assessing its value in portal hypertension have been performed and tissue stiffness has been the only viscoelastic parameter assessed. Nedredal et al. showed in a canine model of cholestatic chronic liver disease that there was a correlation between spleen stiffness and the HVPG [22]. Recently, Yin et al. reinforced those results by reporting a significant spleen stiffness increase in pigs with acute portal hypertension [23]. In humans, Talwalkar et al. reported the feasibility of the spleen stiffness measurements with MR elastography in healthy volunteers and patients with various degrees of liver fibrosis [24], and Yin et al. described postprandial hepatic stiffness augmentation in patients with chronic liver disease [25]. No study thus far has focused on the diagnostic value of more detailed viscoelastic parameters accessible with three-dimensional multifrequency MR elastography in patients with portal hypertension. Therefore, the aim of our study was to prospectively assess the value of the liver and spleen viscoelastic parameters at three-dimensional multifrequency MR elastography for determining the degree of portal hypertension and presence of high-risk varices in a consecutive series of patients with cirrhosis having HPVG measurements.
Patients and methods
Patient selection and design of the study
This prospective monocentric study was conducted between January and September 2012 in the radiology department of our tertiary university hospital. Informed consent was obtained from each patient included in the study. Its protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki and the study was approved by our local ethics committee.
Forty-five patients with histologically proven hepatic cirrhosis (F4 in the METAVIR classification) and evaluated for liver transplantation were prospectively included. Clinical and laboratory data including presence and severity of ascites (mild or severe), platelet count, body mass index, international normalised ratio, serum levels of gamma-glutamyl transpeptidase (GGT), alkaline phosphatase (AP), aspartate aminotransferase (AST), alanine aminotransferase (ALT), albumin and bilirubin were recorded. Patients were classified according to the Child-Pugh score [27]. MELD was calculated according to the United Network for Organ Sharing [28].
HVPG and three-dimensional multifrequency MR elastography for measuring the liver and spleen viscoelastic parameters were performed within 1 week. Upper tract endoscopy was performed within 6 months of MR elastography. All operators worked independently and were not aware of the results of the other investigations.
Out of the 45 initial patients, 3 (7 %) did not have HVPG measurements and were excluded. Six other patients (14 %) did not complete the MR elastography examinations because of movements, claustrophobia or inability to maintain apnoea and were also excluded. The final population consisted of 36 patients (Fig. 1).
The number of patients reached the minimum required to allow at least 90 % power for detecting an increase in HVPG of 1 mmHg using spleen stiffness measurement, with a two-sided 5 % significance level. According to the results from the power analysis, a total of 35 patients had to be included in the study.
Measurements of HVPG
After overnight fasting, the patients were transferred to the hepatic haemodynamic laboratory. All patients underwent measurement of their HVPG under local anaesthesia by one of our experienced hepatologists. An 8 F venous introducer was placed into the right internal jugular using the Seldinger technique. Under fluoroscopic control and continuous electrocardiographic and arterial pressure monitoring, a 7 F catheter (Torcon NB Advantage, Cook Medical, Cook, Bloomington, IN) was placed into the right hepatic vein. The HVPG was calculated as the difference between wedged and free hepatic venous pressures. Patients were considered as having severe portal hypertension if HVPG was ≥12 mmHg. All measurements were performed in three different sites, and permanent tracings were recorded.
Upper gastrointestinal endoscopy
Endoscopy was performed by one of our endoscopy operators with expertise in the assessment of patients with cirrhosis. According to international expert recommendations, endoscopic findings were graded as follows: grade I, varices flattened by insufflation; grade II, nonconfluent varices protruding in the lumen despite insufflation; grade III, confluent varices not flattened by insufflation [29]. The presence of red signs was also recorded. High-risk oesophageal varices were defined according to the Baveno V criteria as grade II to III varices or grade I varices with red colour signs or Child-Pugh class C [3, 19].
