Abstract
Objectives
Susceptibility weighted imaging (SWI) may have the potential to depict the perivenous extent of white matter lesions (WMLs) in multiple sclerosis (MS). We aimed to assess the discriminatory value of the “central vein sign” (CVS).
Methods
In a 3-T magnetic resonance imaging (MRI) study, 28 WMLs in 14 patients with at least one circumscribed lesion >5 mm and not more than eight non-confluent lesions >3 mm were prospectively included. Only WMLs in FLAIR images with a maximum diameter of >5 mm were correlated to their SWI equivalent for CVS evaluation.
Results
Five patients fulfilled the revised McDonald criteria for MS and nine patients were given alternative diagnoses. Nineteen MS-WMLs and nine non-MS-WMLs >5 mm were detected. Consensus reading found a central vein in 16 out of 19 MS-WMLs (84 %) and in one out of nine non-MS-WMLs (11 %), respectively. The CVS proved to be a highly significant discriminator (P < 0.001) between MS-WMLs and non-MS-WMLs with a sensitivity, specificity, positive and negative predictive value and accuracy of 84 %, 89 %, 94 %, 73 % and 86 %, respectively. Inter-rater agreement was good (κ = 0.77).
Conclusions
Even though the CVS is not exclusively found in MS-WMLs, SWI may be a useful adjunct in patients with possible MS.
Key Points
• MRI continues to yield further information concerning MS lesions.
• SWI adds diagnostic information in patients with possible MS.
• The “central vein sign” was predominantly seen in MS lesions.
• The “central vein sign” helps discriminate between MS and non-MS lesions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Multiple sclerosis (MS) is a chronic, multifocal, inflammatory, demyelinating disease affecting the central nervous system (CNS) [1]. It generally, though not exclusively, affects young adults. Childhood onset has been reported in a large European study to occur in about 2.2 % [2]. With an extremely variable natural course, MS typically leads to severe and irreversible disability. In about 85 % of MS cases, the patient presents with a clinically isolated syndrome involving the optic nerve, brainstem or spinal cord [1]. The incidental imaging finding of brain lesions with features highly suggestive of MS in asymptomatic individuals has been termed “radiologically isolated syndrome” (RIS) [3]. Although radiological progression may be observed in almost three out of five RIS patients, only about one-fourth have been shown to convert to either clinically isolated syndrome (CIS) or clinically definite MS (CDMS) over time [3]. The diagnosis of MS can be made upon clinical and laboratory assessments alone [4]; however, magnetic resonance imaging (MRI) of the CNS can support, supplement or even replace some clinical criteria [5]. The emphasis is on demonstrating dissemination of lesions in space (DIS) and time (DIT) and to exclude alternative diagnoses [4]. The McDonald scheme—after incorporating the Barkhof/Tintoré MRI criteria—has resulted in earlier diagnosis of MS and its 2010 revision has simplified the diagnostic process including the interpretation of MRI [6, 7].
Dual-echo and fluid-attenuated inversion-recovery (FLAIR) sequences have a high sensitivity for detection of MS lesions, which appear as focal areas of hyperintensity on T2-weighted images [8]. However, since oedema, inflammation, demyelination, remyelination, axonal loss and gliosis may all lead to a similar appearance, a lack of specificity has to be considered [8]. One of the most common causes of white matter lesions (WMLs), which may even be found in a large percentage of clinically healthy elderly people, is hypertensive microangiopathy [9–11]. In the clinical context of suspected MS, suggestive morphological and topographic features of WMLs can increase the specificity of conventional MRI [8, 12–14]. Postmortem studies have shown that the typical distribution and pattern of MS plaques can be explained by their perivenous localisation [12, 13, 15].
Recent work has shown that T2* weighted MRI at 7 Tesla (7 T) allows the identification of small parenchymal veins within WMLs [16–18]. So-called susceptibility weighted imaging (SWI) may therefore have the potential to depict the perivenous extent of MS-WMLs [16, 17, 19]. However, Lummel et al. [20], using 3-T MRI, recommended that the detection of a central vein within a WML should not be considered a specific finding for MS.
The aim of this study was to assess the discriminatory value of the so-called “central vein sign” (CVS) in a subset of patients where this SWI feature may possibly add to the differential diagnostic characterisation of defined MS-WMLs and non-MS-WMLs at the time of first imaging.
Materials and methods
The study was approved by our local ethics commission.
