Abstract
Objective
We evaluated the performance of manual measures of coronary plaque volumes and atherosclerotic plaque features from coronary CT angiography (CTA), using intravascular ultrasound (IVUS) as the reference.
Methods
Thirty individual coronary plaques with suitable fiduciary markers were identified. Plaque volumes on coronary CTA were manually quantified by two observers and compared to IVUS plaque volumes as interpreted by an independent laboratory. The presence of adverse plaque characteristics—low attenuation plaque (LAP), positive remodelling (PR) and spotty calcification (SC)—on coronary CTA was evaluated and compared to IVUS.
Results
High correlation in plaque volumes was detected between observers (r = 0.94, P < 0.0001; 95 % limits of agreement <48.7 mm3, bias 6.6 mm3). Excellent correlation (r = 0.95, P < 0.0001) was noted in plaque volume between independent observers and IVUS (95 % limits of agreement <40.6 mm3, bias −4.4 mm3) and did not differ from IVUS (105.0 ± 56.7 vs. 109.4 ± 60.7 mm3, P = 0.2). The frequency of LAP (10 % vs. 17 %), PR (7 % vs. 10 %) and SC (27 % vs. 33 %) was similar between coronary CTA and IVUS (all P = NS).
Conclusions
Plaque volume on coronary CTA determined by manual methods demonstrates high correlation and modest agreement to IVUS. Further, coronary CTA demonstrates high accuracy for the identification of adverse plaque characteristics, including LAP, PR and SC.
Key Points
• Coronary CT angiography is a non-invasive test that enables coronary plaque assessment
• Plaque quantification by coronary CT angiography correlates well with intravascular ultrasound findings
• Coronary CT angiography can identify adverse plaque characteristics
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Direct determination of coronary atherosclerotic plaque morphology and plaque burden by invasive methods enhances risk assessment of future major adverse cardiac events (MACE) and monitoring of coronary artery disease (CAD) progression [1–3]. Coronary CT angiography (CTA) has emerged as a potential non-invasive alternative to coronary plaque characterisation and quantification [4–6]. These measures require manual tracing of contours, discriminating epicardial fat from vessel walls and circumscribing noncalcified and calcified plaque components, techniques that are time intensive and may be prone to variability [7, 8]. Therefore, quantification of coronary atherosclerotic plaque volume by coronary CTA has not been extensively investigated. The purpose of this study was to evaluate the performance of manual CT measures of coronary plaque volumes—including both calcified and noncalcified components—employing grayscale intravascular ultrasound (IVUS) as a reference standard as well as the performance of coronary CTA to identify plaque characteristics.
Materials and methods
Study patients and plaque selection
Study patients consisted of consecutive individuals who underwent elective invasive coronary angiography (ICA), IVUS and coronary CTA who were observed by coronary CTA to have a stenosis within the proximal or mid part of a major epicardial coronary artery. Coronary CTA was performed for suspected or known CAD prior to clinically indicated ICA. IVUS was performed as part of the research protocol. Patients were excluded if any of the following was present: individuals unable to provide informed consent, acute ST elevation myocardial infarction, left ventricular ejection fraction <40 %, primary myocardial or valvular disease, presence of collateral vessels by coronary CTA or ICA, or angiographically visible thrombus at the site of the lesion interrogated by IVUS. The Institutional Review Board of the study centres approved the study and all patients provided written informed consent.
Coronary CTA
Coronary CTA was performed using a dual–source CT scanner (Somatom Definition, Siemens, Forchheim, Germany) in direct accordance with the Society of Cardiovascular CT Guidelines on performance of coronary CTA [9]. All patients received nitroglycerin and those with a heart rate >65 bpm were administered beta blockers, unless contraindicated. An intravenous bolus (60–90 ml) of contrast agent (350 mg iodine/ml, Iomeprol) was injected at a flow rate of 6 ml/s. The CT parameters included 2 × 64 × 0.6 mm collimation, gantry rotation time 330 ms, reference tube current of 400 mAs per rotation and tube voltage 100 or 120 kVp. CT was performed using ECG-based tube current modulation whenever possible; otherwise sequential (prospective) image acquisition was used. Axial images were reconstructed with 0.6–0.75-mm slice thickness, 0.3–0.4-mm slice increment and a medium soft convolution kernel (B26f). For helical CT data acquisition, the position of the reconstruction window within the cardiac cycle was individually selected to minimise artefacts. Motion-free data sets, typically in mid-diastole, were collected for analysis. The estimated effective radiation dose for coronary CTA ranged from 2 mSv to 10 mSv using the dose-length product with an organ-specific conversion factor k of 0.014 mSv/mGy/cm [10].