MR elastography
After overnight fasting, the MR elastography examinations were performed on a 1.5-T clinical MR system (Intera, Philips Healthcare, Best, The Netherlands) equipped with a four-element surface coil array. Waves were generated with two electromagnetic mechanical transducers (Philips Healthcare, Hamburg, Germany) driven by a function generator with amplified output. The two transducers were placed against the right and left hypochondria to explore both the liver and spleen, respectively.
T2-weighted MR imaging of the liver and spleen was performed first for proper positioning of the MR elastography slices. A gradient echo MR elastography sequence with fractional encoding was applied in the three spatial directions of motion to obtain volumetric images of the propagating mechanical waves inside the body. The imaging parameters included field of view 40 × 40 cm, nine slices, slice thickness of 4 mm (overlap = 0), in-plane resolution: 5 × 3 mm, 8 phase offsets, TR/TE: 111/9 ms, encoding fraction q = 0.23, 0.47, 0.7, and acquisition time of 4 × 16 s with breathholding. The MR elastography acquisitions were obtained with three different mechanical excitation frequencies of 28, 56 and 84 Hz applied simultaneously [30].
The complex-valued shear, storage and loss moduli [G*, Gd and Gl respectively (kPa)] were calculated by fitting a polynomial function to the displacement values and inversion of the local time-harmonic wave equation, as previously described [26].
On the T2-weighted images, one large region of interest (ROI) was positioned on the central section of the liver and one on the central section of the spleen. The ROIs were propagated to the other slices. Care was taken to exclude large vessels and hilum and to include only the organ parenchyma. The ROIs were then copied on the parametric maps at all frequencies to obtain measurements of G*, Gd and Gl in the liver and the spleen. The viscoelastic parameters were calculated as the mean of the values obtained in the ROIs of the three contiguous central slices. Moreover, the relation between the viscoelastic parameters and the excitation frequency was fitted on a power law and the exponent of the law (γ) was extracted [26].
The post-processing of the MR elastography images was performed by a radiologist with 7-year experience in abdominal radiology. The reproducibility of the liver Gd measurements has been reported previously [21].
Statistical analysis
Values are expressed as median and range, or percentage, as appropriate. The viscoelastic parameters of the liver and spleen were correlated with the measurements of the HVPG using non-parametric Spearman correlation coefficients and multiple regressions. Differences between patients without/with severe portal hypertension and high-grade varices were assessed using nonparametric two-sided Mann–Whitney tests and logistic regressions.
The discriminative ability of the different viscoelastic parameters for the identification of patients with severe portal hypertension and high-risk oesophageal varices was assessed with receiver-operating characteristic (ROC) curve analysis and expressed as areas under the ROC curve (AUROC). The AUROCs were considered significant when they differed significantly from the non-discrimination line (AUROC of 0.50). Comparisons between AUROCs were performed with the DeLong test. Sensitivities and specificities for optimal cutoff values were calculated by maximising the Youden index. P-values ≤ 0.05 were considered significant. All analyses were performed with the Statistical Package for the Social Sciences software (version 20.0, SPSS Inc., Chicago, IL).
Results
Patient characteristics
The clinical and laboratory features of the 36 patients are summarised in Table 1. Briefly, the population had a median age of 56 years (31–69 years) and consisted mostly of men (n = 28, 78 % of the patients). Cirrhosis was mainly related to alcohol consumption (n = 16, 44 %) and hepatitis C virus infection (n = 9, 25 %). The Child score was A (n = 7, 19 %), B (n = 13, 36 %) and C (n = 16, 45 %). The median MELD score was 15 (range: 6–33).
The patients had a median HVPG of 16 mmHg (range: 6–36 mmHg). Thirty (83 %) and 25 (69 %) patients had HVPG ≥10 and ≥12 mmHg, respectively. Ascites were detected in 25 patients (69 %). Twenty-seven patients had oesophageal varices (75 %) and 26 (72 %) had oesophageal varices at high risk of bleeding.
