Abstract
Objectives
To investigate whether CT-derived vascular parameters in primary breast cancer predict complete pathological response (pCR) to neoadjuvant chemotherapy (NAC).
Methods
Twenty prospective patients with primary breast cancer due for NAC underwent volumetric helical perfusion CT to derive whole tumour regional blood flow (BF), blood volume (BV) and flow extraction product (FE) by deconvolution analysis. A pCR was achieved if no residual invasive cancer was detectable on pathological examination. Relationships between baseline BF, BV, FE, tumour size and volume, and pCR were examined using the Mann–Whitney U test. Receiver operating characteristic (ROC) curve analysis was performed to assess the parameter best able to predict response. Intra- and inter-observer variability was assessed using Bland–Altman statistics.
Results
Seventeen out of 20 patients completed NAC with four achieving a pCR. Baseline BF and FE were higher in patients who achieved a pCR compared with those who did not (P = 0.032); tumour size and volume were not significantly different (P > 0.05). ROC analysis revealed that BF and FE were able to identify responders effectively (AUC = 0.87; P = 0.03). There was good intra- and inter-observer agreement.
Conclusions
Primary breast cancers which exhibited higher levels of perfusion before treatment were more likely to achieve a pCR to NAC.
Key Points
• CT-derived vascular parameters may be useful in breast cancer treatment.
• Perfusion CT can help predict response to neoadjuvant chemotherapy in breast cancer.
• Baseline blood flow and flow extraction product are higher in complete pathological responders.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Neoadjuvant chemotherapy (NAC) is well established in the treatment of primary breast cancer with the ability to downstage large tumours for breast-conserving surgery, and with the potential to eradicate occult micrometastatic disease. Patients who achieve a complete pathological response (pCR) to NAC have better long-term outcomes [1] and pCR is therefore commonly regarded as a surrogate parameter for treatment efficacy. The neoadjuvant setting also offers the opportunity to study tumour perfusion and vascular permeability in vivo; in breast cancer, the latter is of particular importance given the overexpression of vascular endothelial growth factor (VEGF), a potent mediator of angiogenesis [2].
Dynamic contrast-enhanced computed tomography (perfusion CT) is an attractive technique which captures anatomical and pathophysiological information in vivo and is becoming increasingly important in lesion characterization and detection, and in therapeutic monitoring. Quantitative vascular parameters such as regional blood flow (BF), blood volume (BV) and flow extraction product (FE) can be estimated in addition to qualitative data, such as curve-pattern and peak enhancement [3, 4]. Typically, regional BF provides an indication of the delivery rate of oxygen. Regional BV and FE reflect the functional volume and permeability, respectively, and may be surrogates for neoangiogenesis which in turn may be an indication of the vascular adaptation to tumour hypoxia [5]. US Food and Drug Administration (FDA)-approved software platforms available in standard CT reporting workstations and technical advances have enabled whole-tumour assessment, and facilitated the post-processing and analysis of data [6].
Studies have demonstrated correlations between contrast enhancement parameters and measurements of microvessel density and/or VEGF in various cancers including lung, renal, colorectal, pancreatic and breast tumours, validating perfusion CT as a biomarker of angiogenesis [6–13]. Thus the advantage of perfusion CT is its ability to provide insight into whole tumour angiogenesis without the need for invasive tissue biopsies, as well as its potential to provide prognostic information by way of biomarkers of response to treatment in breast cancer. The ability to predict those patients who might benefit most from NAC may enable individualization of therapy and a more efficacious use of therapies.
The objective of this study was to investigate whether volumetric helical perfusion CT derived vascular parameters in patients with primary breast cancer before NAC may predict pCR, as defined by pathological evaluation of the specimen.
