Abstract
Objectives
Assessment of cartilage lesions and osteoarthritis (OA) of the patellofemoral joint in patients following lateral patellar dislocation using magnetic resonance imaging (MRI).
Methods
MR images of 129 knees (mean age 26 years, range 11–56) grouped as acute (A), recurrent (B), and chronic (C) dislocators were analysed regarding the prevalence and severity of patellofemoral cartilage lesions. Grades of OA were assessed using modified WORMS.
Results
In groups A, B, and C the prevalence of cartilage lesions was 71%, 82%, and 97%, respectively. Most lesions were located on the central patella in groups A and B (central 69% and 78%; medial 56% and 47%; lateral 31% and 42%), whereas group C revealed all regions affected (73%, 61%, and 67%). Of group A, 14% had mild OA and 64% of group B. Group C showed mild OA in 62% and moderate OA in 18%. Cartilage defect size and prevalence of OA was correlated with number of dislocations (r = 0.41 and r = 0.59; P < 0.001).
Conclusions
Cartilage lesions and early OA are common after patellar dislocation and appear to increase with the frequency of dislocation. Both conditions should be considered when interpreting MRI in such patients, because of implications for treatment.
Key Points
• Cartilage lesions are very common after patellar dislocation.
• The severity of cartilage lesions increases with number of dislocations.
• Osteoarthritis is common after recurrent patellar dislocation, even in young patients.
• Detecting cartilage lesions is important after patellar dislocation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lateral patellar dislocation (LPD) primarily occurs in young and active persons [8, 33]. Dislocation of the patella damages surrounding soft tissue structures and leads to chondral and osteochondral defects. They result from sliding of the posterior patellar surface over the lateral trochlear and subsequent contusion against the lateral femoral condyle [5, 7]. Damage to articular surfaces can result in severe chronic pain, limited mobility and reduced quality of life, especially with chronic patellofemoral instability [14].
Progression of joint destruction can be prevented or slowed by adequate treatment. Magnetic resonance imaging (MRI) has shown to be a reliable diagnostic tool for quantitative assessment of cartilage damage [4]. Dedicated MR analysis of cartilage damage, therefore, can provide crucial information for selecting the best treatment. Therapeutic options include conservative measures and different arthroscopic and surgical interventions [1].
The prevalence of cartilage damage following LPD reported in the literature varies widely and appears to depend on how many episodes of dislocation a patient has experienced and the diagnostic procedure used. Studies using MRI and arthroscopy identified cartilage defects after dislocation in 40-96% of cases [7, 9, 24, 29, 37]. Cartilage defects appear to become more severe over time [23], and an association of OA with LPD has been suspected [16] (Fig. 2).
While osteochondral fracture of the inferomedial patella has long been known to be a typical concomitant injury of acute LPD, little attention has been paid to cartilage lesions of the remaining patellar surface and of the trochlea, the effects of recurrent dislocation on the patellofemoral articular surface and the occurrence of osteoarthritis (OA).
The aim of this study was to assess the prevalence, severity and localisation of cartilage damage and osteoarthritis in patients with acute, recurrent, and chronic LPD, correlating the occurrence of patellofemoral joint defects with the frequency of dislocation episodes.
Materials and methods
Study population
After approval by the institutional review board, we searched the clinical database for patients with patellar dislocation who underwent an MRI knee-examination at one of three sites of a university medical centre from July 2000 through June 2011. Patients included had a convincing history of patellar dislocation and/or typical signs at MRI (e.g. oedema of the inferomedial patella and lateral condyle, tear of the medial patellofemoral ligament). Patients who had a prior knee intervention with metal implantation close to the patellofemoral joint were excluded. Mean interval between the last episode of patellar dislocation and MRI was 21 days (range, 1–93 days).