ICA and IVUS
Selective ICA was performed by standard catheterisation in accordance with the American College of Cardiology Guidelines for Coronary Angiography [11]. IVUS was performed in a standard fashion using an automated motorised pullback system (0.5 mm/s) with commercially available imaging catheters (Boston Scientific/SCIMED, Minneapolis, MN, USA). The IVUS catheter was inserted into the most distal position of the selected vessel that could be safely reached, and the catheter location was documented with cine angiography. Intracoronary nitroglycerin (100–200 μg) was administered immediately prior to IVUS.
Plaque volume measurements and characterisation by coronary CTA
Each identified plaque lesion was quantified manually by two independent level III experienced observers, both of whom were blinded to the IVUS results. Study results were computed as the average of two observers. Another experienced independent observer who was not involved in the later comparative analysis evaluated coronary CTA and ICA, IVUS images side by side. To ensure that identical plaques evaluated with IVUS were assessed, easily identifiable anatomic landmarks (side branches and bifurcations) were selected to define the proximal and distal fiduciary points. Plaques that lacked a proximal reference segment because of an ostial location as well as those within large side branches were not considered. After identification of plaques referenced to fiduciary markers, contiguous cross-sectional reconstructions were rendered along a vessel centerline using a slice thickness of 1.0 mm and an increment of 0.5 mm on a dedicated 3D workstation (AW Advantage; GE Healthcare) [8]. To allow for optimal detection of plaque and outer vessel boundaries, we used a window width representing 155 % of the mean intensity within the lumen and at a window level representing 65 % of the mean intensity for each lesion, as previously described [4]. The outer vessel contour was defined as the visualised border at which point low attenuation (epicardial fat) was observed, with lumen areas manually traced in each cross-section. The difference between vessel area and contrast-enhanced lumen area was defined as the plaque area. Plaque volume was obtained by the summation of all contiguous plaque areas and multiplied by the slice increment [12].
In addition, the presence of adverse plaque characteristics was evaluated as follows: Low attenuation plaque (LAP) was defined as visually distinct intraplaque hypodensities containing <30 HU; positive arterial remodelling (PR) was defined as the maximal outer arterial wall diameter along the plaque exceeding proximal reference by ≥5 %; spotty calcification (SC) was discrete calcification ≤3 mm in length and occupying a ≤90° arc when viewed in the vessel short axis [13].
Plaque volume measurements and characterisation by IVUS
IVUS images were analysed in blinded fashion by an independent core laboratory (Seoul National University Hospital), according to established and validated standards [14]. Lumen and external elastic membrane (vessel) contours were manually traced at every 0.5-mm cross-sectional frame using commercially available planimetry software (echoPlaque™ system, Indec Systems Inc., Mountain View, CA), generating volumetric data in a manner similar to that described for coronary CTA. Plaque volume was calculated as vessel volume minus lumen volume. In addition, the presence of echolucent plaque, PR and SC was evaluated as previously described [15, 16].
Statistical analysis
Continuous variables were expressed as mean ± SD and range. Comparisons between volumes were performed using a paired t test. Correlation and agreement between two observers on coronary CTA and coronary CTA to IVUS measurements were evaluated by Pearson’s correlation and Bland-Altman analysis. Averaged values between two observers on coronary CTA were used for the comparison of IVUS measurements. The frequency of LAP, PR and SC was compared between CT and IVUS using the chi-square test for trend. A P value of less than 0.05 was considered indicative of statistical significance. Statistical analysis was performed using dedicated statistical software (SPSS 19.0, SPSS, Chicago, IL, USA).
Results
We identified 30 individual coronary atherosclerotic plaques in 27 consecutive individuals. The patient characteristics are shown in Table 1. The distribution of stenoses was: 18 in the left anterior descending artery, 5 in the left circumflex artery and 7 in the right coronary artery. The average plaque length measured by coronary CTA was 14.7 ± 7.7 mm. The mean value of window width and window level was 795 ± 198 HU and 334 ± 83 HU. Processing times ranged from 15 to 30 min for CT manual quantification.