Subjects
In a prospective, lesion-based study, 28 defined WMLs in 14 patients (11 women, 3 men; mean age, 33.8 years; age median, 35.5 years; range, 4–62 years) were included. The MR examination of interest was the first with regard to the respective indication for brain imaging in all patients. None of these patients had any evidence or prior diagnosis of major cardiovascular morbidity, cerebrovascular event or other neurological disease. Furthermore, all patients with acute or subacute ischaemic brain lesions and a history of malignant disease were excluded. The minimum age for inclusion into the study was three years since complete white matter myelination can be assumed at this stage of life [21]. Regarding the number and characteristics of WMLs, the following inclusion criteria were adopted with respect to clinical utility: (1) at least one lesion with a maximum diameter of >5 mm; (2) not more than eight non-confluent lesions >3 mm.
MR image acquisition
Imaging was conducted on a Philips Achieva 3.0-T MR system (Philips Medical Systems, Best, The Netherlands) using a SENSE eight-channel head coil. Our standard protocol, including a 3D FLAIR sequence with multiplanar reconstructions, was extended by an axial SWI sequence [19]. The FLAIR sequence was acquired in the sagittal plane with the following imaging parameters: TR, 4,800 ms; TI, 1,650 ms; TE, 277 ms; FOV, 250 × 250 × 180 mm (FH × AP × RL); acquired voxel size, 1.12 × 1.12 × 1.12 mm; reconstructed voxel size, 0.56 × 0.56 × 0.56 mm; acquisition matrix, 224 × 224 mm; reconstructed slice thickness, 3.0 mm (acquired, 1.12 mm); acquisition time, 4 min 52 s. The SWI sequence, a T2*-weighted 3D gradient echo pulse sequence with multiple echoes during one TR period at different time points, was acquired with: TR, 45 ms; TE, 25 ms; receiver bandwidth, 35 Hz; flip angle, 17°; FOV, 230 × 184 x 100 mm (FH × AP × RL); acquired voxel size, 0.6 × 0.71 × 1.00 mm; reconstructed voxel size, 0.45 × 0.45 × 1.00 mm; acquisition matrix, 384 × 258 mm; slab thickness of minimum intensity projections (mIP), 8 mm (acquired, 1.0 mm); acquisition time, 7 min 45 s.
Image analysis
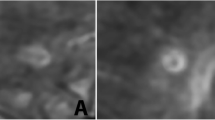
The MR data sets were analysed independently by two readers following thorough preparation for the purpose of this study. Both readers were blinded to all clinical data and patient identification information. Image interpretation was performed on a standard picture archiving and communication system (PACS) workstation (IMPAX EE, Agfa HealthCare, Mortsel, Belgium). FLAIR images served as the “gold standard” for the identification of WMLs, which were then classified as periventricular, subcortical or infratentorial. Only defined WMLs with a maximum diameter of >5 mm were correlated to their SWI mIP equivalent for further analysis. Thus, each lesion of interest was evaluated for the CVS [20]. For co-registration of SWI and FLAIR images, the synchronisation tool of our PACS was used. Depending on the slice angle, the hypointense vein was either depicted as a thin line coursing through the centre of the lesion or as a centrally located dot on contiguous slices (Fig. 1). In oval WMLs, only veins running in the direction of the long axis of a lesion were counted. The CVS was only rated positive if the most prominent vein was located in the centre of a lesion (Fig. 2). The final rating was based on consensus reading.
Axial FLAIR (a) and corresponding susceptibility weighted image (b) of a juxtacortical WML (white arrows) rated positive for the central vein sign (black arrow). In this large MS plaque, the hypointense vein (black arrow) could even be depicted on a T2-weighted, coronal image (c) as a thin line coursing through the centre of the lesion
Statistics
For both patient groups (MS-WMLs and non-MS-WMLs), descriptive statistics including the mean total lesion load were calculated. We compared lesion sizes with the two-sided Wilcoxon Mann–Whitney U test. Inter-rater reliability was assessed using kappa statistics. The potential of the CVS to discriminate between MS-WMLs and non-MS-WMLs was assessed by means of sensitivity, specificity, positive and negative predictive value and accuracy, including confidence intervals (CI). The data were analysed using Statistics Online Computational Resource (SOCR), a freely available online software tool provided by a University of California, Los Angeles, platform (www.socr.ucla.edu) [22]. A value of P < 0.05 was considered significant.