A total of 129 knees (72 left, 57 right) of 125 patients (68 women, 59 men) were included (Table 1). The mean age was 26 years (range, 11–56 years). The number of patellar dislocations for each patient was determined from the medical records or retrospective personal questioning. Based on the number of dislocations, patients were assigned to the following groups: first-time dislocators (group A) with one dislocation; recurrent dislocators with two to nine dislocations (group B); and chronic dislocators with ten or more dislocations or chronically subluxated patella (group C).
Information on prior surgery for patellofemoral instability was available for 33 subjects of groups B and C. Nineteen patients (57.6%) had repair of the medial patellofemoral ligament, ten patients (30.3%) had tibial tuberosity advancement and five patients had lateral release (15.2%). None of the patients had prior trocheloplasty or derotational osteotomy.
MRI
MRI was performed on one of six MRI systems, using either dedicated knee coil or an extremity coil with clinical routine standard protocols. MRI systems, sequence specifications and coil combinations are described in Table 2. Since some sequence specifications were adjusted over the 11 years of the study period, the last-used parameters are described. All patients were imaged in the supine position with the leg in full extension.
MRI evaluation
MR images were evaluated separately on a PACS workstation (RadiForce R22; Eizo Nanao Corporation, Hakui, Japan). Images were assessed for cartilage defects and OA by two radiologists in consensus (reader 1 had 8 years’ experience in musculoskeletal imaging and reader 2 had 3 years’ experience). Retrospective reading was done in chronological order of MRI examinations. The readers were blinded to number of LPD and other patient data.
MRI evaluation of cartilage defects
Cartilage defects were evaluated in three anatomic subregions of the patellofemoral joint as previously published [37]: the central dome, medial and lateral facets of patella; the central, medial and lateral aspects of trochlea. As in other studies [27, 34, 37], the Outerbridge classification [25] was used to assess the depth of cartilage defects; briefly: grade 0—normal cartilage; grade I—smooth cartilage surface but focal signal heterogeneity; grade II—partial-thickness defect not deeper than 50%; grade III—defect of > 50% of cartilage thickness; grade IV—full-thickness defect and osteochondral defect (Fig. 1). In all subregions, the lesion with the highest grade was documented.
Classification of cartilage lesions. Patients after lateral patellar dislocation; MRI: axial, T2-weighted, fat saturated TSE sequences (a, b, d) and axial, PD-weighted, fat saturated FSE sequence (c). a Grade I: intact cartilage surface, altered signal intensity of cartilage layer in patellar dome and lateral facet. b Grade II: local cartilage lesion, < 50% of cartilage thickness, in patellar dome. c Grade III: erosion of cartilage surface, > 50% of cartilage thickness, in patellar dome. d Grade IV: full surface defect of patellar cartilage in central dome
Morphologically, cartilage defects were categorised using arthroscopic terminology [22, 23]: category I—fissure; II—fibrillation; III—erosion. This classification was supplemented by three types of cartilage defects typically found after LPD: IV—lamination; V—dislocated cartilage fragment; VI—osteochondral defect. Additionally, the largest extent of the cartilage defect was measured on axial and sagittal images and the area was calculated (mm²).
Inter- and intraobserver reliability with regard to Outerbridge classification of cartilage defects was evaluated by analysis of 25 randomly selected subjects. Weighted Cohen’s Kappa statistics showed good intraobserver (κ = 0.89) and interobserver agreement (κ = 0.84).
MRI evaluation of osteoarthritis
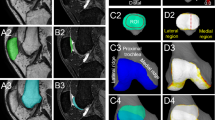
The severity of OA of the patellofemoral joint was evaluated using a modified WORMS classification [26]. Briefly, WORMS is a semi-quantitative scoring method for evaluation of the knee in osteoarthritis based on MRI findings using 14 different features, including meniscal, ligamentous, cartilage and bony lesions. These features are graded by severity and summed to a total score. Here we used a reduced version of WORMS since only the patellofemoral joint was investigated. The patella and trochlea were evaluated for articular cartilage integrity, subarticular bone marrow abnormality, subarticular cysts, subarticular bone attrition and marginal osteophytes (Fig. 2). In the full version, a total score of 332 can be assigned versus 88 in our version. Scores were summarised in three grades: grade 1 (mild)—scores 1–29; grade 2 (moderate)—scores 30–58; grade 3 (severe)—scores 59–88. The intraclass correlation coefficient (ICC) showed an excellent intra- and interobserver reliability using the modified WORMS classification (intraobserver reliability 0.98; interobserver reliability 0.98).
