Abstract
Objectives
To compare the costs of CT- and MR-guided lumbosacral nerve root infiltration for minimally invasive treatment of low back pain and radicular pain.
Methods
Ninety patients (54 men, 36 women; mean age, 45.5 ± 12.8 years) underwent MR-guided single-site periradicular lumbosacral nerve root infiltration with 40 mg of triamcinolone acetonide. A further 91 patients (48 men, 43 women; mean age, 59.1 ± 13.8 years) were treated under CT fluoroscopy guidance. Prorated costs of equipment use (purchase, depreciation and maintenance), staff costs based on involvement times and expenditure for disposables were identified for MR- and CT-guided procedures.
Results
Mean intervention time was 20.6 min (14–30 min) for MR-guided and 14.3 min (7–32 min) for CT-guided treatment. The average total costs per patient were €177 for MR-guided and €88 for CT-guided interventions. These consisted of (MR/CT guidance) €93/29 for equipment use, €43/35 for staff and €41/24 for disposables.
Conclusions
Lumbosacral nerve root infiltration using MRI guidance is still about twice as expensive as infiltration using CT guidance. Given the advantages of no radiation exposure and possible future decrease in prices for MRI devices and MR-compatible injection needles, MR-guided nerve root infiltration may become a promising alternative to the CT-guided procedure.
Key Points
• MR-guided nerve root infiltration therapy is now technically and clinically established.
• Costs using MRI guidance are still about double those for CT guidance.
• MR guidance involves no radiation exposure to patients and personnel.
• MR-guided nerve root infiltration may become a promising alternative to CT.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
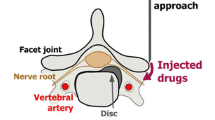
Chronic low back pain and radicular pain are very common in developed countries and are associated with a considerable economic burden resulting from absenteeism from work and disability [1–4]. Patients with radicular pain who do not achieve adequate pain relief with conservative management including oral analgesics and physical treatment can be effectively treated by nerve root infiltration of corticosteroids and anaesthetics under fluoroscopy or computed tomography (CT) guidance [5, 6]. Both methods of guidance have excellent bone-soft tissue contrast and provide near-real-time monitoring for precise and rapid interventional injection. However, both fluoroscopic and CT guidance involve radiation exposure [7–10]. In addition, when using contrast medium for monitoring the correct distribution of the therapeutic injection patients are exposed to the risk of an allergoid reaction [11].
With the advent of MR-compatible injection cannulae with adequate needle artefacts and the development of fast sequences, MRI has become an alternative imaging technique for interventional monitoring without radiation exposure [12–15]. Open MRI systems provide easy access for the interventionalist and are well accepted by patients [13, 16].
Despite these advantages, there are still several obstacles to the widespread use of this technology: open MRI systems are not generally available and MRI is perceived to be more expensive owing to higher imager use cost, longer intervention duration and more expensive MR-compatible instruments [17]. In recent years, there has been a considerable drop in the price of MRI systems and above all of MR-compatible injection cannulae. With the use of faster MRI sequences and shorter intervention duration, it is expected that there have been improvements in workflow and a considerable drop in overall costs.
The aim of our study was to evaluate the current costs of nerve root infiltration using MRI guidance compared with CT-guided treatment.
Materials and methods
Patients
A total of 90 patients (54 men, 36 women; mean age, 45.5 ± 12.8 years; range, 14–73 years) who underwent a single-segment lumbar nerve root infiltration (L1, L2, L3, L4, L5, or S1) using imaging guidance in an open 1.0-T MRI system (Panorama HFO, Philips, Best, The Netherlands) in the period from July 2009 to December 2011 were retrospectively identified. In addition, we identified 91 patients (48 men, 43 women; mean age, 59.1 ± 13.8 years; range, 25–90) who underwent identical interventions except that CT fluoroscopy was used for guidance (Somatom Definition 64, Siemens, Erlangen, Germany) in a time period from November 2010 to December 2011. Patients who had received a periradicular infiltration treatment or underwent surgery before were excluded. All patients had radicular pain and respective findings at preinterventional MRI. They were referred for treatment at our department by neurosurgeons or orthopaedic surgeons. Written informed consent was given by all patients before the treatment procedure, possible complications and alternative treatment options had been explained to them. The local ethics committee had approved the procedure of MR-guided nerve root infiltration. The retrospective data analysis was approved by the institutional review board.