Results
Five patients (four women, one man; age median, 47 years; range, 20–57 years) fulfilled the revised McDonald criteria for MS [7] after a follow-up period of at least 6 months. Nine patients (seven women, two men; age median, 34 years; range, 4–62 years) without any clinical or paraclinical signs of MS were given an alternative diagnosis. A total of 28 lesions >5 mm (19 MS-WMLs and 9 non-MS-WMLs) were detected. Ten lesions were located in the periventricular white matter (7 MS-WMLs, 3 non-MS-WMLs), 17 lesions subcortically (12 MS-WMLs, 5 non-MS-WMLs) and a single non-MS-WML in the cerebellum. We evaluated two to seven lesions (median, four MS-WMLs) per MS patient. In all other patients, each non-MS-WML was analysed for the CVS.
Consensus reading found a central vein in 16 out of 19 MS-WMLs (84 %) and in one out of nine non-WMLs (11 %), respectively. The lesion size was similar in both groups, with a median maximum diameter of 9 mm and a mean value of 9.8 mm for MS-WMLs and 9.7mm for non-MS-WMLs, respectively (Fig. 3). Lesions positive for the CVS were on average larger than negative ones (median, 10 mm versus 8 mm in MS-WMLs and 11 mm versus 6.5 mm in non-MS-WMLs, respectively). Considering both groups (MS-WMLs and non-MS-WMLs), the difference in lesion size was not statistically significant (P = 0.180). The frequency of CVS positive lesions was highest in the periventricular zone (70 %, 7/10), even more markedly in MS-WMLs alone (86 %, 6/7) (Fig. 4). Table 1 gives an overview of indications for MR examination, diagnoses, number of evaluated WMLs per patient and CVS ratings.
Considering all 28 lesions, a good inter-rater agreement (κ = 0.77) was calculated. The CVS proved to be a highly significant discriminator (P < 0.001) between MS-WMLs and non-MS-WMLs with a sensitivity, specificity, positive and negative predictive value and accuracy of 84 % (CI, 62–95 %), 89 % (CI, 57–98 %), 94 % (CI, 73–99 %), 73 % (CI, 43–90 %) and 86 % (CI, 67–96 %), respectively.
Discussion
The discriminatory power of a “central vein sign” on susceptibility weighted images was found to be good for the differentiation of white matter lesions associated with multiple sclerosis and non-MS-WMLs. The CVS, as defined in this study, was positive in 84 % of MS-WMLs and in 11 % of non-MS-WMLs. Its frequency was highest in periventricular MS-WMLs (86 %) and CVS positive lesions were generally larger than negative ones.
Our primary results are in discordance with findings published by Lummel et al. [20]. Their recommendation that a central vein within a WML should not be considered a specific finding for MS was based on the evaluation of an average of 61 lesions per patient, each >3 mm in diameter. A threshold of 3 mm for sharply delineated high-signal-intensity WMLs is commonly employed also in major treatment trials in order to overcome the risk of counting unspecific white matter changes [23, 24]. We aimed to choose inclusion criteria that, in our experience, better reflect equivocal cases in clinical routine. Therefore, the overall number of WMLs >3 mm was limited to a maximum of eight, which is a critical lesion load regarding the Barkhof criteria for the diagnosis of MS [25]. It is known from comparative studies that, compared with 3-T MRI, imaging at ultra-high field is advantageous in demonstrating detailed structural anatomy of MS lesions [17]. In order to reduce the risk of impaired visualisation, target lesions had to be >5 mm in the largest diameter. Since small veins are ubiquitous and may traverse any type of WML coincidentally, the definition of a positive CVS was refined for the purpose of this study [13, 18, 20, 26]. In addition to its central location within a WML, a CV required to be the most prominent vein within the lesion and to run along a lesion’s long axis. The orientation and shape of MS lesions are known to usually correspond with the course of a vein, confirming their perivenous origin [13, 26]. In this regard, the initial inflammatory process of MS has been shown by histochemical and immunocytochemical methods to start around small veins [27]. While the visibility of cerebral veins in SWI has been reported to be diminished in long-standing MS, this may not play a major role in the early stage as relevant to this study [28, 29].