Liver and spleen viscoelastic parameters
Table 2 details the distribution of the different viscoelastic parameters for the whole study population. The values of G*, Gd and Gl increased with increasing frequency and were higher in the spleen than in the liver.
Regarding the liver viscoelastic parameters, significant correlations were observed between Gd, Gl and HVPG, but only at 84 Hz (r = 0.41, p = 0.025; and r = 0.37, p = 0.047, respectively). No correlation was found between G* or γ and HVPG (Table 2). At multiple regression, only Gl at 84 Hz correlated with HVPG (r = 0.44, p = 0.017).
Regarding the spleen measurements, there were significant correlations between G*, Gd and Gl on the one hand and HVPG on the other at both 56 and 84 Hz, but not at 28 Hz (r = 0.42, p = 0.028; r = 0.44, p = 0.022; and r = 0.53, p = 0.0041, respectively, at 56 Hz; and r = 0.43, p = 0.024; r = 0.41, p = 0.034; and r = 0.47, p = 0.014 at 84 Hz) (Table 2). Again, there was no correlation between γ and HVPG. At multiple regression, only Gl at 56 Hz and 84 Hz correlated with HVPG (r = 0.57, p = 0.002, and r = 0.48, p = 0.012, respectively).
Patients with severe portal hypertension and high-risk oesophageal varices
The liver viscoelastic parameters did not significantly differ between patients with and without severe portal hypertension. In contrast, the spleen G*, Gd, and Gl were significantly higher in patients with severe portal hypertension than in patients without it, both at 56 and 84 Hz (p < 0.04) (Table 3 and Fig. 2). At logistic regression, only Gl at 56 Hz was found to be associated with severe portal hypertension (p = 0.019).
Box plots of the spleen shear (G*), storage (Gd) and loss (Gl) moduli at 56 and 84 Hz. Viscoelastic parameters are significantly higher in patients with severe portal hypertension relative to patients without it. Line within boxes represents median; lower and upper limits of boxes represent 25th and 75th percentiles respectively. Whiskers represent 10 and 90 percentiles and outliers are represented as individual points. Grey boxes correspond to patients with severe portal hypertension (HVPG ≥12 mmHg)
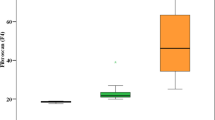
Similarly, the hepatic viscoelastic parameters were not able to differentiate between patients with and without high-risk oesophageal varices. In the spleen, G* at 84 Hz and Gl at 56 Hz and 84 Hz were significantly higher in patients with high-risk varices (p = 0.03, p = 0.0025 and p = 0.0003, respectively) (Table 3, Fig. 3.). At logistic regression, only Gl at 84 Hz was associated with high-risk oesophageal varices (p = 0.042).
Box plot of spleen shear (G*), storage (Gd) and loss (Gl) moduli at 56 and 84 Hz. Viscoelastic parameters are significantly higher in patients with high-risk varices (+) than in patients without them at 84 Hz, as are Gd and Gl at 56 Hz. Line within boxes represents median, lower and upper limits of boxes represent 25th and 75th percentiles respectively. Whiskers represent 10th and 90th percentiles. Grey boxes correspond to patients with high-risk varices
ROC analysis for severe portal hypertension
Differentiation between patients with and without severe portal hypertension could be obtained at ROC curve analysis of the spleen viscoelastic parameters with AUROCs ranging from 0.73 to 0.81 and p-values ranging from 0.01 to 0.0035 (Table 4). The comparison between the AUROCs of G*, Gd and Gl showed no significant difference between them (p-values ranging from 0.25 to 0.75). However, Gd and Gl at 56 Hz had the largest AUROCs: 081 ± 0.07.