Methods of nerve root infiltration
MRI fluoroscopy-guided nerve root infiltration
The technique of MR fluoroscopy-guided nerve root infiltration therapy has been described in detail by Streitparth et al. [13]. In brief, the patient was placed in a lateral position, and a multipurpose loop coil was fixed to the back in an orthogonal position to B0, resulting in a maximum signal yield. An interactive PD-w fast spin echo (FSE) sequence (TR/TE 600/10 ms, FOV 200 × 157 mm, matrix 224 × 72 mm, SL 5 mm, TA 2) was used for localising the target anatomy and subsequent multiplanar real-time needle guidance. Two millilitres of Xylonest 1 % (lidocaine and 1 % adrenaline, AstraZeneca, Wedel, Germany) were subcutaneously administered for local anaesthesia. An MR-compatible 20-G needle (MReye™, Cook, Bloomington, IN, USA) was inserted at the predefined dorsolateral entry point. In proximity to the nerve root, the needle position was controlled once more and adjusted in its directionality, if necessary (Fig. 1). After aspiration, a solution of 1 ml Triam (40 mg/ml; triamcinolone acetonide; Winthrop, Fürstenfeldbruck, Germany) and 2 ml Carbostesin 0.5 % (bupivacaine; AstraZeneca, Wedel, Germany) was injected into the periradicular space. A heavily T2-weighted fat-saturated SPIR (Spectral Presaturation with Inversion Recovery) sequence (TR/TE 1,500/100 ms, FOV 200 × 200 mm, NOS 6, matrix 224 × 216 mm, TF 24, SL 3 mm, TA 4.16 s) was acquired to monitor the correct injectant distribution as a means of interventional control. Procedures were defined as technically successful if the injectant was monitored in the perineural sheath of the targeted spinal nerve. The patients were discharged after the puncture site was bandaged with a patch and 30 min of post-interventional monitoring if no increase in pain or discomfort occurred. Interventions were performed by two radiologists with experience of at least 2 years for MR-guided nerve root infiltrations.
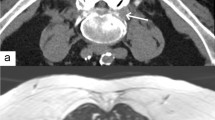
Example of an MR-guided periradicular nerve root infiltration. Sagittal (a) and axial (b) proton density-weighted (PDw) fast spin echo (FSE) sequences (TE/TR 10/600, TA 2 s) with final position of the needle tip close to the left first sacral root (arrows). A strongly T2-weighted spectral presaturation with inversion recovery (SPIR) sequence confirms the correct application and distribution of the cortico-analgesic injection fluid in the periradicular space (c)
CT fluoroscopy-guided nerve root Infiltration
Before the intervention, patients were positioned prone on the CT table for pre-interventional scout imaging of the lumbar spine. A metal wire was placed on the skin parallel to the spine on the side of the symptoms to facilitate exact localisation of the target nerve root and to plan the access path on individual CT slices. Finally, the desired puncture site was marked on the skin. The area was covered with sterile drapes, and skin disinfectant was applied, followed by application of superficial local anaesthesia (Xylonest 1 %, AstraZeneca, Wedel, Germany). Under CT fluoroscopy guidance, a 20-G puncture needle (Becton Dickinson SA, S. Agustin del Gualdix, Spain) was advanced until the tip reached the posterior edge of the neuroforamen of the target nerve root (Fig. 2). Following removal of the trocar, a mixture of 2 ml Carbostesin (0.5 %) and 1 ml iodine-based contrast medium (Accupaqe 240, GE Healthcare, Munich, Germany) was administered to document the correct periradicular contrast medium distribution. Finally, 1 ml with 40 mg of triamcinolone acetonide (Winthrop Arzneimittel, Mühlheim, Germany) was administered. Correct positioning of the needle close to the nerve root and adequate periradicular distribution of contrast medium were classified as a technical success. The dose-length product (in mGy*cm) was documented for all patients to calculate approximate effective radiation doses (in millisievert, mSV) using the software CT-expo version 2.0.1. Interventions were performed by three different radiologists with at least 1 year of experience in CT-guided nerve root infiltrations.