Materials and methods
Patients
Institutional review board approval was obtained for this prospective study and informed consent obtained from all participating patients. Women with histologically proven primary breast cancer suitable for NAC were recruited consecutively from January 2009 to August 2010. Inclusion criteria for this study were (1) female patients 18–70 years, (2) adequate bone marrow function for chemotherapy and (3) adequate renal function. Exclusion criteria were (1) pregnant women and (2) any contraindications to CT imaging. Patients were required to undergo a baseline perfusion CT before the commencement of chemotherapy. Twenty patients (median age 45 years; range 35–58) were examined. The neoadjuvant regimen consisted of three cycles of FEC (5-fluorouracil 500 mg/m2, epirubicin 100 mg/m2, cyclophosphamide 500 mg/m2 intravenously every 21 days) followed by three cycles of docetaxel (100 mg/m2 intravenously every 21 days). Nine patients also received a single cycle of the anti-angiogenic agent bevacizumab (15 mg/kg intravenously) before chemotherapy as part of another trial. Patients then went on to receive surgery which was either mastectomy or breast-conserving surgery.
Response assessment
Pathological response was evaluated by a consultant pathologist from the histological comparison of the diagnostic core biopsy with the surgical specimen obtained after the completion of NAC. A pCR was defined as no residual invasive disease remaining in the breast and axillary nodes at surgery; residual in situ carcinoma was allowed which is a commonly accepted definition [14]. pCR was chosen as the primary endpoint in this study as it is associated with a better long-term prognosis and therefore is often used as a surrogate marker of efficacy. Patients who achieved a response other than a pCR were deemed non-responders in this study.
Perfusion CT acquisition and analysis
Patients underwent CT consisting of consecutive helical acquisitions using a ‘4D-adaptive spiral mode’ (Dual Source CT, Somatom Definition, Siemens Healthcare, Forchheim, Germany). A breath-held low dose planning acquisition to locate the breast tumour was performed initially (80 kV, effective mAs 70, rotation time 0.5 s, detectors 24 × 1.2 mm, pitch 1.2, slice collimation 5 mm, SFOV 300–450 mm, matrix 512 mm). Then 50 ml of iodinated contrast agent (ioversol 350 mg/ml iodine; Optiray, Covidien, Mansfield, MA, USA) at 6 ml/s was administered intravenously using a dual-headed pump injector, followed by a 50-ml saline flush.
After a delay of 4 s from the start of injection, a volumetric perfusion CT acquisition consisting of successive craniocaudal and caudocranial acquisitions was obtained (80 kV, 100 or120 mA, rotation time 0.33 s, 4D adaptive spiral with a variable pitch (consisting of a pitch of 1 at the central point of table travel and less than 1 in the accelerating and decelerating portions, thereby guaranteeing a full rotation was always available for reconstruction), cycle time of 1.5 s for the first 30 s, decreased to a cycle time of 3.0 s between helical acquisitions after 30 s for the remaining 15 s; z-axis coverage 11.4 cm; reconstructed slice thickness 3 mm, increment 2 mm; total acquisition time 45 s; effective dose 8.5–10.2 mSv; Fig. 1).
The volumetric perfusion CT acquisition was a breath-held acquisition: the patient was asked to hyperventilate before the acquisition, then instructed to breath-hold in expiration for the first 30 s, then instructed to take a single breath in and out (<3 s) before a second breath-hold for the remainder of the dynamic acquisition (Fig. 1). Image analysis was performed using validated commercially available software (Deconvolution algorithm, vPCT; Siemens Healthcare, Forchheim, Germany) [15]. Initial post-processing steps within the software platform included (1) motion correction and (2) application of the noise reduction filter. Motion correction was based on a non-rigid deformable registration technique [16], previously validated for lung and kidney data [17, 18]. The arterial time–attenuation curve was defined by placing a region of interest (ROI) within the thoracic aorta. Parametric maps of regional BF (ml/min/100 ml), BV (ml/100 ml) and FE (ml/min/100 ml) were then generated in axial, coronal and sagittal planes. Regional tumour BF represents the delivery rate of contrast agent to the tumour (blood flow per unit volume or mass of tissue); regional tumour BV represents the proportion of tissue that is comprised of flowing blood; FE reflects the rate of transfer of contrast agent from the intravascular to extravascular space.