For Gd and Gl at 56 Hz, cutoff values of 4.5 kPa and 2.5 kPa enabled diagnosing severe portal hypertension with sensitivities of 65 % and 64 % and specificities of 85 and 92 %, respectively. The positive predictive values were 80 % and 80 % and the negative predictive values 44 % and 47 %, respectively.
ROC analysis for high-risk varices
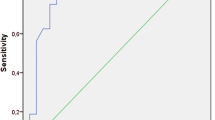
For the diagnosis of high-risk oesophageal varices, spleen Gl at 56 Hz, and G* and Gl at 84 Hz had significant AUROCs ranging from 0.76 to 0.93 (p-values ranging from 0.023 to 0.001) (Table 4 and Fig. 4). Gl at 84 Hz had the largest AUROC. The difference in AUROCs between Gl and G* at 84 Hz reached statistical significance (p = 0.05). Similarly, Gl at 56 Hz had a larger AUROC than G* and Gd at the same frequency, without reaching the level of significance.
With a cutoff value of 4.2 kPa, Gl at 84 Hz enabled identifying high-risk varices with sensitivity and specificity of 54 % and 100 %. The corresponding positive predictive and negative predictive values were 33 % and 79 %.
Discussion
The results of our study in patients with cirrhosis show that three-dimensional multifrequency MR elastography of the spleen is a promising method for assessing severe portal hypertension and high-risk oesophageal varices. Among the viscoelastic parameters that were assessed, the loss modulus Gl was the most accurate.
Most studies about elastography in portal hypertension have been performed with ultrasound elastography. The liver stiffness assessed at one-dimensional transient elastography has been shown to correlate with the HVPG in several studies [15, 31, 32]. Regarding the spleen, Colecchia et al. showed that the spleen stiffness assessed with one-dimensional transient elastography presented a better correlation with HVPG than the liver stiffness and could be used for the noninvasive assessment of portal hypertension and detection of oesophageal varices in patients with cirrhosis [16]. These results correlated with those obtained with real time tissue elastography by Hirooka et al. [33]. Takuma et al. used ARFI, another ultrasound-based elastography method, and reported similar results [19].
Publications about MR elastography in portal hypertension remain scarce. The correlations we observed between the viscoelastic parameters and HVPG are consistent with those published in animal models by Nedredal et al. and Yin et al. [22, 23]. In humans, the study published by Talwalkar et al. showed the feasibility of spleen stiffness measurements with MR elastography and reported significantly differences in stiffness between patients harbouring varices or not [24]. Moreover, Yin et al. described a postprandial hepatic stiffness increase in patients with chronic liver disease [25]. However, in these studies there was no measurement of HVPG.
In our series of patients with HVPG measurements, we showed that the viscoelastic parameters of the spleen, but not the liver, allowed the identification of patients with severe portal hypertension and those with high-risk varices, with acceptable performances. Those results are in line with published data regarding ultrasound methods [19, 33]. The inferiority of the liver parameters might be explained by the fact that the liver viscoelastic parameters reflect not only the variations of portal hypertension, but also the presence of extensive hepatic fibrosis [21, 23, 24, 34]. Some histological changes in the spleen have also been described in portal hypertension, including fibrosis, but are usually less advanced than in the liver.
A potential advantage of three-dimensional MR elastography over current ultrasound elastography methods is its ability to decompose the viscoelastic parameters in different components, namely G* reflecting the stiffness, but also Gd reflecting the elasticity and Gl related to the viscosity. Moreover, the multifrequency approach allows the calculation of the exponent of the frequency power law, γ, which reflects the architectural organisation of the tissue [26]. The spleen loss modulus Gl was the best viscoelastic parameter for identifying patients with severe portal hypertension and high-risk varices. This might be explained by the spleen congestion in portal hypertension, while tissue organisation is not dramatically modified. Conversely, Gd and γ are less likely to be influenced by congestion.