Signs of osteoarthritis after LPD. Patients after recurrent (a, b) and chronic (c, d) lateral patellar dislocation; MRI: axial, T2-weighted, fat saturated TSE sequences (a, b, d) and PD-weighted, fat saturated FSE sequence (c). a Erosive changes of patellar and trochlear cartilage, cyst-like lesion in patella (arrow). b Erosive changes in patellar and trochlear cartilage, focal bone marrow lesion in patella (arrow). c Erosive changes in patellar and trochlear cartilage, small osteophyte at lateral trochlea (arrow). d Nearly complete erosive destruction of patellar and trochlear cartilage, bone erosion, bone marrow lesion and osteophytes
Statistical analysis
Descriptive statistics of cohort and prevalence of cartilage defects and OA were calculated. The chi-squared test was used to compare prevalence between groups and anatomical regions. Linear regression analysis using ANOVA was done to assess associations between number of LPD and cartilage defect areas, as well as WORMS. Adjustments for age and sex were made by multivariate analysis. Statistical analysis, calculations and drawings of boxplot diagrams were done with SPSS version 15.0 (SPSS, Chicago, IL).
Results
Cartilage defects
Cartilage defects of different grades and morphology were identified in 79.8% of cases (103/129 cases) (Table 3). The central patellar dome was the most common site of cartilage defects, accounting for 75% of instances (P = 0.026). Of patients with a central dome defect, 51.9% had an additional medial defect (40/77) and 48.1% an additional lateral defect (37/77). An isolated defect of the lateral or medial compartment in an otherwise intact patella was present in 10.6% (11/103) and 4.9% (5/103) of cases, respectively. A total of 21.4% (22/103) had defects of all patellar subregions.
Trochlear cartilage defects were less common than patellar defects (32.0% trochlea vs 80.2% patella, P < 0.001) and were most common in lateral cartilage, which were seen in one third of the cases (P < 0.001). Only 4.8% of patients had trochlear cartilage defects without concomitant patellar lesion.
In group A, approximately two-thirds of patients had cartilage defects. Cartilage defects of the central dome were primarily grades II and III (Table 4). These defects were primarily due to erosion (31.6%) or fibrillation (26.3%). Defects of the medial facet were exclusively due to osteochondral defects. The most common trochlear lesions were Outerbridge II defects of the lateral aspect (37.5% of cases).
In group B, 79.5% of patients had cartilage defects, most commonly involving central and medial patellar regions; however, one-third of patients had concomitant defects of the lateral facet (Table 4). Most central dome lesions were Outerbridge II lesions, predominantly with fibrillation and erosion (52.9% and 35.3%, respectively). In the medial region were Outerbridge IV lesions typically due to osteochondral defects (85.7%), along with Outerbridge II and III lesions. The lateral trochlea predominantly showed erosive Outerbridge II and III lesions.
Patients of group C had highest prevalence of cartilage defects (93.3%, P = 0.009). As in the other two groups, cartilage defects were mostly found in the central dome, slightly less commonly in the medial and lateral facets (Table 4). Erosive cartilage lesions were most common in all patellar subregions with partially severe lesions in medial facet and otherwise predominantly moderate lesions (Outerbridge II or III). Erosive lesions were also common in all three trochlear subregions compared with groups A and B (Tables 3, 4 and 5).
The total area of cartilage defects was largest in group C (Fig. 3). Regression analysis of the total defect area and the number of LPDs yielded a regression coefficient B of 27.889 (standard error, 6.022; r = 0.413; P < 0.001). This corresponds to a 27.9 mm² increase in the total defect area per dislocation. With age and sex as cofactors, we calculated regression coefficients of B = 20.184 (standard error, 5.804; r = 0.552; P < 0.001) and B = 30.645 (standard error, 6.638; r = 0.423; P < 0.001), respectively.