Definition and determination of costs
For a disaggregation of the costs of MR- and CT-guided interventional procedures, we documented different types of costs, grouping them into three categories: costs for use of equipment, staff costs and expenditure on disposables.
Costs of equipment use included purchase costs, depreciation and maintenance costs. Prorated costs for equipment use were calculated on the basis of 7-year use and linear depreciation in accordance with German tax laws. For the calculation of proportionate costs for the use of imaging techniques (CT device and open MRI system) including maintenance costs, we determined the total annual use of these techniques as well as their use for specific examinations.
The calculation of expenditure for disposables required for each treatment procedure (e.g. MR-compatible injection needle, sterile drapes) was based on the purchase prices provided by the hospital administration. For the calculation of staff costs (physicians, technologists), we established process models of all steps involved in the two therapeutic procedures [18]. For each step, we assigned the staff involved (physicians, technologists) and the duration of involvement in minutes. Times for all staff (in minutes) involved in the pre- and post-interventional phases were measured prospectively by documentation of the duration of all steps during ten CT- and MR-guided infiltration procedures with calculation of mean values. The length of the interventional procedure for each patient was calculated from the documented DICOM headers of the CT acquisitions and MR imaging sequences (survey, T2w SE for planning, interactive PDw TSE and postintervantional T2w TSE SPIR). Intervention time was defined from the beginning of the localising imaging until retraction of the needle in CT and to the ending of the post-interventional T2-w SPAIR FSE sequence in MRI. This did not include the time required for patient and operation room preparation or for post-interventional observation. Staff costs were then calculated on the basis of the tariffs for German civil service employees (TVöD-Besonderer Teil Krankenhäuser, Tarifgebiet West) and for physicians working in university hospitals (TV-Ärzte an Universitätskliniken, Tarifgebiet West). From the monthly gross wages—averaged from the variable pay scales based on length of employment—and after subtraction of an average number of vacation days and average absenteeism (illness, advanced training), we calculated staff costs per minute for the physicians and technologists involved in the interventions. The total costs were computed by addition of costs for use of equipment, staff costs and expenditure on disposables. For simplicity, we did not take costs for the use of rooms, cleaning and energy into account.
Results
All MR- and CT-guided interventions were technically successful. Figure 3 provides an overview of the numbers of interventions performed at each spinal level with either guidance technique. In the MRI guidance group, the mean intervention time was 20.7 ± 3.4 min (range, 14–30 min) with a mean of 27 min for pre-interventional preparation and 9 min for post-interventional care. In the CT fluoroscopy guidance group, the mean intervention time was 14.4 ± 5.2 min (range, 7–32 min) with a mean of 25 min for pre-interventional preparation and 9 min for post-interventional care. The mean dose-length product was 38.2 mGy*cm (range, 14–263 mGy*cm). In this group, a short-spiral CT acquisition for localisation was obtained before the intervention in 14 of the 91 patients. The mean dose-legth product in this subgroup was 163.9 mGy*cm (range, 38–263), which was significantly higher than in the subgroup without a pre-interventional localisation CT acquisition (t test, P < 0.001).
According to the tariffs for physicians and public employees at German university hospitals, staff costs per minute were €0.77 for the radiologist and €0.35 for the technologist. Based on the staff involvement times we measured for both types of treatment, average staff costs were €43.07 for MR-guided and €35.43 for CT-guided interventions (Table 1). Mean expenditure for disposables was €40.74 per patient for MR-guided and €23.81 per patient for CT-guided nerve root infiltration (Tables 2 and 3). Proportionate costs for equipment used were €92.83 per patient for MR-guided procedures and €28.86 per patient for CT-guided interventions (Table 4). Mean total costs per patient were €176.64 for MR-guided and €88.10 for CT-guided nerve root infiltration (see Table 5).