New ultrasound elastography methods, such as the recently introduced multifrequency shear wave dispersion ultrasound vibrometry method, allow calculating the elasticity and viscosity [35] in addition to the stiffness. This means that the better diagnostic performance that we observed in the current study regarding spleen Gl (viscosity) relative to Gd (elasticity) and G* (stiffness) might also be relevant to ultrasound elastography.
Finally, we observed better results with the viscoelastic parameters measured at 56 Hz and 82 Hz than at 28 Hz. This may be explained by the long wavelength at 28 Hz, causing some boundary effects, especially in smaller organs such as the spleen [36].
While there are some technical issues that may hamper its applicability, mainly the cost and the accessibility of the method in comparison to ultrasound-based approaches, three-dimensional MR elastography has several advantages including the combination of volumetric assessment of the viscoelastic parameters of whole organs (taking into considerations local viscoelastic heterogeneities) and precise local assessment (avoiding the impact of reflected shear waves caused by the border of the organs that may induce artefacts in stiffness values as estimated using a time-of-flight approach [36]. Whether or not those theoretical advantages of three-dimensional MR elastography relative to current ultrasound elastography methods will translate into better performance for the assessment of portal hypertension remains to be investigated.
Our study suffers from several limitations. First, our population is relatively limited. However, the number of patients was considered to be sufficient based on our statistical power calculation. Moreover, this limitation is counterbalanced by the prospective design of the study and the extensive evaluation performed including not only endoscopic evaluation but also HVPG measurements.
Second, the patients frequently had severe portal hypertension as illustrated by the high median HVPG. However, we were able to observe differences between patients with and without severe portal hypertension and between those with and without high-risk varices [32]. Regarding the latter patients, the sensitivity of Gl for the identification of high-risk varices was not high. This might be partially explained by the definition of such varices, including grade I varices with red colour signs, difficult to identify with MR elastography alone.
Third, we did not study the influence of a meal on the viscoelastic parameters [25, 37]. All the MR elastography procedures were performed after overnight fasting to avoid potential confounding effects caused by eating. Fourth, we did not include a control group composed of patients without portal hypertension. It would have been interesting to compare our results with those obtained in a normal population, but it would not have been ethical to obtain HVPG in these subjects. Finally, in our population, the liver/spleen MR elastography analysis was incomplete or impossible in six patients. If we exclude the three patients who did not undergo HVPG measurements, this leads to a feasibility rate of 86 %. This percentage is lower than the reported feasibility of liver MR elastography (95 % in the study of Huwart et al. [21]), but is similar to that of liver/spleen ultrasound elastography (88 % in the study of Colecchia et al. [16]). Further studies comparing the feasibility of liver/spleen MR and ultrasound elastography in the same population should be performed.
In conclusion, the liver and the spleen viscoelastic parameters assessed with three-dimensional multifrequency MR elastography were correlated with HVPG in patients with cirrhosis, and the spleen loss modulus Gl was with the best parameter for diagnosing severe portal hypertension and high-risk oesophageal varices.
