Abstract
Objective
To evaluate the diagnostic accuracy of contrast-enhanced MR angiography (CE-MRA) and the added benefit of unenhanced proton MR angiography compared with CT pulmonary angiography (CTPA) in patients with chronic thromboembolic disease (CTE).
Methods
A 2 year retrospective study of 53 patients with chronic thromboembolic pulmonary hypertension who underwent CTPA and MRI for suspected pulmonary hypertension and a control group of 36 patients with no CT evidence of pulmonary embolism. The MRI was evaluated for CTE and the combined diagnostic accuracy of ce-MRA and unenhanced proton MRA was determined. CE-MRA generated lung perfusion maps were also assessed.
Results
The overall sensitivity and specificity of CE-MRA in diagnosing proximal and distal CTE were 98% and 94%, respectively. The sensitivity improved from 50% to 88% for central vessel disease when CE-MRA images were analysed with unenhanced proton MRA. The CE-MRA identified more stenoses (29/18), post-stenosis dilatation (23/7) and occlusions (37/29) compared with CTPA. The CE-MRA perfusion images showed a sensitivity of 92% for diagnosing CTE.
Conclusion
CE-MRA has high sensitivity and specificity for diagnosing CTE. The sensitivity of CE-MRA for visualisation of adherent central and lobar thrombus significantly improves with the addition of unenhanced proton MRA which delineates the vessel wall.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chronic thromboembolic pulmonary hypertension (CTEPH) is a serious complication of thromboembolic disease. It is one of the leading causes of severe pulmonary hypertension (PH) and is associated with considerable morbidity and mortality [1]. The natural history of CTEPH is not fully understood, but it is related to intraluminal thrombus organisation leading to remodelling of the pulmonary arteries [2]. This subsequently increases pulmonary vascular resistance (PVR) resulting in pulmonary hypertension and progressive right heart failure. It is estimated that 3.8% of patients suffering a symptomatic acute pulmonary embolism (PE) will develop CTEPH at 2 years [3]. Even among those who receive appropriate treatment during their acute episode, the thrombus may not resolve completely, resulting in CTEPH [4–6].
The diagnosis of chronic thromboembolic disease (CTE) is made principally through imaging. Traditionally, invasive pulmonary angiography is considered to be the definitive investigation for the diagnosis of CTE, especially to evaluate the disease extent before surgical planning [7, 8]. With the advent of multidetector-row CT, CT pulmonary angiography (CTPA) has replaced pulmonary angiography [9–11]. In the last decade, MRI techniques such as pulmonary MR angiography (MRA), lung perfusion imaging, assessment of right ventricular haemodynamics and hyperpolarised noble-gas imaging have proved to be promising for the evaluation of patients with CTEPH [12, 13, 26].
The aim of our study was to evaluate the diagnostic accuracy of contrast-enhanced MR angiography (CE-MRA) and also to assess the added benefit of unenhanced proton MRA using a 2D balanced Steady State Free Precession (bSSFP) sequence compared with the gold standard, CTPA, in patients with suspected proximal and distal chronic thromboembolic disease. To our knowledge, there have been no published studies analysing the added utility of this unenhanced proton MRA in demonstrating chronic thromboembolism.
Methods
All patients referred to our institute for the evaluation of CTEPH in the period from January 2008 to March 2010 were considered for this study. The study patients belonged to the following two groups: 53 patients with CTEPH and 36 patients with normal pulmonary artery pressure (mPAP < 25) on right heart catheterisation and no CT evidence of embolic disease. All patients included in this study underwent CT and MRI within a time interval of 48 h. The study had local research ethics committee approval.
Magnetic resonance imaging was performed at 1.5 Tesla (HDx system, GE Healthcare, Milwaukee, WI, USA).
Contrast-enhanced perfusion images were acquired using a time-resolved 3D spoiled gradient echo sequence with view sharing (TRICKS sequence) [Korosec, 1996 #339]. The sequence parameters were: coronal orientation, TE 1.1 ms, TR 2.5 ms, flip angle of 30°, FOV = 48 cm2, 2x Asset, 125 kHz bandwidth, slice thickness of 5 mm, average of 32 slices and frame rate was two 3D volumes per second. Acquisition times for the complete perfusion angiogram sequence were on average 30 s. This sequence was acquired during breathhold after intravenous administration of 0.05 mL/kg of Gadovist (Schering, Berlin) at 5 mL/s, which was followed by a 20-mL saline flush. MR perfusion images were created by subtracting one contrast-enhanced image from its corresponding unenhanced image on a MR workstation.