Discussion
When two alternative therapeutic procedures with very similar outcomes are available, it is necessary to compare the costs [19]. For patients in whom results of conservative treatment are not satisfying, fluoroscopy- and CT-guided periradicular nerve root infiltration is a safe and effective treatment [5]. As an alternative, MR-guided nerve root infiltration has been clinically established and a technical success rate of nearly 100 % has been reported [12–14, 20]. This is confirmed by our results: MRI guidance enabled correct positioning of the injection needle and uncomplicated infiltration in all 90 patients.
However, our cost analysis found that the MR-guided interventions were still twice as expensive as the same interventions performed with CT guidance (€176 vs. 88, 2.01-fold). The main source of the higher total costs was the markedly higher cost of equipment use in MR-guided interventions (€93 vs. 29, 3.2-fold) as open high-field MR systems operating at 1.0 or 1.5 T obviously have much higher purchase costs. These costs led to relatively higher equipment costs even though technical developments in recent years with improved hardware and software allow an improved workflow with faster sequences and even though the MR system is shared among different departments in our clinic. Therefore, equipment costs per intervention may be lowered by performing more interventions. When using an open low-field MR system at 0.23 T for bone biopsies, Alanen et al. [17] observed only 1.82-fold higher costs of MR equipment compared with CT-guided biopsies. An alternative sensitivity analysis assuming the use of a low-field scanner in our setting (Table 4) shows that this could substantially decrease equipment costs to €37 per MR-guided intervention leading to only 1.29-fold higher equipment costs (€37 vs. 29) und only 1.30-fold higher overall costs (€121 vs. 88, Tables 4 and 5) compared with CT guidance. On the other hand, CT costs may also be reduced. There is no need to use the latest 64-slice multidetector CT for CT-guided interventions. Theoretically, when using standard 16-slice multidetector CT, equipment costs may be as low as €15 per intervention (Table 4). In this alternative situation, the resulting overall costs of an MR-guided intervention with low-field MR technology are 1.63 times higher than the costs of an intervention using 16-slice multidetector CT (€121 vs. 75, Tables 4 and 5).
One factor contributing to the higher costs was the higher personnel costs for MR guidance due to a slightly longer duration of the procedure, although the difference was small (MR 20.7 vs. CT 14.4 min). MR-guided interventions in our study were markedly shorter than in the study of Ojala et al. (mean 32 min; range, 12–62 min) [20]. Sequeiros et al. [14] reported a mean overall intervention time of 33 min (range, 9–84 min) and a mean puncture time of 12 min, whereas Fritz et al. [12] stated an overall mean table time of 42 min (range, 23–75 min) and Streitparth et al. [13] 27 min (range, 19–67 min) for selective nerve root injections. These differences in procedure times may be attributable to a learning curve of the interventionalist [13]. Ojala et al. found the interventions to become shorter as the interventionalist’s experience increased from a mean intervention time of 34 min for the first five and of 23 min for the last five interventions [20]. MR-guided treatment in our study was performed by two interventionalists who were already very experienced in the technique, and this is reflected in similar mean intervention times for the first ten patients (21.1 min) and the last ten treatments (22.4 min). The absence of a learning curve may possibly explain the shorter MR invention times in our department. As the CT procedures were performed by three experienced interventionalists, there was also no significant learning curve for CT-guided treatments (mean overall intervention time of 11.5 min for the first 10 and 12.4 min for the last 10 treatments). By comparison, the mean intervention time for CT guidance in our patient group was markedly longer than in a study by Wagner et al. (mean 7 min; range, 5–16 min) [6]. Compared with the literature, these longer mean intervention times may be due to time expenses for a pre-interventional CT acquisition for localisation in 14 of 91 patients with severe spinal degeneration.