Abbreviations
- MR:
-
magnetic resonance
- HVPG:
-
hepatic venous pressure gradient
- G*:
-
complex shear modulus
- Gd:
-
storage modulus
- Gl:
-
loss modulus
- ROC:
-
receiver-operating characteristics
- INR:
-
international normalised ratio
- GGT:
-
gamma-glutamyl transpeptidase
- AP:
-
alkaline phosphatase
- AST:
-
aspartate aminotransferase
- ALT:
-
alanine aminotransferase
- AUROC:
-
area under the ROC curve
References
Bosch J, Abraldes JG, Berzigotti A, Garcia-Pagan JC (2009) The clinical use of HVPG measurements in chronic liver disease. Nat Rev Gastroenterol Hepatol 6:573–582
Ripoll C, Groszmann R, Garcia-Tsao G et al (2007) Hepatic venous pressure gradient predicts clinical decompensation in patients with compensated cirrhosis. Gastroenterology 133:481–488
de Franchis R, Baveno VF (2010) Revising consensus in portal hypertension: report of the Baveno V consensus workshop on methodology of diagnosis and therapy in portal hypertension. J Hepatol 53:762–768
Groszmann RJ, Wongcharatrawee S (2004) The hepatic venous pressure gradient: anything worth doing should be done right. Hepatology 39:280–282
Thomopoulos KC, Labropoulou-Karatza C, Mimidis KP, Katsakoulis EC, Iconomou G, Nikolopoulou VN (2003) Non-invasive predictors of the presence of large oesophageal varices in patients with cirrhosis. Dig Liver Dis 35:473–478
Schepis F, Camma C, Niceforo D et al (2001) Which patients with cirrhosis should undergo endoscopic screening for esophageal varices detection? Hepatology 33:333–338
Pilette C, Oberti F, Aube C et al (1999) Non-invasive diagnosis of esophageal varices in chronic liver diseases. J Hepatol 31:867–873
Madhotra R, Mulcahy HE, Willner I, Reuben A. Prediction of esophageal varices in patients with cirrhosis. J Clin Gastroenterol;34:81–85
Giannini E, Botta F, Borro P et al (2003) Platelet count/spleen diameter ratio: proposal and validation of a non-invasive parameter to predict the presence of oesophageal varices in patients with liver cirrhosis. Gut 52:1200–1205
Cottone M, D’Amico G, Maringhini A et al (1986) Predictive value of ultrasonography in the screening of non-ascitic cirrhotic patients with large varices. J Ultrasound Med 5:189–192
Berzigotti A, Rossi V, Tiani C et al (2011) Prognostic value of a single HVPG measurement and Doppler-ultrasound evaluation in patients with cirrhosis and portal hypertension. J Gastroenterol 46:687–695
Sebastiani G, Tempesta D, Fattovich G et al (2010) Prediction of oesophageal varices in hepatic cirrhosis by simple serum non-invasive markers: Results of a multicenter, large-scale study. J Hepatol 53:630–638
Thabut D, Trabut JB, Massard J et al (2006) Non-invasive diagnosis of large oesophageal varices with FibroTest in patients with cirrhosis: a preliminary retrospective study. Liver Int 26:271–278
Berzigotti A, Gilabert R, Abraldes JG et al (2008) Noninvasive prediction of clinically significant portal hypertension and esophageal varices in patients with compensated liver cirrhosis. Am J Gastroenterol 103:1159–1167
Castera L, Pinzani M, Bosch J (2012) Non invasive evaluation of portal hypertension using transient elastography. J Hepatol 56:696–703
Colecchia A, Montrone L, Scaioli E et al (2012) Measurement of spleen stiffness to evaluate portal hypertension and the presence of esophageal varices in patients with HCV-related cirrhosis. Gastroenterology 143:646–654
Berzigotti A, Seijo S, Arena U et al (2013) Elastography, spleen size, and platelet count identify portal hypertension in patients with compensated cirrhosis. Gastroenterology 144:102–111, e1
Sharma P, Kirnake V, Tyagi P et al (2013) Spleen stiffness in patients with cirrhosis in predicting esophageal varices. Am J Gastroenterol 108:1101–1107
Takuma Y, Nouso K, Morimoto Y et al (2013) Measurement of spleen stiffness by acoustic radiation force impulse imaging identifies cirrhotic patients with esophageal varices. Gastroenterology 144:92–101, e2
Bavu E, Gennisson JL, Couade M et al (2011) Noninvasive in vivo liver fibrosis evaluation using supersonic shear imaging: a clinical study on 113 hepatitis C virus patients. Ultrasound Med Biol 37:1361–1373