High correlation existed for vessel volume, lumen volume and plaque volume (r = 0.97, r = 0.96, r = 0.95, all P < 0.0001) for coronary CTA compared to IVUS. Bland-Altman limits of agreement for vessel volume, lumen volume and plaque volume ranged from −53.7 to 53.1 mm3 with a bias of −0.3 mm3, −24.1 to 32.2 mm3 with a bias of 4.0 mm3 and −40.6 to 31.8 mm3 with a bias of −4.4 mm3, respectively (Fig. 1). There were no significant differences in vessel, lumen and plaque volume between coronary CTA and IVUS (191.0 ± 101.9 vs. 191.3 ± 108.3 mm3, P = 0.9; 86.0 ± 50.4 vs. 82.0 ± 51.4 mm3, P = 0.1; 105.0 ± 56.7 vs. 109.4 ± 60.7 mm3, P = 0.2, respectively). Per artery results for plaque volume are summarised in Table 2. There were high correlations with no significant differences in plaque volume between coronary CTA and IVUS measurements in the left anterior descending artery (LAD), left circumflex artery (LCX) and right coronary artery (RCA), respectively.
Correlations were high for vessel volume, lumen volume and plaque volume (r = 0.99, r = 0.97, r = 0.94, all P < 0.0001) between two observers. Bland-Altman limits of agreement for vessel volume, lumen volume and plaque volume ranged from −29.1 to 43.8 mm3 with a bias of 7.4 mm3, −23.0 to 24.5 mm3 with a bias of 0.8 mm3 and −35.5 to 48.7 mm3 with a bias of 6.6 mm3, respectively (Fig. 2). The bias in plaque volume between two observers corresponded to 10.8 %. There were no significant differences in lumen and plaque volume between two observers (85.6 ± 50.0 vs. 86.4 ± 51.5 mm3, P = 0.7, 101.7 ± 62.1 vs. 108.3 ± 53.0 mm3, P = 0.1). There were no significant differences in plaque volume between each observer and IVUS (101.7 ± 62.1 vs. 109.4 ± 60.7 mm3, P = 0.1, 108.3 ± 53.0 vs. 109.4 ± 60.7 mm3, P = 0.8). There were significant differences in vessel volume between two observers (187.3 ± 105.2 vs. 194.7 ± 99.3 mm3, P = 0.04). All 30 individual plaques are described in detail (Supplementary Table).
The frequency of LAP/echolucent plaque (10 % vs. 17 %), PR (7 % vs. 10 %) and SC (27 % vs. 33 %) was similar with no significant differences between coronary CTA and IVUS (all P = NS) (Fig. 3). A representative case of the images with IVUS and coronary CTA are shown in Figs. 4 and 5. Adverse plaque characteristics on coronary CTA are depicted in Fig. 6.
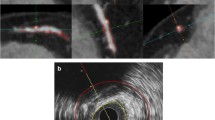
Images show quantification of proximal right coronary artery plaque in a 68-year-old male with hyperlipidaemia. a Longitudinal IVUS view of the plaque. White arrow Position of cross-sectional views. b Cross-sectional IVUS view with (c) traced external elastic membrane (red arrow) and lumen-intima border (yellow arrow). d Same plaque imaged on coronary CTA with longitudinal view. White dotted line Position of cross-sectional views. e Cross-sectional coronary CTA view with (f) traced outer vessel border (red arrow) and lumen (orange arrow). In this leasion, plaque volume is calculated as 79.7 mm3 for IVUS and 83.4 mm3 for coronary CTA. Abbreviations as in Fig. 1
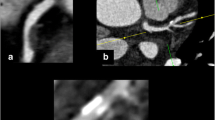
Images: Top row shows example of noncalcified plaque (a) and calcified plaque (b) on coronary CTA. Bottom row shows the corresponding image of IVUS for noncalcified plaque (c) and calcified plaque (d). Yellow allow Noncalcified plaque, Red allow Calcified plaque. Abbreviations are the same as in Fig. 1
Discussion
In the present study, we observed a high correlation between total plaque volumes quantified from coronary CTA as compared to an IVUS reference standard. Further, plaque measures by coronary CTA demonstrated low biases with generally favourable limits of agreement. Importantly, we noted no significant differences in plaque volume quantified by IVUS and coronary CTA. Finally, we examined the performance of coronary CTA to identify plaque characteristics associated with worsened prognosis, including LAP, PR and SC. These present data may suggest a role for coronary CTA as a noninvasive method for accurate quantification and characterisation of atherosclerotic plaque.