In our study, material costs were 1.7-fold higher for MRI guidance than for CT guidance (€ 41 vs. 24). MR-compatible disposables are still more expensive, which is another factor contributing to the higher costs of MR-guided nerve root infiltration. However, prices have already markedly decreased in recent years and we expect a further decrease within the next years. This price difference for disposables is already much smaller than that reported by Alanen et al. [17], who analysed MR- and CT-guided biopsies and found the costs of MR-compatible disposables to be 5.57 times higher than those of CT-guided biopsies.
Another important issue besides costs is radiation exposure, which may have a hazardous effect on both patients and medical staff [8–10]. Radiation exposure is of particular concern in this field because many patients require repeated infiltrations to achieve an optimal outcome. The low-dose CT protocol used in our study resulted in a mean dose-length product of 38.2 mGy*cm. Hoang et al. [21] and Schmid et al. [22] used a phantom model to investigate exact effective doses of CT fluoroscopy-guided lumbar nerve root infiltrations and found a mean effective dose of 0.45 mSV and between 0.22 and 0.43 mSv, respectively. Although it was not the aim of our study to investigate exact values for effective doses and we did not use a phantom, we were at least able to calculate an approximate mean effective dose of 0.73 mSv in the group with CT-guided interventions. A low-dose CT protocol alone, however, is not sufficient to reduce radiation exposure. The pre-interventional acquisition required in some patients significantly contributes to the overall exposure. Hoang et al. [21] reported an average increase in the effective dose of 2.90 mSv when pre-imaging was necessary. In our study, the 14 patients who required a pre-interventional short-spiral CT scan to improve orientation in the presence of severe degenerative changes had a significant increase in mean dose-length product of 125.7 mGy*cm (approximate increase of 2.36 mSV; t test, P < 0.001).
MRI guidance of nerve root infiltration involves no radiation exposure of patients or medical staff. Therefore, when choosing the method of treatment those patients with a high probability of severe degenerative changes should get a treatment with MR guidance to avoid the additional radiation of the scan needed for planning. When performing the procedure in an open MRI system, the interventionalist has good access to the patient and the option of interactive control of the procedure [13, 23]. Owing to multiplanar navigation capabilities, needle positioning is facilitated, improving the workflow, accuracy and speed of the intervention. A further technical advantage of MR guidance is the high soft tissue contrast, allowing injection control to be performed without contrast medium, which is used by some proceduralists for CT guidance. Hence, MRI guidance completely avoids the risk of a reaction to contrast agents.
Our study has limitations: The cost analysis for both CT and MR guidance was performed in Germany. Costs of materials, personnel and equipment may differ widely among European countries. Although there may be differences in absolute costs, we nevertheless expect that relative cost differences are much smaller. As the reimbursement policy is already complex in a single European country such as Germany and depends on the patient’s health insurance, we did not take reimbursement into account in our study. We are aware that many institutions still perform fluoroscopy-guided nerve root injections. We did not take the costs of fluoroscopic guidance into account as these procedures are performed in the orthopaedic department of our hospital and data on process time were not available.
The limited availability of open MRI devices is still an obstacle to the wider use of MR-guided interventions. However, as open high-field systems such as Siemens’ 1.5-T Magnetom Espree (Siemens Medical Solutions, Erlangen, Germany) and the 1.0-T Philips Panorama used in our study show promising results with an improved workflow [12] and a high level of patient acceptance [16], we expect interventional MRI to become more widely available in the future.
In conclusion, lumbosacral nerve root infiltration under MRI guidance involves no radiation exposure for patients and personnel but is at this stage not a cost-effective alternative. It is still about twice as expensive per procedure as CT guidance, which is mainly attributable to higher costs of equipment and materials. Only with further decreases in prices for MRI devices and for MR-compatible disposables will MR-guided therapeutic nerve root infiltration perhaps become a promising alternative to the CT-guided procedure.