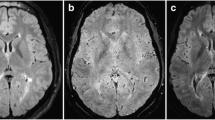
The limited number of WMLs did not allow for a location based subanalysis. However, we can confirm from our experience that the quality of SW images may be reduced near air-tissue interfaces, i.e. around the sinuses or in the mastoid and infratentorial regions [26] (Fig. 5). Further susceptibility effects potentially influence SWI patterns. Grabner et al. [18], analysing 3-T FLAIR/7-T SWI phase data overlays in eight MS patients, reported iron depositions to be found in nearly one-third of all plaques, accompanied by penetrating veins of any location and orientation in about 15 %. So-called susceptibility weighted (SW) sequences take advantage of the high susceptibility of materials with strong paramagnetic properties; for example, iron and venous blood [30, 31]. Technical aspects of susceptibility weighted imaging are described elsewhere in the literature.
Discordance between both readers in this case of an infratentorial WML (black arrow) may possibly be attributable to some degree of uncertainty in the co-registration of FLAIR (a) and susceptibility weighted (b) images when evaluating it for the presence of a central vein. Consensus reading rated this lesion negative for the CVS (white arrow)
Some study limitations may be addressed. Although we performed a lesion based analysis in a considerable number of defined WMLs, both the MS and non-MS group were small. A subanalysis as to lesion size and location cannot be expected to lead to significant results. Second, the mean number of lesions to be evaluated was higher in MS patients than in non-MS patients (four versus one per patient). This may potentially be a bias even in readers that are blinded to clinical information. As another limitation to our study, the problem of synchronisation of SWI and FLAIR images with different slice thickness has to be mentioned (Fig. 5). It may be argued that this method entails the risk of misregistration when evaluating WMLs for a possible CVS. Finally, an acquisition time of almost 8 min for our SWI sequence has to be acknowledged.
In conclusion, the results of our study indicate that even though the CVS is not exclusively found in MS-WMLs, SWI may be a useful adjunct in patients with possible MS.
Abbreviations
- AP:
-
Antero-posterior
- CDMS:
-
Clinically definite multiple sclerosis
- CIS:
-
Clinically isolated syndrome
- CV:
-
central vein
- CVS:
-
Central vein sign
- DD:
-
Differential diagnosis
- DIS:
-
Dissemination in space
- DIT:
-
Dissemination in time
- FH:
-
Feet-head
- FLAIR:
-
Fluid-attenuated inversion-recovery
- mIP:
-
Minimum intensity projection
- MS:
-
Multiple sclerosis
- PACS:
-
Picture archiving and communication system
- RIS:
-
Radiologically isolated syndrome
- RL:
-
Right-left
- SOCR:
-
Statistics Online Computational Resource
- SWI:
-
Susceptibility weighted imaging
- WML:
-
white matter lesion
References
Noseworthy JH, Lucchinetti C, Rodriguez M, Weinshenker BG (2000) Multiple sclerosis. N Engl J Med 343:938–952
Renoux C, Vukusic S, Mikaeloff Y et al (2007) Natural history of multiple sclerosis with childhood onset. N Engl J Med 356:2603–2613
Okuda DT, Mowry EM, Beheshtian A et al (2009) Incidental MRI anomalies suggestive of multiple sclerosis: the radiologically isolated syndrome. Neurology 72:800–805
Poser CM, Paty DW, Scheinberg L et al (1983) New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann Neurol 1:227–231
McDonald WI, Compston A, Edan G et al (2001) Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol 50:121–127
Tintoré M, Rovira A, Río J et al (2003) New diagnostic criteria for multiple sclerosis. Application in first demyelinating episode. Neurology 60:27–30
Polman CH, Reingold SC, Banwell B et al (2011) Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 69:292–302
Filippi M, Rocca MA (2011) MR imaging of multiple sclerosis. Radiology 259:659–681
Fazekas F, Kleinert R, Offenbacher H et al (1991) The morphologic correlate of incidental punctate white matter hyperintensities on MR images. AJNR Am J Neuroradiol 12:915–921
Breteler MM, van Swieten JC, Bots ML et al (1994) Cerebral white matter lesions, vascular risk factors, and cognitive function in a population-based study: the Rotterdam Study. Neurology 44:1246–1252