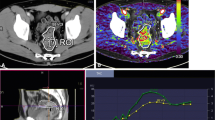
Volumetric regions of interest (VOI) were defined by a single radiologist with over 10 years of experience of perfusion CT techniques (observer 1), by manually outlining the margin of the tumour in axial, sagittal and coronal planes simultaneously thus generating parameter mean values for the entire tumour volume of interest (Fig. 2). Analysis was repeated in the same manner at least 4 weeks after the initial analysis, and the mathematical average of the two separate readings was used. VOIs were also outlined by an oncology fellow (observer 2) for the purposes of measurement of intra- and inter-observer variability. Maximum tumour dimension and volume were also recorded for each patient.
Statistical analysis
Statistical analysis was performed using the StatsDirect software package (Sale, Cheshire, UK). Median values for perfusion CT derived vascular parameters and tumour size and volume were compared between patients who achieved a complete response at surgery (pCR) versus those that did not, using the Mann–Whitney U test. Statistical significance was set at a two-sided P value of less than 0.05. Relationships between vascular parameters were evaluated using Spearman’s rank coefficient (ρ). Receiver operating characteristic (ROC) curve analysis was used to compare test performance for predicting final response at surgery.
For the measurement of intra- and inter-observer agreement, the mean differences, standard deviation and the 95 % limits of agreement for each of the perfusion parameters for each observer were calculated using Bland–Altman analysis [19]. The within-subject coefficient of variation and intra-class correlation were also determined.
Results
Seventeen of 20 patients successfully underwent NAC and were available for the purposes of response assessment. Of the three who were excluded, one received only two cycles of NAC after experiencing significant toxicity, another was still undergoing NAC at the time this was written and the third patient was not suitable for NAC after recruitment and instead received neoadjuvant hormonal therapy. The median total number of cycles of NAC received was 6 (range 4–6). Four patients achieved a pCR at surgery (23.5 %), whereas the remaining 13 achieved either a partial or no response. Of these 13 patients who were deemed to have a non-complete response, two did not have surgery at the completion of their NAC owing to a diagnosis of metastatic disease shortly after recruitment: their final clinical response was such that complete response at surgery was unlikely. Patient and disease characteristics are summarised in Table 1. These characteristics were not significantly different between responders and non-responders.
Values for BF and FE were significantly higher in patients who attained a complete response compared with those who did not (median BF 74.4 vs 46.4, P = 0.03; median FE 24.9 vs 16.9, P = 0.03). Representative images of a responder and non-responder are shown in Fig. 3. BV was also higher in responders but this did not reach statistical significance (median 7.7 vs 5.7, P = 0.08). There was a positive correlation between blood flow and volume (ρ = 0.79; P = 0.0002) across the group overall. Neither measurements of pretreatment tumour size nor volume were significantly different between the two groups (P = 0.16 and P = 0.08, respectively; Table 2).
Representative parametric maps of blood flow in a responder (top row) compared with a non-responder (bottom row) in the i axial, ii sagittal and iii coronal planes with the tumour volume of interest (VOI) outlined and the iv corresponding time–density curves for artery (red) and tumour (white) for whole tumour VOI. Both patients had a grade 3, T2N0M0 oestrogen receptor negative, progesterone receptor negative breast adenocarcinoma
ROC analysis revealed that both BF and FE were capable of identifying patients more likely to have a complete response following completion of chemotherapy (AUC: BF 0.87, P = 0.03; FE 0.87, P = 0.03) in comparison with BV and initial tumour size and volume (Table 2, Fig. 4).
The mean (and standard deviation) of tumour size, BF, BV and FE for observers 1 and 2 are summarized in Table 3. Measurements for intra- and inter-observer agreement are demonstrated in Table 4, with within-subject coefficients of variation ranging from 5.5 to 16.8 % and the intra-class correlation coefficient from 0.92 to 0.99, for all perfusion parameters.
Discussion
Recent technological advances including wide detector arrays (e.g. 320 MDCT) and volumetric helical perfusion CT techniques (e.g. 4D adaptive spiral; helical shuttle mode) have facilitated the clinical application of perfusion CT by allowing the whole tumour or more than one target lesion to be assessed. Clinical application in oncologic practice has primarily been in head and neck [20–25], lung [26–30], colorectal [31–36], upper gastrointestinal tract [12, 37, 38] and renal cancers [39–41] with only a few studies in breast cancer [7, 42]. Perfusion CT has been used in not only the assessment of response to various chemotherapeutic agents but also in proof of mechanistic studies of novel targeted agents such as the anti-VEGF inhibitor bevacizumab [34, 43] and the multikinase inhibitor sorafenib [29, 44].