The high-resolution CE-MRA was preceded by a bolus of contrast agent which was used for timing purpose. Approximately 15 mL of contrast agent was used for CE-MRA ensuring the total dose of contrast does not exceed 0.3 mL/kg. High-resolution CE-MRA was performed with following parameters: 3D Coronal Spoiled Gradient Echo, TE 1.0 ms, TR 2.8 ms, flip angle of 30°, FOV = 48 cm2, 2 × Asset, 300 × 200 Matrix, 125 kHz bandwidth, slice thickness of 3 mm and average of 60 slices. Both these breath-hold sequences were acquired during inspiration.
Unenhanced proton MRA was performed as a stack of coronal 2D SSFP images, with the following parameters: TR 2.8 ms, TE 1.0 ms, flip angle of 50°, FOV = 48 cm × 43.2 cm, 256 × 256 matrix, 125 kHz bandwidth and slice thickness of 10 mm. This sequence was performed at full inspiration before the CE-MRA with a total breath-hold time of 12 s.
The CTPA was acquired on 64-slice MDCT (Light-Speed General Electric Medical Systems) during a single breath-hold and standard acquisition parameters were used: 100 mA with automated dose reduction, 120 kV, 1 pitch, rotation time 0.5 s and 0.625-mm collimation. The field of view was 400 × 400 mm with an acquisition matrix of 512 × 512. 100 mL of intravenous contrast agent (Ultravist 300; Bayer Schering, Berlin, Germany) was administered at a rate of 5 mL/s.
The CTPA was interpreted by two radiologists (C.H. and C.D.) and was evaluated for evidence of thromboembolic disease. The volumetric MRA dataset and maximum intensity projection (MIP) images were reviewed on a standard GE workstation by two independent radiologists (S.R. and A.S.) blinded to the CT findings. In cases of discrepancy between observers, a consensus read was performed jointly by the two observers and this was used in all further analysis. The image quality was assessed subjectively and graded for artefacts as “none”, “mild”, “moderate” or “non-diagnostic”.
The CE-MRA images were examined for the presence of thromboembolic disease at central, lobar, segmental and sub-segmental vessels. The presence or absence of signs of chronic thromboembolism in the pulmonary artery such as complete or partial obstruction, adherent thrombus, bands, webs and post-stenotic dilatation was also studied. The unenhanced proton MRA was similarly evaluated for features of chronic thromboembolism and the combined diagnostic accuracy of CE-MRA and unenhanced proton MRA was assessed.
Finally, the CE-MR perfusion images were qualitatively evaluated for the presence or absence of segmental perfusion defects. Statistical analyses were performed with SPSS software (Chicago, IL, USA) and a p value ≤ 0.05 was considered to indicate a statistically significant difference. Direct comparison was made between MR and CTPA, using CTPA as the reference method. A Chi-squared test was used to establish sensitivity, specificity, positive and negative predictive values for detecting the presence and absence of PE. The Kappa statistic was used to determine the level of agreement between the independent observers. In cases of discrepancy between observers regarding abnormality detection, a final interpretation was obtained by consensus during a second session.
Results
One hundred and six patients underwent MRI and CT for suspected CTEPH. There were 63 patients with CTEPH and 43 patients with no evidence of pulmonary hypertension or pulmonary embolism and for the purposes of this study were considered ‘normals’. The mean age of the patients was 61 years and the female to male ratio was 1.2. In nine patients the renal function was impaired hence a CE-MRA was not performed. Based on qualitative visual assessment of image resolution, clarity and breathing artefact, 33 MRI examinations were deemed to have mild artefacts, 17 moderate artefacts and eight were considered to be non-diagnostic. Eighty-nine MRI were of diagnostic quality (53 patients with CTEPH and 36 ‘normal’ patients).
Of the 53 patients with CTEPH, 31 patients had proximal disease (involving central and lobar vessels) and 22 had distal disease (distal to lobar vessels). The overall sensitivity and specificity of CE-MRA in diagnosing CTE were 98% and 94% respectively with a positive predictive value of 96% and negative predictive value of 97% (Table 1). One patient with isolated distal CTEPH was missed on CE-MRA and two patients were misdiagnosed to have CTEPH on CE-MRA.
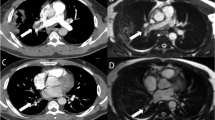
The pulmonary vasculature was analysed for CTE at the central, lobar, segmental and sub-segmental levels and the ability of CE-MRA to identify disease at each of these levels is summarised in Table 2 (Fig. 1). Our results showed that the sensitivity for recognising lobar and segmental disease was 74% and 81%, respectively. The sensitivity of CE-MRA for appreciating central disease was comparatively low at 50%, the reason being the poor contrast of smooth thromboembolic material adherent to the pulmonary vessel wall on CE-MRA images. With the addition of the unenhanced MRA sequence, which depicts the vessel wall better, the sensitivity improved significantly from 50% to 88% (Fig. 2). However we also found that using unenhanced proton MRA in isolation resulted in poor sensitivity (45%) and high false-positive rates for identifying proximal disease as shown in Table 3. 12 of the unenhanced MRA were non-diagnostic.