Osteoarthritis
Overall, we identified patellofemoral OA of variable severity in about 50% of the study population (Table 6). Nearly all patients with degenerative damage had low-grade disease; however, four patients in group C (3.1% of total) had moderate OA. High-grade OA (WORMS > 58) of patellofemoral joint was not detected in this study population consisting of predominantly young patients.
In relation to the number of dislocations, the proportion of OA was highest in group C (76.7%, P < 0.001). In group B, two-thirds of patients had low-grade OA, in group A OA rarely was detected (Fig. 4).
Patellofemoral osteoarthritis following lateral patellar dislocation. Boxplots showing the WORMS scores and grades of patellofemoral osteoarthritis in first-time (n = 51), recurrent (n = 44), and chronic dislocators (n = 34). The maximum osteoarthritis score in the modified WORMS system is 88 points
Regression analysis of WORMS and number of dislocations yielded B = 1.556 (standard error 0.208; r = 0.590; P < 0.001). With each dislocation in the previous medical history, there was an increase in the WORMS of 1.556 points. The results were similar when sex was taken into account (B = 1.578; r = 0.590; P < 0.001). With age as a cofactor, the regression coefficient was slightly smaller (B = 1.251; r = 0.695; P < 0.001), identifying age as an additional influence. It is noteworthy that we observed a few cases of OA in very young patients. A 16-year-old youth in group A had first signs of early OA (WORMS of 7). A 26-year-old patient with chronic LPD had marked signs of OA (WORMS of 44)
Discussion
The aim of this study was to determine the prevalence and severity of cartilage defects and osteoarthritis of the patellofemoral joint on MRI in relation to the number of LPDs. Nearly all patients had cartilage lesions and more than half of the patients had at least mild or moderate OA, which is a significant finding regarding the low mean age of the study population. Cartilage lesions in first-time and recurrent dislocators were primarily accounted for erosions and fissures of the central patellar dome and osteochondral defects of the medial facet. Chronic dislocators additionally had severe defects of the lateral patella facet and of trochlear cartilage. Overall, the prevalence of cartilage defects and OA was highest in chronic patellar dislocators. The extent of cartilage defects and severity of OA correlated moderately with the number of prior LPDs.
A normal population without a history of LPD has a markedly lower prevalence of cartilage damage or OA, even at a higher age [18]. The mechanism of LPD, with slipping out of the trochlear groove, sliding of the patellar cartilage surface over the lateral trochlear edge, and contusion of the medial patella at the lateral femoral condyle is associated with abnormal loading of the articular surface of the patella. Although we did not obtain longitudinal data in our study, we may safely assume that both first-time LPD and, above all, recurrent dislocation are considerable risk factors for cartilage defects of patellofemoral joint. Chondral and osteochondral lesions have been reported as complications of LPD in arthroscopic and radiological studies [2, 3, 7, 9, 13, 17, 21, 22, 24, 29–32, 35–37]. Earlier investigators identified the inferomedial patella as the main site of articular defects resulting from patellar impaction on the condyle [7, 13, 24]. During dislocation, the cartilage of the central dome of the patella is exposed to strong shear forces and pressure. This might explain the high prevalence of central and medial patellar cartilage defects [11, 22, 24]. Lateral patellar damage primarily is observed in chronic luxation and is attributable to persistence of the patella in lateral subluxation. With the patella in this position, its lateral portion glides down the lateral trochlear, resulting in abrasion of patellar cartilage. Damage to the trochlear groove in acute dislocation appears to be less severe, probably because the groove is protected by the concavity of its surface.