To our knowledge, this is the first study to demonstrate the potential of CT perfusion in the prediction of response to NAC in human breast cancer. In this study, higher values of regional BF and FE were observed in patients who achieved a pCR to chemotherapy in comparison with those who did not. Therefore patients with breast tumours that are better perfused (increased blood flow) and which exhibit elevated levels of neoangiogenesis (increased FE) before treatment may derive the greatest benefit from NAC. Other studies have also reported favourable responses from tumours with higher baseline BVs [25, 35]; however, this was not statistically significant in our study despite there being a positive correlation between BV and BF. Measurements of BV reflect both mature and immature, newly formed leaky blood vessels and therefore may not be as sensitive as BF as a predictive marker. Our findings suggest that patients with less well perfused breast tumours may experience worse outcomes and early monitoring of response may be important for these patients. pCT parameters may be used for the stratification of non-responding patients into alternative treatment strategies. The addition of novel targeted therapies to conventional chemotherapy in this group of patients may help to improve their chances of achieving a pCR.
Our findings may be due to a combination of factors. Evidence in vitro indicates that the endothelial cells of newly forming blood vessels are highly and selectively sensitive to even very low doses of chemotherapeutic drugs compared with genetically more stable mature vessels and therefore tumours which display higher levels of neoangiogenesis before treatment may benefit the most from NAC [45]. As such, the more aggressive and highly proliferative nature of these highly vascular tumours makes them more susceptible to the anti-proliferative effects of cytotoxic agents. In clinical practice, women with large, high grade, hormone receptor negative breast cancers, i.e. more aggressive tumours, are the most likely to respond to preoperative chemotherapy [46, 47]. Furthermore, tumours which are less well perfused may have a higher degree of hypoxia resulting in the clonal selection of more aggressive tumour cells that are more chemo- and radioresistant [24, 26]. Hypoxia is an important upregulator of the angiogenic pathway via the stimulation of hypoxia inducible factor (HIF-1α)-induced VEGF expression leading to enhanced vascular proliferation [36]. Lastly, higher regional perfusion and permeability allows for more efficient chemotherapy delivery and distribution into the tumour creating higher intratumoural drug concentrations and potentially a better response [38, 48].
Similarly other perfusion CT studies, albeit using non-table moving techniques, have shown in several tumour sites that highly perfused tumours may in fact have better responses. In a study by Makari et al. [38] of 55 patients with advanced oesophageal cancers undergoing NAC, high blood flow before treatment was associated with a good response, whereas BV was not. Similarly, Wang et al. [26] demonstrated in 35 patients with non-small cell lung cancer that baseline blood flow in responders to either chemotherapy, radiotherapy or chemoradiotherapy was significantly higher than those of non-responders (P = 0.023). Bellomi et al. [35] found that baseline BF and BV in seven patients with rectal carcinoma who did not respond to neoadjuvant chemoradiation to be significantly lower than those of the 17 who did. Furthermore, patients with renal cell carcinoma treated with anti-angiogenic therapy experienced more than a twofold likelihood of response if they exhibited higher baseline tumour BF and BV [40]. Details of these and other studies are shown in Table 5.
Other functional imaging techniques including [18F]-fluorodeoxy-d-glucose positron emission tomography (FDG-PET) and diffusion-weighted MRI (DW-MRI) have been applied in breast cancer and demonstrated similar results [49, 50]. In a study of 30 patients with locally advanced breast cancer, Smith et al. [49] found that pretreatment values of semi-quantitative dose uptake on FDG-PET were higher in the eight primary breast lesions that achieved a pCR than those from less responsive lesions (P = 0.037). Mankoff et al. [51] evaluated tumour blood flow and glucose metabolism simultaneously in 37 patients, demonstrating that a low ratio of FDG metabolic rate to blood flow (MRFDG/BF) was the best predictor of complete response to NAC. They suggested that an elevated ratio was indicative of high glucose extraction by tumour in the setting of a hypoxic, poorly perfused environment, which confers resistance to treatment.