Qualitative plaque characterisation by coronary CTA has been evaluated by Pundziute et al. [17], who demonstrated that non-calcified, calcified and mixed exhibit significantly different compositions when assessed by IVUS with radiofrequency backscatter analysis; however, the quantitative relationship of coronary CTA to IVUS was not evaluated in this study. Prior studies have demonstrated generally high correlation between cardiac coronary CTA and IVUS for assessment of plaque volume [4, 6, 12, 18–22]. In these studies, overall plaque volumes were underestimated with coronary CTA when compared with IVUS [4, 12]. These findings may relate to the results of a recent meta-analysis [23], which observed that coronary CTA slightly overestimates lumen area, presumably in part because of partial volume effects that lead to overestimation of the size of very bright structures (such as the contrast-enhanced lumen). Nevertheless, in these analyses, plaque area and volume were similar between coronary CTA and IVUS. The differences between coronary CTA and IVUS volumetric measurements could be attributed to several technical and methodological factors. The spatial resolution of 64-detector CT is less than IVUS, and the accuracy of coronary CTA may be mitigated by inaccuracies in edge discrimination of coronary CTA arterial images. The present study results observed a high correlation for quantitative assessment of plaque volume by coronary CTA, with a low bias of only 4.4 mm3. However, Bland-Altman limits of agreement were generally wide, suggesting a modest agreement between the coronary CTA and IVUS. One distinction between the present study and prior published investigations is that we observed the interobserver variability to be generally low [24, 25]. These differences may be explained by a number of potential factors, including the methodology of evaluation (vessel specific window width and window level), the experience of observers and the use of consensus to minimise outlier measurements. In addition to careful outer vessel wall segmentation, pre-defined vessel-specific windowing and leveling may therefore be important for accurate plaque quantification.
While still constrained by limited temporal and spatial resolution, coronary CTA appears to be emerging as an accurate non-invasive method for not only stenosis assessment, but also for comprehensive characterisation and quantification of coronary plaque features, including volume. In this regard, coronary CTA may hold the potential to serve as a non-invasive method for plaque characterisation in lieu of IVUS for both quantification and characterisation. Accordingly, future studies may consider the use of coronary CTA for monitoring the progression and regression of coronary atherosclerosis as an alternative to invasive methods. If proven, coronary CTA may represent a highly useful quantitative non-invasive technique that may allow the careful study of a significantly greater number of individuals than could be done by invasive methods. These efforts may allow for improved stratification of risk beyond traditional angiographic measures of stenosis severity and may allow for potential discrimination of individuals with more aggressive forms of atherosclerosis or who have responded optimally to medical therapy. However, as we know, plaque imaging by CT remains a challenge in daily routine practice. Depending on the characteristics of the patient and the CT system used, plaque visualisation and vessel contour detection in an individual patient may become very difficult and sometimes even impossible. This is especially true when protocols for reducing radiation (low tube voltage, prospective ECG-gating) are applied. The impossibility to reconstruct data sets at different points within the cardiac cycle using a prospectively triggered “step and shoot” mode also remains a matter of concern for plaque imaging.
We also examined several non-volumetric characteristics of atherosclerotic plaques. The features chosen were done so based upon prior invasive and non-invasive studies that have identified a clinical importance to LAP, PR and SC. In comparative histological studies, echolucent appearance by IVUS was correlated with the lipid content of plaques [26], a finding that has been associated with unstable as opposed to stable angina [27]. Similarly, PR has also been associated with unstable coronary syndromes [15]. A final atherosclerotic feature, namely SC, has been demonstrated by Ehara et al. to be of greater predictive value for acute coronary syndromes than the overall volume of atherosclerotic plaque [16]. CT density, based upon Hounsfield unit methods, also allows for assessment of plaque that demonstrates high echogenicity by IVUS, and these low attenuation CT plaques are those thought by comparison to IVUS to be rich in an intraplaque lipid core. Motoyama et al. demonstrated in a large, retrospective coronary CTA study that culprit lesions of acute coronary syndrome had a higher proportion of PR (87 % vs. 12 %, P < 0.0001), more frequently had components with LAP (attenuation values <30 HU) (79 % vs. 9 %, P < 0.0001) and typically had SC [28]. These prognostic findings have been assumed to be based upon the highly accurate diagnostic performance of coronary CTA to identify these plaque characteristics, and the present study results confirm the overall feasibility of high accuracy measures.