de Leeuw FE, de Groot JC, Achten E et al (2001) Prevalence of cerebral white matter lesions in elderly people: a population based magnetic resonance imaging study. The Rotterdam Scan Study. J Neurol Neurosurg Psychiatry 70:9–14
Putnam TJ (1933) The pathogenesis of multiple sclerosis: a possible vascular factor. N Engl J Med 209:786–790
Fog T (1965) The topography of plaques in multiple sclerosis with special reference to cerebral plaques. Acta Neurol Scand Suppl 15:1–161
Horowitz AL, Kaplan RD, Grewe G, White RT, Salberg LM (1989) The ovoid lesion: a new MR observation in patients with multiple sclerosis. AJNR Am J Neuroradiol 10:303–305
Filippi M, Rocca MA, Barkhof F et al (2012) Attendees of the Correlation between Pathological MRI findings in MS workshop. Association between pathological and MRI findings in multiple sclerosis. Lancet Neurol 11:349–360
Ge Y, Zohrabian VM, Grossman RI (2008) Seven-Tesla magnetic resonance imaging: new vision of microvascular abnormalities in multiple sclerosis. Arch Neurol 65:812–816
Tallantyre EC, Morgan PS, Dixon JE et al (2009) A comparison of 3T and 7T in the detection of small parenchymal veins within MS lesions. Invest Radiol 44:491–494
Grabner G, Dal-Bianco A, Schernthaner M, Vass K, Lassmann H, Trattnig S (2011) Analysis of multiple sclerosis lesions using a fusion of 3.0 T FLAIR and 7.0 T SWI phase: FLAIR SWI. J Magn Reson Imaging 33:543–549
Haacke EM, Xu Y, Cheng YC, Reichenbach JR (2004) Susceptibility weighted imaging (SWI). Magn Reson Med 52:612–618
Lummel N, Boeckh-Behrens T, Schoepf V, Burke M, Brückmann H, Linn J (2011) Presence of a central vein within white matter lesions on susceptibility weighted imaging: a specific finding for multiple sclerosis? Neuroradiology 53:311–317
Carmody DP, Dunn SM, Boddie-Willis AS, DeMarco JK, Lewis M (2004) A quantitative measure of myelination development in infants, using MR images. Neuroradiology 46:781–786
Dinov ID (2006) SOCR: Statistics Online Computational Resource. J Stat Softw 16:1–16
Jacobs DL, Beck RW, Simon JH et al (2000) Intramuscular interferon beta-1a therapy initiated during a first demyelinating event in multiple sclerosis. N Engl J Med 343:898–904
Enzinger C, Smith S, Fazekas F et al (2006) Lesion probability maps of white matter hyperintensities in elderly individuals: results of the Austrian stroke prevention study. J Neurol 253:1064–1070
Barkhof F, Filippi M, Miller DH et al (1997) Comparison of MRI criteria at first presentation to predict conversion to clinically definite multiple sclerosis. Brain 120:2059–2069
Tan IL, van Schijndel RA, Pouwels PJ, van Walderveen MA, Reichenbach JR, Manoliu RA, Barkhof F (2000) MR venography of multiple sclerosis. AJNR Am J Neuroradiol 21:1039–1042
Adams CWM, Poston RN, Buk SJ (1989) Pathology, histochemistry and immunocytochemistry of lesions in acute multiple sclerosis. J Neurol Sci 92:291–306
Ge Y, Zohrabian VM, Osa EO et al (2009) Diminished visibility of cerebral venous vasculature in multiple sclerosis by susceptibility-weighted imaging at 3.0 Tesla. J Magn Reson Imaging 29:1190–1194
Zivadinov R, Poloni GU, Marr K et al (2011) Decreased brain venous vasculature visibility on susceptibility-weighted imaging venography in patients with multiple sclerosis is related to chronic cerebrospinal venous insufficiency. BMC Neurol 11:128
Reichenbach JR, Venkatesan R, Schillinger DJ, Kido DK, Haacke EM (1997) Small vessels in the human brain: MR venography with deoxyhemoglobin as an intrinsic contrast agent. Radiology 204:272–277
Hodel J, Rodallec M, Gerber S et al (2012) Susceptibility weighted magnetic resonance sequences “SWAN, SWI and VenoBOLD”: technical aspects and clinical applications. J Neuroradiol 39:71–86
Acknowledgements
The abstract of this scientific paper (ctrl no. 2123) has been accepted for oral presentation at ECR 2013.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kau, T., Taschwer, M., Deutschmann, H. et al. The “central vein sign”: is there a place for susceptibility weighted imaging in possible multiple sclerosis?. Eur Radiol 23, 1956–1962 (2013). https://doi.org/10.1007/s00330-013-2791-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-013-2791-4