On diffusion-weighted MRI, Park et al. [50] demonstrated significantly lower pretreatment ADC values in responders to NAC compared with non-responders indicating that tumours that are more cellular, i.e. are of higher grade, have a higher chance of response. On the other hand, conflicting results have been seen with DCE-MRI in breast cancer. Ah-See et al. [52] demonstrated that although significant reductions in K trans were seen in pathological responders to NAC, they failed to find a correlation between baseline K trans and response. K trans, a transfer constant, is a parameter which describes the rate of transendothelial flow of contrast medium from plasma into the interstitium and has been used to characterise microvascular permeability quantitatively [3]. Similar results were reported in a study of 30 patients by Martincich et al. [53] who did not find any correlation between baseline contrast enhancement patterns on DCE-MRI and histopathological response. However it should be noted that these DCE-MRI studies were limited in their tumour volume coverage. In contrast the entire tumour volume was assessed on perfusion CT, which may provide a more reproducible and accurate measure of perfusion parameters, in particular within heterogeneous tumours when compared to two-dimensional ROI analysis on MRI [54].
In current practice patients are imaged with conventional MRI before NAC for the purposes of defining the extent of disease in the breast such that the presence of multifocal disease would negate the option of breast-conserving surgery. Patients may then be imaged again at the completion of NAC to evaluate morphologic response and to aid surgical excision. Whilst we recognise that anatomical MRI remains the gold standard for breast evaluation, functional imaging techniques that assess tumour biology in vivo such as those mentioned above are rapidly gaining clinical acceptance for monitoring tumour response to both chemotherapeutic and anti-angiogenic agents. CT, however, remains a widely performed technique for staging (assessment of distant metastases) and therapeutic response assessment.
Perfusion CT can be readily incorporated into existing CT protocols with good reproducibility and relative ease of parameter quantification compared with DCE-MRI. Advantages for the patient include the shorter duration of data acquisition and less chance of claustrophobia that is typically associated with MRI. Perfusion CT can also be readily integrated with PET with current integrated PET/CT systems, to provide a flow-metabolic evaluation. With regards to radiation dose, the radiation exposure associated with the diagnostic perfusion CT examination is relatively small compared with the radiotherapy dose that patients undergoing NAC will eventually receive as part of their breast cancer management. Patients who are suitable for NAC typically have tumours which are larger, of higher grade with many also having lymph node metastases and would therefore benefit from radical, post-operative radiotherapy after their surgery (either radical mastectomy or a wide local excision). Nevertheless, we appreciate that there remains a need to limit the radiation burden to the patient, in line with the ALARA (as low as reasonably achievable) principle. With the recent development of novel dedicated breast CT technology leading to doses equivalent to or lower than that of conventional mammography as demonstrated by Kalender et al. [55], perfusion CT may become even more attractive, although further work is needed to confirm these results.
Good intra- and inter-observer agreement was also observed in this study. Mean values varied by less than 10 % between both observers suggesting that VOI analysis was reproducible irrespective of the level of observer experience. In terms of intra-observer agreement, the mean difference and 95 % limits of agreement were also small for both observers. The within-subject coefficient of variation, a measure of the error relative to parameter size, ranged from 5.5 to 7.1 % for BF, 7.2 to 8.2 % for BV and 8.7 to 16.8 % for FE. Although observer 2 demonstrated a higher coefficient of variation (16.8 %) for FE, this was found to be due to a single outlier who had a small infiltrative tumour. Moreover, this figure remains acceptable within the range quoted by current literature (15–30 %) for perfusion CT [56, 57]. Furthermore, the intra-class correlation coefficients for both observers were largely comparable.
There were limitations in this study. Patient numbers were small and therefore, the results from this study should be interpreted with caution, warranting future confirmation in larger studies. For the same reason, it was not possible to perform a multivariate analysis. Not all patients underwent surgery. Two patients did not undergo surgery at the completion of their NAC and were subsequently deemed to not have had achieved a pCR. However, it was felt that this was acceptable as their final clinical response was incompatible with a pCR. Although some patients received anti-angiogenic therapy with bevacizumab, only one single dose was given and proportionally this was not significant between responders and non-responders. Notwithstanding, this study was exploratory in nature and hypothesis generating. Future studies investigating the relationship between changes in perfusion and permeability with treatment on repeat CT imaging and response would be worthwhile.