While seemingly intuitive, plaque quantification and characterisation can, at present, only be performed in images with excellent image quality. As such, its greatest present application will be for selected patients and in vessels that are relatively large. Furthermore, well-defined coronary CTA image acquisition parameters for plaque characterisation may require modestly higher radiation doses, as very low dose scans may result in too much noise—particularly for smaller vessels—and thus make detection and quantification of plaque difficult. To date, however, initial reports evaluating the accuracy of coronary CTA for coronary atherosclerotic plaque measurements have been reported as high even in low radiation dose scans with good interobserver agreements [29].
This study is not without limitations. Given the time-intensive nature of plaque quantification and characterisation, the present study analyses were conducted retrospectively on a relatively small study sample. Our non-significant findings may be the result of a small sample size. Further, important sub-analyses such as examination of potential over- or underestimation of plaque volumes of CT in smaller versus larger plaques or calcified versus non-calcified plaques could not be performed. Future prospective multicentre studies to confirm our findings now appear warranted. Further, our comparison of coronary CTA to IVUS was primarily restricted to plaque including both noncalcified as well as calcified components. Given the imaging limitations of IVUS for quantification of calcified plaque due to acoustic shadowing in areas of calcification for the view of the outer vessel borders, differences between coronary CTA and IVUS in the present study may be related not solely to the limitations of coronary CTA but also the limitations of IVUS. A similar concern about the exact overlay of the coronary CTA to IVUS remains despite our careful attempts to ensure fiduciary consistency. While future studies may be helpful to discriminate these differences, no current invasive method allows for more accurate evaluations. With regards to plaque quantification, prior studies have identified Hounsfield unit overlap in LAP, other non-calcified plaque thought to be fibrous in nature and even calcified plaque. These findings could have affected the quantification of atherosclerotic plaque in the present study.
Using current generation CT technology, several limitations are also inherent in contrast-enhanced coronary CTA itself, including constraints by spatial and temporal resolution. Partial volume effects caused by large coronary calcifications and lumen contrast enhancement may evoke over- as well as under-estimation of atherosclerotic plaque volume or characterisation of atherosclerotic plaque characteristics. Furthermore, contrast enhancement of the arterial lumen during coronary CTA may overlap with calcified plaque Hounsfield unit densities and, based upon partial volume effects as well as overlap, may conceal, superficial and smaller calcified deposits encroaching upon the coronary arterial lumen. Finally, manual plaque quantification largely depends on the individual operator and is time consuming. A modern software tool with favourable agreement with manual CT plaque volume measurements or IVUS measurements has been recently described [20, 30–32]. However, these tools remain semi-automated in nature and still require manual expert observer correction and accurate delineation of the coronary vessel wall from surrounding epicardial fat. In this regard, future studies evaluating forthcoming fully automated software may be helpful for quantification and characterisation of coronary plaque in routine clinical settings.
At present, plaque volume quantification is not reliable for daily clinical practice at this stage for the aforementioned reasons, but may serve as a potentially valuable research tool to evaluate not only the presence, quantity and type of coronary artery plaque but also in the serial examination of at-risk patients. However, given the present study results, identification of adverse plaque characteristics by coronary CTA, including LAP, PR and SC, can be easily applied and may help risk stratification.
In conclusion, manual measures of coronary artery plaque volume on coronary CTA demonstrate high correlation and modest agreement compared to IVUS. Further, coronary CTA demonstrates high performance for the identification of adverse plaque characteristics, including LAP, PR and SC.