Differences in anatomy may influence the severity of cartilage damage. Most important factors are trochlear dysplasia, patellar height, and possibly also lateralisation of the force vector caused by an abnormal trochlear-groove-to-tibial-tuberosity distance. Additionally, several forms of soft tissue hyperlaxity (e.g. Ehlers-Danlos syndrome, Marfan syndrome) can predispose to LPD. These conditions may be associated with lower mechanical loading of articular cartilage. Hence, not all dislocators will develop higher-grade cartilage damage. Future studies should investigate whether there is an association between the severity of anatomic risk factors and the severity of chondral lesions and OA.
Reports of prevalence of cartilage defects after LPD vary widely. In an arthroscopy-based study, Nomura and Inoue [22] detected medial patellar defects in two-thirds of the cases and lesions of the central dome in half of the cases. Slightly lower prevalence was found in the arthroscopy-based study of Virolainen et al. [36]. The arthroscopic findings in both studies were obtained at time of arthroplasty or resection of the osteochondral fragment [24], indicating mainly patients with severe trauma and probably severe damage were included. Our population may include more patients with less severe trauma, resulting in lower rates of severe osteochondral lesions.
Several longitudinal studies have shown that cartilage defects progress with further dislocations [22, 23]. The high prevalence of cartilage lesions of 96% that Nomura and Inoue [23] found in an arthroscopic study of chronic LPD correlates well with the rate in our study. Moreover, the prevalence of lesions of the central patellar dome (77%) is slightly higher than the prevalence reported here (66%), confirming the frequent occurrence of damage at this site, in particular in chronic dislocators. In a follow-up investigation, Nomuraand Inoue [23] found a marked increase in cartilage defects. To our knowledge, this is the first MRI study analysing the prevalence of OA in patients with LPD. Our results show that there is a correlation between OA and the number of LPD and that even adolescents may develop mild or moderate OA. In contrast, in young, healthy adults OA appears unlikely [19]. Although, the significance of small cartilage defects with respect to clinical symptoms and progression of OA is still unclear, there is strong evidence that abnormal loading of cartilage can lead to chondropathy and degenerative disease over time [10, 12] Ding et al. [6] showed that the prevalence and severity of knee cartilage defects are significantly associated with tibiofemoral osteophytes, knee cartilage volume, suggesting an important role of cartilage defects in early OA. An experimental model in rabbits confirms an association between induced cartilage defects and development of OA [15]. The high prevalence of OA in the group of chronic dislocators might thus be attributable to cartilage lesions occurring during LPD.
Demonstration of cartilage damage after LPD is pivotal for therapeutic decision-making. The choice between conservative and surgical management crucially depends on the morphology, size, and depth of cartilage defects. However, a consistent international therapeutic approach is still lacking [20]. Smaller and more superficial cartilage defects are treated conservatively. Extensive and deeper defects can be managed by a variety of arthroscopic and open surgical options [28]. Therefore, precise documentation of cartilage condition in the radiologist’s report is central for treatment planning.
Our study has some limitations. We analysed MRI findings without arthroscopic correlation, primarily because only patients with osteochondral or significant chondral defects underwent arthroscopy and also because, due to the retrospective study setting, there was no consistent documentation of cartilage damage by surgeons at the three participating hospitals. However, published studies have shown excellent agreement of MRI grading of cartilage damage with arthroscopy as the “gold standard”, while suggesting that MRI may have limitations in identifying low-grade cartilage damage [37]. Moreover, our study patients were examined on different MR systems using routine clinical pulse sequences. Since the isotropic three-dimensional pulse sequence was not acquired in all patients, only the standard pulse sequences were included in the analysis in order to use identical images in all cases. This might have resulted in a lower sensitivity for the detection of low-grade cartilage lesions. Moreover, the analysis did not include prior therapeutic procedures in recurrent and chronic dislocators, which might also have influenced our results. Moreover, the grouping of the patients was done in such a way to separate patients with recurrent dislocations (less than ten) from those with a chronically luxated patella. It must be noted that there is no clinical consensus regarding the number of previous episodes that defines a recurrent or chronic dislocator.