In summary, perfusion CT has the potential to identify patients with primary breast cancer who may achieve a pCR to NAC, with those exhibiting elevated levels of vascular permeability and perfusion before treatment most likely to do so. However larger studies are required to confirm these findings.
References
Fisher B, Bryant J, Wolmark N et al (1998) Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J Clin Oncol 16:2672–2685
Sledge GW Jr (2002) Vascular endothelial growth factor in breast cancer: biologic and therapeutic aspects. Semin Oncol 29:104–110
Jeswani T, Padhani AR (2005) Imaging tumour angiogenesis. Cancer Imaging 5:131–138
Kambadakone AR, Sahani DV (2009) Body perfusion CT: technique, clinical applications, and advances. Radiol Clin North Am 47:161–178
Miles KA, Williams RE (2008) Warburg revisited: imaging tumour blood flow and metabolism. Cancer Imaging 8:81–86
Miles KA (2003) Perfusion CT for the assessment of tumour vascularity: which protocol? Br J Radiol 76:S36–S42
Park CM, Goo JM, Lee HJ et al (2009) FN13762 murine breast cancer: region-by-region correlation of first-pass perfusion CT indexes with histologic vascular parameters. Radiology 251:721–730
Tateishi U, Kusumoto M, Nishihara H et al (2002) Contrast-enhanced dynamic computed tomography for the evaluation of tumor angiogenesis in patients with lung carcinoma. Cancer 95:835–842
Li Y, Yang ZG, Chen TW et al (2008) Peripheral lung carcinoma: correlation of angiogenesis and first-pass perfusion parameters of 64-detector row CT. Lung Cancer 61:44–53
Ma SH, Le HB, Jia BH et al (2008) Peripheral pulmonary nodules: relationship between multi-slice spiral CT perfusion imaging and tumor angiogenesis and VEGF expression. BMC Cancer 8:186
Goh V, Halligan S, Daley F et al (2008) Colorectal tumor vascularity: quantitative assessment with multidetector CT—do tumor perfusion measurements reflect angiogenesis? Radiology 249:510–517
d'Assignies G, Couvelard A, Bahrami S et al (2009) Pancreatic endocrine tumors: tumor blood flow assessed with perfusion CT reflects angiogenesis and correlates with prognostic factors. Radiology 250:407–416
Wang JH, Min PQ, Wang PJ et al (2006) Dynamic CT evaluation of tumor vascularity in renal cell carcinoma. AJR Am J Roentgenol 186:1423–1430
Jones RL, Lakhani SR, Ring AE et al (2006) Pathological complete response and residual DCIS following neoadjuvant chemotherapy for breast carcinoma. Br J Cancer 94:358–362
Haberland U, Klotz E, Abolmaali N (2010) Performance assessment of dynamic spiral scan modes with variable pitch for quantitative perfusion computed tomography. Invest Radiol 45:378–386
Saddi KA, Chefd'hotel C, Cheriet F (2007) Large deformation registration of contrast-enhanced images with volume-preserving constraint. In: Pluim JPW, Reinhardt JM (eds) Medical imaging 2007: image processing. Proc SPIE, vol 6512. The International Society for Optical Engineering (SPIE), Bellingham
Goetti R, Leschka S, Desbiolles L et al (2010) Quantitative computed tomography liver perfusion imaging using dynamic spiral scanning with variable pitch: feasibility and initial results in patients with cancer metastases. Invest Radiol 45:419–426
Reiner CS, Goetti R, Eberli D et al (2012) CT perfusion of renal cell carcinoma: impact of volume coverage on quantitative analysis. Invest Radiol 47:33–40
Bland JM, Altman DG (1986) Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1:307–310
Mukherji SK, Castelijns JA (2010) CT perfusion of head and neck cancer: why we should care versus why should we care! AJNR Am J Neuroradiol 31:391–393
Gandhi D, Chepeha DB, Miller T et al (2006) Correlation between initial and early follow-up CT perfusion parameters with endoscopic tumor response in patients with advanced squamous cell carcinomas of the oropharynx treated with organ-preservation therapy. AJNR Am J Neuroradiol 27:101–106