Abbreviations
- Coronary CTA:
-
Coronary computed tomographic angiography
- ICA:
-
Invasive coronary angiography
- IVUS:
-
Intravascular ultrasound
- LAD:
-
Left anterior descending artery
- LAP:
-
Low attenuation plaque
- LCX:
-
Left circumflex artery
- MACE:
-
Major adverse cardiac events
- PR:
-
Positive arterial remodelling
- RCA:
-
Right coronary artery
- SC:
-
Spotty calcification
References
Stone GW, Maehara A, Lansky AJ et al (2011) A prospective natural-history study of coronary atherosclerosis. N Engl J Med 364:226–235
Nissen SE, Nicholls SJ, Sipahi I et al (2006) Effect of very high-intensity statin therapy on regression of coronary atherosclerosis: the ASTEROID trial. JAMA 295:1556–1565
Nissen SE, Tuzcu EM, Schoenhagen P et al (2004) Effect of intensive compared with moderate lipid-lowering therapy on progression of coronary atherosclerosis: a randomized controlled trial. JAMA 291:1071–1080
Leber AW, Becker A, Knez A et al (2006) Accuracy of 64-slice computed tomography to classify and quantify plaque volumes in the proximal coronary system: a comparative study using intravascular ultrasound. J Am Coll Cardiol 47:672–677
Leber AW, Knez A, von Ziegler F et al (2005) Quantification of obstructive and nonobstructive coronary lesions by 64-slice computed tomography: a comparative study with quantitative coronary angiography and intravascular ultrasound. J Am Coll Cardiol 46:147–154
Petranovic M, Soni A, Bezzera H et al (2009) Assessment of nonstenotic coronary lesions by 64-slice multidetector computed tomography in comparison to intravascular ultrasound: evaluation of nonculprit coronary lesions. J Cardiovasc Comput Tomogr 3:24–31
Burgstahler C, Reimann A, Beck T et al (2007) Influence of a lipid-lowering therapy on calcified and noncalcified coronary plaques monitored by multislice detector computed tomography: results of the New Age II Pilot Study. Invest Radiol 42:189–195
Schmid M, Achenbach S, Ropers D et al (2008) Assessment of changes in non-calcified atherosclerotic plaque volume in the left main and left anterior descending coronary arteries over time by 64-slice computed tomography. Am J Cardiol 101:579–584
Abbara S, Arbab-Zadeh A, Callister TQ et al (2009) SCCT guidelines for performance of coronary computed tomographic angiography: a report of the Society of Cardiovascular Computed Tomography Guidelines Committee. J Cardiovasc Comput Tomogr 3:190–204
Hausleiter J, Meyer T, Hermann F et al (2009) Estimated radiation dose associated with cardiac CT angiography. JAMA 301:500–507
Scanlon PJ, Faxon DP, Audet AM et al (1999) ACC/AHA guidelines for coronary angiography. A report of the American College of Cardiology/American Heart Association Task Force on practice guidelines (Committee on Coronary Angiography). Developed in collaboration with the Society for Cardiac Angiography and Interventions. J Am Coll Cardiol 33:1756–1824
Schepis T, Marwan M, Pflederer T et al (2010) Quantification of non-calcified coronary atherosclerotic plaques with dual-source computed tomography: comparison with intravascular ultrasound. Heart 96:610–615
Shmilovich H, Cheng VY, Tamarappoo BK et al (2011) Vulnerable plaque features on coronary CT angiography as markers of inducible regional myocardial hypoperfusion from severe coronary artery stenoses. Atherosclerosis 219:588–595
Mintz GS, Nissen SE, Anderson WD et al (2001) American College of Cardiology clinical expert consensus document on standards for acquisition, measurement and reporting of intravascular ultrasound studies (IVUS). A report of the American College of Cardiology task force on clinical expert consensus documents. J Am Coll Cardiol 37:1478–1492
Schoenhagen P, Ziada KM, Kapadia SR, Crowe TD, Nissen SE, Tuzcu EM (2000) Extent and direction of arterial remodeling in stable versus unstable coronary syndromes: an intravascular ultrasound study. Circulation 101:598–603
Ehara S, Kobayashi Y, Yoshiyama M et al (2004) Spotty calcification typifies the culprit plaque in patients with acute myocardial infarction: an intravascular ultrasound study. Circulation 110:3424–3429