In conclusion, cartilage lesions are very common after LPD, and their severity correlates with the number of previous dislocations. Moreover, many patients with chronic patellar subluxation have degenerative changes of the patellofemoral joint, which is a significant outcome considering the young age of these individuals. We conclude that it is important to put emphasis on cartilage lesions in MRI reports and allow for a more dedicated management strategy to prevent subsequent damage to the joint.
References
Ahmed TA, Hincke MT (2010) Strategies for articular cartilage lesion repair and functional restoration. Tissue engineering. Part B. Reviews 16:305–329
Ahstrom JP Jr (1965) Osteochondral fracture in the knee joint associated with hypermobility and dislocation of the patella. Report of eighteen cases. J Bone Joint Surg Am 47:1491–1502
Dainer RD, Barrack RL, Buckley SL, Alexander AH (1988) Arthroscopic treatment of acute patellar dislocations. Arthroscopy 4:267–271
Dandy DJ, Griffiths D (1989) Lateral release for recurrent dislocation of the patella. J Bone Joint Surg Br 71:121–125
Diederichs G, Issever AS, Scheffler S MR imaging of patellar instability: injury patterns and assessment of risk factors. Radiographics 30:961–981
Ding C, Garnero P, Cicuttini F, Scott F, Cooley H, Jones G (2005) Knee cartilage defects: association with early radiographic osteoarthritis, decreased cartilage volume, increased joint surface area and type II collagen breakdown. Osteoarthritis Cartilage 13:198–205
Elias DA, White LM, Fithian DC (2002) Acute lateral patellar dislocation at MR imaging: injury patterns of medial patellar soft-tissue restraints and osteochondral injuries of the inferomedial patella. Radiology 225:736–743
Fithian DC, Paxton EW, Stone ML et al (2004) Epidemiology and natural history of acute patellar dislocation. Am J Sports Med 32:1114–1121
Guerrero P, Li X, Patel K, Brown M, Busconi B (2009) Medial patellofemoral ligament injury patterns and associated pathology in lateral patella dislocation: an MRI study. Sports Med Arthrosc Rehabil Ther Technol 1:17
Hunter DJ, Zhang YQ, Niu JB et al (2007) Patella malalignment, pain and patellofemoral progression: the Health ABC Study. Osteoarthritis Cartilage 15:1120–1127
Insall J, Falvo KA, Wise DW (1976) Chondromalacia Patellae. A prospective study. J Bone Joint Surg Am 58:1–8
Kalichman L, Zhang Y, Niu J et al (2007) The association between patellar alignment and patellofemoral joint osteoarthritis features—an MRI study. Rheumatology (Oxford) 46:1303–1308
Kirsch MD, Fitzgerald SW, Friedman H, Rogers LF (1993) Transient lateral patellar dislocation: diagnosis with MR imaging. AJR Am J Roentgenol 161:109–113
Kodali P, Islam A, Andrish J (2011) Anterior knee pain in the young athlete: diagnosis and treatment. Sports Med Arthrosc 19:27–33
Lefkoe TP, Trafton PG, Ehrlich MG et al (1993) An experimental model of femoral condylar defect leading to osteoarthrosis. J Orthop Trauma 7:458–467
Maenpaa H, Lehto MU (1997) Patellofemoral osteoarthritis after patellar dislocation. Clin Orthop Relat Res:156–162
Mashoof AA, Scholl MD, Lahav A, Greis PE, Burks RT (2005) Osteochondral injury to the mid-lateral weight-bearing portion of the lateral femoral condyle associated with patella dislocation. Arthroscopy 21:228–232
McAlindon TE, Snow S, Cooper C, Dieppe PA (1992) Radiographic patterns of osteoarthritis of the knee joint in the community: the importance of the patellofemoral joint. Ann Rheum Dis 51:844–849
Merchant AC (1988) Classification of patellofemoral disorders. Arthroscopy 4:235–240
Mouzopoulos G, Borbon C, Siebold R (2011) Patellar chondral defects: a review of a challenging entity. Knee Surg Sports Traumatol Arthrosc 19(12):1990-2001