Petralia G, Preda L, Giugliano G et al (2009) Perfusion computed tomography for monitoring induction chemotherapy in patients with squamous cell carcinoma of the upper aerodigestive tract: correlation between changes in tumor perfusion and tumor volume. J Comput Assist Tomogr 33:552–559
Bisdas S, Rumboldt Z, Surlan-Popovic K et al (2010) Perfusion CT in squamous cell carcinoma of the upper aerodigestive tract: long-term predictive value of baseline perfusion CT measurements. AJNR Am J Neuroradiol 31:576–581
Hermans R, Meijerink M, Van den Bogaert W et al (2003) Tumor perfusion rate determined noninvasively by dynamic computed tomography predicts outcome in head-and-neck cancer after radiotherapy. Int J Radiat Oncol Biol Phys 57:1351–1356
Zima A, Carlos R, Gandhi D et al (2007) Can pretreatment CT perfusion predict response of advanced squamous cell carcinoma of the upper aerodigestive tract treated with induction chemotherapy? AJNR Am J Neuroradiol 28:328–334
Wang J, Wu N, Cham MD, Song Y (2009) Tumor response in patients with advanced non-small cell lung cancer: perfusion CT evaluation of chemotherapy and radiation therapy. AJR Am J Roentgenol 193:1090–1096
Tacelli N, Remy-Jardin M, Copin MC et al (2010) Assessment of non-small cell lung cancer perfusion: pathologic-CT correlation in 15 patients. Radiology 257:863–871
Ng QS, Goh V, Fichte H et al (2006) Lung cancer perfusion at multi-detector row CT: reproducibility of whole tumor quantitative measurements. Radiology 239:547–553
Lind JS, Meijerink MR, Dingemans AM et al (2010) Dynamic contrast-enhanced CT in patients treated with sorafenib and erlotinib for non-small cell lung cancer: a new method of monitoring treatment? Eur Radiol 20:2890–2898
Ng QS, Goh V, Carnell D et al (2007) Tumor antivascular effects of radiotherapy combined with combretastatin a4 phosphate in human non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 67:1375–1380
Goh V, Halligan S, Gharpuray A et al (2008) Quantitative assessment of colorectal cancer tumor vascular parameters by using perfusion CT: influence of tumor region of interest. Radiology 247:726–732
Goh V, Halligan S, Gartner L et al (2006) Quantitative colorectal cancer perfusion measurement by multidetector-row CT: does greater tumour coverage improve measurement reproducibility? Br J Radiol 79:578–583
Sahani DV, Kalva SP, Hamberg LM et al (2005) Assessing tumor perfusion and treatment response in rectal cancer with multisection CT: initial observations. Radiology 234:785–792
Koukourakis MI, Mavanis I, Kouklakis G et al (2007) Early antivascular effects of bevacizumab anti-VEGF monoclonal antibody on colorectal carcinomas assessed with functional CT imaging. Am J Clin Oncol 30:315–318
Bellomi M, Petralia G, Sonzogni A et al (2007) CT perfusion for the monitoring of neoadjuvant chemotherapy and radiation therapy in rectal carcinoma: initial experience. Radiology 244:486–493
Goh V, Halligan S, Wellsted DM, Bartram CI (2009) Can perfusion CT assessment of primary colorectal adenocarcinoma blood flow at staging predict for subsequent metastatic disease? A pilot study. Eur Radiol 19:79–89
Park MS, Klotz E, Kim MJ et al (2009) Perfusion CT: noninvasive surrogate marker for stratification of pancreatic cancer response to concurrent chemo- and radiation therapy. Radiology 250:110–117
Makari Y, Yasuda T, Doki Y et al (2007) Correlation between tumor blood flow assessed by perfusion CT and effect of neoadjuvant therapy in advanced esophageal cancers. J Surg Oncol 96:220–229
Ng CS, Wang X, Faria SC et al (2010) Perfusion CT in patients with metastatic renal cell carcinoma treated with interferon. AJR Am J Roentgenol 194:166–171