Huwart L, Sempoux C, Vicaut E et al (2008) Magnetic resonance elastography for the noninvasive staging of liver fibrosis. Gastroenterology 135:32–40
Nedredal GI, Yin M, McKenzie T et al (2011) Portal hypertension correlates with splenic stiffness as measured with MR elastography. J Magn Reson Imaging 34:79–87
Yin M, Kolipaka A, Woodrum DA et al (2013) Hepatic and splenic stiffness augmentation assessed with MR elastography in an in vivo porcine portal hypertension model. J Magn Reson Imaging 38:809–15
Talwalkar JA, Yin M, Venkatesh S et al (2009) Feasibility of in vivo MR elastographic splenic stiffness measurements in the assessment of portal hypertension. AJR Am J Roentgenol 193:122–127
Yin M, Talwalkar JA, Glaser KJ et al (2011) Dynamic postprandial hepatic stiffness augmentation assessed with MR elastography in patients with chronic liver disease. AJR Am J Roentgenol 197:64–70
Sinkus R, Siegmann K, Xydeas T, Tanter M, Claussen C, Fink M (2007) MR elastography of breast lesions: understanding the solid/liquid duality can improve the specificity of contrast-enhanced MR mammography. Magn Reson Med 58:1135–1144
Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R (1973) Transection of the oesophagus for bleeding oesophageal varices. Br J Surg 60:646–649
Wiesner RH, McDiarmid SV, Kamath PS et al (2001) MELD and PELD: application of survival models to liver allocation. Liver Transpl 7:567–580
Qureshi W, Adler DG, Davila R et al (2005) ASGE Guideline: the role of endoscopy in the management of variceal hemorrhage, updated July 2005. Gastrointest Endosc 62:651–655
Garteiser P, Sahebjavaher RS, Ter Beek L et al (2013) Rapid acquisition of multifrequency, multislice and multidirectional magnetic resonance elastography data with a fractionally encoded gradient echo sequence. NMR Biomed 26:1326–35
Carrion JA, Navasa M, Bosch J, Bruguera M, Gilabert R, Forns X (2006) Transient elastography for diagnosis of advanced fibrosis and portal hypertension in patients with hepatitis C recurrence after liver transplantation. Liver Transpl 12:1791–1798
Vizzutti F, Arena U, Romanelli RG et al (2007) Liver stiffness measurement predicts severe portal hypertension in patients with HCV-related cirrhosis. Hepatology 45:1290–1297
Hirooka M, Ochi H, Koizumi Y et al (2011) Splenic elasticity measured with real-time tissue elastography is a marker of portal hypertension. Radiology 261:960–8
Huwart L, Peeters F, Sinkus R et al (2006) Liver fibrosis: non-invasive assessment with MR elastography. NMR Biomed 19:173–179
Chen S, Sanchez W, Callstrom MR, Gorman B, Lewis JT, Sanderson SO et al (2013) Assessment of liver viscoelasticity by using shear waves induced by ultrasound radiation force. Radiology 266:964–970
Deffieux T, Gennisson JL, Bercoff J, Tanter M (2011) On the effects of reflected waves in transient shear wave elastography. IEEE Trans Ultrason Ferroelectr Freq Control 58:2032–2035
Arena U, Platon ML, Stasi C et al (2013) Liver stiffness is influenced by a standardized meal in patients with chronic hepatitis C virus at different stages of fibrotic evolution. Hepatology 58:65–72
Acknowledgements
We thank Lucile Dehec, Ariane Metei, Maryse Moinat, Maryvonne Monroty and Irène Rua for their help in identifying the patients.
The scientific guarantor of this publication is Bernard E Van-Beers. The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article. The authors state that this work has not received any funding. No complex statistical methods were necessary for this paper. Institutional review board approval was obtained. Written informed consent was obtained from all subjects (patients) in this study. Methodology: prospective, diagnostic or prognostic study, performed at one institution.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ronot, M., Lambert, S., Elkrief, L. et al. Assessment of portal hypertension and high-risk oesophageal varices with liver and spleen three-dimensional multifrequency MR elastography in liver cirrhosis. Eur Radiol 24, 1394–1402 (2014). https://doi.org/10.1007/s00330-014-3124-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-014-3124-y