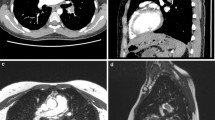
On assessing the various patterns of CTE disease appreciated on CE-MRA and CTPA, CE-MRA helps to recognise more stenosis (29 vs. 18), post-stenosis dilatation (23 vs. 7) and complete vessel obstruction (37 vs. 29) compared with CTPA (Fig. 3). CTPA was superior to CE-MRA in identifying patients who had pulmonary wall adherent thromboembolic material and intra-luminal webs and bands (Table 4). An example is shown in Fig. 4.
Visual evaluation of CE-MRA-generated perfusion images showed a high sensitivity (92%) but a lower specificity (75%) for the detection of CTEPH based on the presence of segmental perfusion defects (Fig. 5). The positive predictive value was 84% and the negative predictive value was 87%. Table 5 shows the overall accuracy of each MR sequence in the diagnosis of CTE.
Discussion
We have demonstrated that CE-MRA has very high sensitivity and specificity in diagnosing the presence or absence of chronic thromboembolic disease in a population of patients with and without CTEPH undergoing evaluation for suspected PH. We have also shown that the addition of unenhanced proton MR improves the sensitivity of MR for the detection of proximal clot and that CE-MRA is superior in representing stenosis and post-stenotic dilatations compared with CTPA. This is consistent with an increasing role for MRI in the assessment of patients with suspected thromboembolic disease.
Thromboembolic occlusion of the pulmonary arteries due to unresolved pulmonary embolism is increasingly recognised as a common cause of PH [1, 3] for which surgery in selected cases can provide a cure.
Traditionally imaging techniques such as nuclear medicine ventilation-perfusion (V/Q) scintigraphy, pulmonary angiography and more recently CTPA have been used in the diagnostic work-up of patients with suspected CTEPH who are considered to be potential surgical candidates. V/Q scintigraphy has a very high negative predictive value and a normal V/Q scintigram practically rules out the presence of CTEPH [14]. However it is known to grossly underestimate the degree of large vessel obstruction and plays a very limited role in the assessment of the extent of disease [15]. Pulmonary angiography is still considered by many to be the definitive investigation for assessment of surgically treatable CTEPH. The main disadvantage of this technique is that the acquisition of high-quality images and the interpretation of angiograms can be challenging. In addition, with the advent of CTPA for the evaluation of acute PTE the number of experienced operators able to provide a high-quality diagnostic service is rapidly diminishing and becoming increasingly confined to specialist pulmonary vascular centres. The invasive nature of this investigation must also be considered as it can cause discomfort and comes with a mortality risk, albeit small [16]. Patients with CTEPH will often require repeated studies to confirm the diagnosis, to assess the course of the disease and to monitor outcome and hence an alternative radiation-free imaging technique would be ideal.
Contrast-enhanced MRA is increasingly recognised as a valuable technique for imaging the pulmonary vasculature in patients with suspected CTEPH [19, 26]. With the application of faster gradients and parallel imaging techniques, the duration of the breath-holding for the MRA sequences are significantly reduced and can rapidly characterise the pulmonary vasculature even in a symptomatic patient (breath-hold <20 s). MRI perfusion imaging can provide information about the presence of perfusion defects at higher spatial resolution than perfusion scintigraphy. These functional haemodynamic-sensitive MR techniques have the potential to quantify the pulmonary blood flow and regional pulmonary vascular resistance and predict the surgical outcome [20, 21]. The presence of dilated bronchial arteries has been shown to correlate with a lower mortality rate after pulmonary thrombo-endarterectomy and CE-MRA has been shown to accurately estimate the flow in the bronchial arteries in patients with CTEPH [22]. An MR technique that can demonstrate pulmonary vasculature without the use of contrast agent would be very useful and bSSFP is particularly suitable as it offers good contrast from the blood pool because of its inherent long T2 compared with tissue. Studies using this sequence in the imaging of coronary arteries have shown promising results [23, 24]. One of the main difficulties of 3D SSFP coverage is the prolonged breath-hold time which can potentially be overcome by using a free-breathing navigator-gated technique [25].