Fournier LS, Oudard S, Thiam R et al (2010) Metastatic renal carcinoma: evaluation of antiangiogenic therapy with dynamic contrast-enhanced CT. Radiology 256:511–518
Jinzaki M, Tanimoto A, Mukai M et al (2000) Double-phase helical CT of small renal parenchymal neoplasms: correlation with pathologic findings and tumor angiogenesis. J Comput Assist Tomogr 24:835–842
Hirasawa H, Tsushima Y, Hirasawa S et al (2007) Perfusion CT of breast carcinoma: arterial perfusion of nonscirrhous carcinoma was higher than that of scirrhous carcinoma. Acad Radiol 14:547–552
Willett CG, Boucher Y, di Tomaso E et al (2004) Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer. Nat Med 10:145–147
Xiong HQ, Herbst R, Faria SC et al (2004) A phase I surrogate endpoint study of SU6668 in patients with solid tumors. Invest New Drugs 22:459–466
Kerbel RS, Klement G, Pritchard KI, Kamen B (2002) Continuous low-dose anti-angiogenic/metronomic chemotherapy: from the research laboratory into the oncology clinic. Ann Oncol 13:12–15
Fisher ER, Wang J, Bryant J et al (2002) Pathobiology of preoperative chemotherapy: findings from the National Surgical Adjuvant Breast and Bowel (NSABP) protocol B-18. Cancer 95:681–695
Wang J, Buchholz TA, Middleton LP et al (2002) Assessment of histologic features and expression of biomarkers in predicting pathologic response to anthracycline-based neoadjuvant chemotherapy in patients with breast carcinoma. Cancer 94:3107–3114
Davies MM, Burke D, Carnochan P et al (2002) Basic fibroblast growth factor infusion increases tumour vascularity, blood flow and chemotherapy uptake. Acta Oncol 41:84–90
Smith IC, Welch AE, Hutcheon AW et al (2000) Positron emission tomography using [(18)F]-fluorodeoxy-D-glucose to predict the pathologic response of breast cancer to primary chemotherapy. J Clin Oncol 18:1676–1688
Park SH, Moon WK, Cho N et al (2010) Diffusion-weighted MR imaging: pretreatment prediction of response to neoadjuvant chemotherapy in patients with breast cancer. Radiology 257:56–63
Mankoff DA, Dunnwald LK, Gralow JR et al (2002) Blood flow and metabolism in locally advanced breast cancer: relationship to response to therapy. J Nucl Med 43:500–509
Ah-See ML, Makris A, Taylor NJ et al (2008) Early changes in functional dynamic magnetic resonance imaging predict for pathologic response to neoadjuvant chemotherapy in primary breast cancer. Clin Cancer Res 14:6580–6589
Martincich L, Montemurro F, De Rosa G et al (2004) Monitoring response to primary chemotherapy in breast cancer using dynamic contrast-enhanced magnetic resonance imaging. Breast Cancer Res Treat 83:67–76
Ng QS, Goh V, Klotz E et al (2006) Quantitative assessment of lung cancer perfusion using MDCT: does measurement reproducibility improve with greater tumor volume coverage? AJR Am J Roentgenol 187:1079–1084
Kalender WA, Beister M, Boone JM et al (2012) High-resolution spiral CT of the breast at very low dose: concept and feasibility considerations. Eur Radiol 22:1–8
Miles KA, Griffiths MR (2003) Perfusion CT: a worthwhile enhancement? Br J Radiol 76:220–231
Goh V, Halligan S, Hugill JA, Bartram CI (2006) Quantitative assessment of tissue perfusion using MDCT: comparison of colorectal cancer and skeletal muscle measurement reproducibility. AJR Am J Roentgenol 187:164–169
Acknowledgements
Kings College London receives funding from the National Institute for Health Research (NIHR) as a Comprehensive Biomedical Research Centre.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, S.P., Makris, A., Gogbashian, A. et al. Predicting response to neoadjuvant chemotherapy in primary breast cancer using volumetric helical perfusion computed tomography: a preliminary study. Eur Radiol 22, 1871–1880 (2012). https://doi.org/10.1007/s00330-012-2433-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-012-2433-2