Nietosvaara Y, Aalto K, Kallio PE (1994) Acute patellar dislocation in children: incidence and associated osteochondral fractures. J Pediatr Orthop 14:513–515
Nomura E, Inoue M (2004) Cartilage lesions of the patella in recurrent patellar dislocation. Am J Sports Med 32:498–502
Nomura E, Inoue M (2005) Second-look arthroscopy of cartilage changes of the patellofemoral joint, especially the patella, following acute and recurrent patellar dislocation. Osteoarthritis Cartilage 13:1029–1036
Nomura E, Inoue M, Kurimura M (2003) Chondral and osteochondral injuries associated with acute patellar dislocation. Arthroscopy 19:717–721
Outerbridge RE (1961) The etiology of chondromalacia patellae. J Bone Joint Surg Br 43-B:752–757
Peterfy CG, Guermazi A, Zaim S et al (2004) Whole-Organ Magnetic Resonance Imaging Score (WORMS) of the knee in osteoarthritis. Osteoarthritis Cartilage 12:177–190
Potter HG, Linklater JM, Allen AA, Hannafin JA, Haas SB (1998) Magnetic resonance imaging of articular cartilage in the knee. An evaluation with use of fast-spin-echo imaging. J Bone Joint Surg Am 80:1276–1284
Redziniak DE, Diduch DR, Mihalko WM et al (2009) Patellar instability. J Bone Joint Surg Am 91:2264–2275
Sanders TG, Paruchuri NB, Zlatkin MB (2006) MRI of osteochondral defects of the lateral femoral condyle: incidence and pattern of injury after transient lateral dislocation of the patella. AJR Am J Roentgenol 187:1332–1337
Sillanpaa PJ, Maenpaa HM, Mattila VM, Visuri T, Pihlajamaki H (2008) Arthroscopic surgery for primary traumatic patellar dislocation: a prospective, nonrandomized study comparing patients treated with and without acute arthroscopic stabilization with a median 7-year follow-up. Am J Sports Med 36:2301–2309
Spahn G, Klinger HM, Baums M, Pinkepank U, Hofmann GO (2011) Reliability in arthroscopic grading of cartilage lesions: results of a prospective blinded study for evaluation of inter-observer reliability. Arch Orthop Trauma Surg 131:377–381
Stanitski CL, Paletta GA Jr (1998) Articular cartilage injury with acute patellar dislocation in adolescents. Arthroscopic and radiographic correlation. Am J Sports Med 26:52–55
Stefancin JJ, Parker RD (2007) First-time traumatic patellar dislocation: a systematic review. Clin Orthop Relat Res 455:93–101
Suh JS, Lee SH, Jeong EK, Kim DJ (2001) Magnetic resonance imaging of articular cartilage. Eur Radiol 11:2015–2025
Vainionpaa S, Laasonen E, Patiala H, Rusanen M, Rokkannen P (1986) Acute dislocation of the patella. Clinical, radiographic and operative findings in 64 consecutive cases. Acta Orthop Scand 57:331–333
Virolainen H, Visuri T, Kuusela T (1993) Acute dislocation of the patella: MR findings. Radiology 189:243–246
von Engelhardt LV, Raddatz M, Bouillon B et al (2010) How reliable is MRI in diagnosing cartilaginous lesions in patients with first and recurrent lateral patellar dislocations? BMC Musculoskelet Disord 11:149
Acknowledgements
The authors are grateful to Dr. Maryna Verba (Institute of Biometry and Clinical Epidemiology, Charité—Universitätsmedizin Berlin, Berlin) for statistical support.
There was no funding used for this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
E. Wiener and G. Diederichs contributed equally to this article
Rights and permissions
About this article
Cite this article
Vollnberg, B., Koehlitz, T., Jung, T. et al. Prevalence of cartilage lesions and early osteoarthritis in patients with patellar dislocation. Eur Radiol 22, 2347–2356 (2012). https://doi.org/10.1007/s00330-012-2493-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-012-2493-3