Pundziute G, Schuijf JD, Jukema JW et al (2008) Head-to-head comparison of coronary plaque evaluation between multislice computed tomography and intravascular ultrasound radiofrequency data analysis. JACC Cardiovasc Interv 1:176–182
Achenbach S, Moselewski F, Ropers D et al (2004) Detection of calcified and noncalcified coronary atherosclerotic plaque by contrast-enhanced, submillimeter multidetector spiral computed tomography: a segment-based comparison with intravascular ultrasound. Circulation 109:14–17
Otsuka M, Bruining N, Van Pelt NC et al (2008) Quantification of coronary plaque by 64-slice computed tomography: a comparison with quantitative intracoronary ultrasound. Invest Radiol 43:314–321
Dey D, Schepis T, Marwan M, Slomka PJ, Berman DS, Achenbach S (2010) Automated three-dimensional quantification of noncalcified coronary plaque from coronary CT angiography: comparison with intravascular US. Radiology 257:516–522
Papadopoulou SL, Neefjes LA, Schaap M et al (2011) Detection and quantification of coronary atherosclerotic plaque by 64-slice multidetector CT: a systematic head-to-head comparison with intravascular ultrasound. Atherosclerosis 219:163–170
Stolzmann P, Schlett CL, Maurovich-Horvat P et al (2012) Variability and accuracy of coronary CT angiography including use of iterative reconstruction algorithms for plaque burden assessment as compared with intravascular ultrasound—an ex vivo study. Eur Radiol 22:2067–2075
Voros S, Rinehart S, Qian Z et al (2011) Coronary atherosclerosis imaging by coronary CT angiography: current status, correlation with intravascular interrogation and meta-analysis. JACC Cardiovasc Imaging 4:537–548
Pflederer T, Schmid M, Ropers D et al (2007) Interobserver variability of 64-slice computed tomography for the quantification of non-calcified coronary atherosclerotic plaque. Rofo 179:953–957
Hoffmann H, Frieler K, Hamm B, Dewey M (2008) Intra- and interobserver variability in detection and assessment of calcified and noncalcified coronary artery plaques using 64-slice computed tomography: variability in coronary plaque measurement using MSCT. Int J Cardiovasc Imaging 24:735–742
Gussenhoven EJ, Essed CE, Lancée CT et al (1989) Arterial wall characteristics determined by intravascular ultrasound imaging: an in vitro study. J Am Coll Cardiol 14:947–952
Hodgson JM, Reddy KG, Suneja R, Nair RN, Lesnefsky EJ, Sheehan HM (1993) Intracoronary ultrasound imaging: correlation of plaque morphology with angiography, clinical syndrome and procedural results in patients undergoing coronary angioplasty. J Am Coll Cardiol 21:35–44
Motoyama S, Kondo T, Sarai M et al (2007) Multislice computed tomographic characteristics of coronary lesions in acute coronary syndromes. J Am Coll Cardiol 50:319–326
Schuhbäck A, Marwan M, Gauss S et al (2012) Interobserver agreement for the detection of atherosclerotic plaque in coronary CT angiography: comparison of two low-dose image acquisition protocols with standard retrospectively ECG-gated reconstruction. Eur Radiol 22:1529–1536
Rinehart S, Vazquez G, Qian Z, Voros S (2009) Coronary plaque imaging with multi-slice computed tomographic angiography and intravascular ultrasound: a close look inside and out. J Invasive Cardiol 21:367–372
Rinehart S, Vazquez G, Qian Z, Murrieta L, Christian K, Voros S (2011) Quantitative measurements of coronary arterial stenosis, plaque geometry, and composition are highly reproducible with a standardized coronary arterial computed tomographic approach in high-quality CT datasets. J Cardiovasc Comput Tomogr 5:35–43
Akram K, Rinehart S, Voros S (2008) Coronary arterial atherosclerotic plaque imaging by contrast-enhanced computed tomography: fantasy or reality? J Nucl Cardiol 15:818–829
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 36 kb)
Rights and permissions
About this article
Cite this article
Nakazato, R., Shalev, A., Doh, JH. et al. Quantification and characterisation of coronary artery plaque volume and adverse plaque features by coronary computed tomographic angiography: a direct comparison to intravascular ultrasound. Eur Radiol 23, 2109–2117 (2013). https://doi.org/10.1007/s00330-013-2822-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-013-2822-1