References
Martin BI, Deyo RA, Mirza SK et al (2008) Expenditures and health status among adults with back and neck problems. JAMA 299:656–664
Deyo RA, Weinstein JN (2001) Low back pain. N Engl J Med 344:363–370
Andersson GB (1999) Epidemiological features of chronic low-back pain. Lancet 354:581–585
van Tulder MW, Koes BW, Bouter LM (1995) A cost-of-illness study of back pain in The Netherlands. Pain 62:233–240
Uhlenbrock D, Arlinghaus J (1997) Results of CT-guided periradicular pain therapy. Rofo 166:528–534
Wagner AL (2004) Selective lumbar nerve root blocks with CT fluoroscopic guidance: Technique, results, procedure time, and radiation dose. AJNR Am J Neuroradiol 25:1592–1594
Botwin KP, Thomas S, Gruber RD et al (2002) Radiation exposure of the spinal interventionalist performing fluoroscopically guided lumbar transforaminal epidural steroid injections. Arch Phys Med Rehabil 83:697–701
Nawfel RD, Judy PF, Silverman SG et al (2000) Patient and personnel exposure during CT fluoroscopy-guided interventional procedures. Radiology 216:180–184
Paulson EK, Sheafor DH, Enterline DS et al (2001) CT fluoroscopy—guided interventional procedures: Techniques and radiation dose to radiologists. Radiology 220:161–167
Hall EJ, Brenner DJ (2008) Cancer risks from diagnostic radiology. Br J Radiol 81:362–378
Pfirrmann CW, Oberholzer PA, Zanetti M et al (2001) Selective nerve root blocks for the treatment of sciatica: Evaluation of injection site and effectiveness–a study with patients and cadavers. Radiology 221:704–711
Fritz J, Thomas C, Clasen S et al (2009) Freehand real-time MRI-guided lumbar spinal injection procedures at 1.5 T: Feasibility, accuracy, and safety. AJR Am J Roentgenol 192:W161–167
Streitparth F, Walter T, Wonneberger U et al (2010) Image-guided spinal injection procedures in open high-field MRI with vertical field orientation: Feasibility and technical features. Eur Radiol 20:395–403
Sequeiros RB, Ojala RO, Klemola R et al (2002) MRI-guided periradicular nerve root infiltration therapy in low-field (0.23-T) MRI system using optical instrument tracking. Eur Radiol 12:1331–1337
Ronkainen J, Blanco Sequeiros R, Tervonen O (2006) Cost comparison of low-field (0.23 T) MRI-guided laser ablation and surgery in the treatment of osteoid osteoma. Eur Radiol 16:2858–2865
Bangard C, Paszek J, Berg F et al (2007) MR imaging of claustrophobic patients in an open 1.0 T scanner: Motion artifacts and patient acceptability compared with closed bore magnets. Eur J Radiol 64:152–157
Alanen J, Keski-Nisula L, Blanco-Sequeiros R et al (2004) Cost comparison analysis of low-field (0.23 T) MRI- and CT-guided bone biopsies. Eur Radiol 14:123–128
Huppertz A, Schmidt M, Wagner M et al (2010) Whole-body MR imaging versus sequential multimodal diagnostic algorithm for staging patients with rectal cancer: Cost analysis. Rofo 182:793–802
Singer ME, Applegate KE (2001) Cost-effectiveness analysis in radiology. Radiology 219:611–620
Ojala R, Vahala E, Karppinen J et al (2000) Nerve root infiltration of the first sacral root with MRI guidance. J Magn Reson Imaging 12:556–561
Hoang JK, Yoshizumi TT, Toncheva G et al (2011) Radiation dose exposure for lumbar spine epidural steroid injections: A comparison of conventional fluoroscopy data and CT fluoroscopy techniques. AJR Am J Roentgenol 197:778–792
Schmid G, Schmitz A, Borchardt D et al (2006) Effective dose of CT- and fluoroscopy-guided perineural/epidural injections of the lumbar spine: A comparative study. Cardiovasc Intervent Radiol 29:84–91
Streitparth F, Hartwig T, Schnackenburg B et al (2011) MR-guided discography using an open 1 Tesla MRI system. Eur Radiol 21:1043–1049
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Maurer, M.H., Schreiter, N., de Bucourt, M. et al. Cost comparison of nerve root infiltration of the lumbar spine under MRI and CT guidance. Eur Radiol 23, 1487–1494 (2013). https://doi.org/10.1007/s00330-012-2757-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-012-2757-y