In our study, CE-MRA had overall high sensitivity and specificity in identifying the presence or absence of proximal and distal chronic thromboembolic disease compared with CTPA and importantly images of a quality sufficient to make a confident diagnosis were made in 92% of cases. The various patterns of thromboembolic diseases were also well depicted on CE-MRA. The main problem arose in viewing adherent thrombotic material that was flushed with the vessel wall. One of the reasons for the insensitivity of CE-MRA in identifying central disease was that the vessel wall is not visualised in axial image data or the MIP images. This makes it difficult to appreciate the wall-adherent changes on CE-MRA in some patients. However, with the addition of a simple unenhanced MRA sequence the vessel wall is clearly delineated, which significantly improved the identification of the central and lobar adherent thrombotic disease in our study. In a 2D-bSSFP image, the thrombus is demonstrated as an area of very low signal intensity compared with hyperintensity surrounding flowing blood. As the vessel wall is clearly visualised with this sequence, any wall-adherent thromboembolic material is readily recognised.
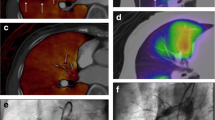
Our study showed that there were false-positive results when unenhanced MRA images were used in isolation. The high false-positive rate was due to the relatively low spatial resolution of the bSSFP sequence (Fig. 6), but this could be improved easily by increasing the data acquisition time or performing repeat imaging at higher resolution on selected slices of interest (Fig. 7). Hence our recommendation is to use unenhanced MRA images as a routine adjunct to CE-MRA especially when assessing for proximal disease. Previous studies in patients with CTEPH have shown misdiagnosis of central wall-adherent thromboembolic material with both pulmonary angiography and CT and this was thought to be due to multiple factors [17, 18]. In chronic CTE, the residual thrombus is incorporated into the vessel wall and is covered by a new epithelial layer smoothing the intimal surface [17]. In CE-MRA, as in DSA and CTPA, the vessel lumen is filled with contrast medium and, in the absence of a wall irregularity, adherent thrombus may easily be missed.
In conclusion, CE-MRA has very high sensitivity and specificity in identifying patients with proximal and distal CTEPH. One of the limitations of CE-MRA, as with DSA, is the inability to accurately identify wall-adherent thromboembolic material. This can be overcome to an extent with the use of an unenhanced bSSFP sequence. With the added benefit of the functional and quantitative information, MRI can provide a holistic picture of the extent and severity of the disease and provides valuable information that complements CTPA in the assessment of patients with suspected surgically accessible CTEPH. In addition the non-ionising nature of this investigation makes it attractive in the initial evaluation of patients with persisting breathlessness following acute PTE and in the follow-up of patients with CTEPH.
References
Riedel M, Stanek V, Widimsky J, Prerovsky I (1982) Longterm follow-up of patients with pulmonary thromboembolism. Late prognosis and evolution of hemodynamic and respiratory data. Chest 81:151–158
Moser KM, Bloor CM (1993) Pulmonary vascular lesions occurring in patients with chronic major vessel thromboembolic pulmonary hypertension. Chest 103:685–692
Pengo V, Lensing AW, Prins MH et al (2004) Incidence of chronic thromboembolic pulmonary hypertension after pulmonary embolism. N Engl J Med 350:2257–2264
Wartski M, Collignon MA (2000) Incomplete recovery of lung perfusion after 3 months in patients with acute pulmonary embolism treated with antithrombotic agents. THESEE Study Group. Tinzaparin ou Heparin Standard: Evaluation dans l’Embolie Pulmonaire Study. J Nucl Med 41:1043–1048
Remy-Jardin M, Louvegny S, Remy J et al (1997) Acute central thromboembolic disease: posttherapeutic follow-up with spiral CT angiography. Radiology 203:173–180
Ribeiro A, Lindmarker P, Johnsson H, Juhlin-Dannfelt A, Jorfeldt L (1999) Pulmonary embolism: one-year follow-up with echocardiography doppler and five-year survival analysis. Circulation 99:1325–1330
Daily PO, Johnston GG, Simmons CJ, Moser KM (1980) Surgical management of chronic pulmonary embolism: surgical treatment and late results. J Thorac Cardiovasc Surg 79:523–531
Jamieson SW, Auger WR, Fedullo PF et al (1993) Experience and results with 150 pulmonary thromboendarterectomy operations over a 29-month period. J Thorac Cardiovasc Surg 106:116–126, discussion 126–117
Kauczor HU, Schwickert HC, Mayer E, Schweden F, Schild HH, Thelen M (1994) Spiral CT of bronchial arteries in chronic thromboembolism. J Comput Assist Tomogr 18:855–861
Wittram C, Kalra MK, Maher MM, Greenfield A, McLoud TC, Shepard JA (2006) Acute and chronic pulmonary emboli: angiography-CT correlation. AJR Am J Roentgenol 186:S421–S429
Remy-Jardin M, Remy J, Wattinne L, Giraud F (1992) Central pulmonary thromboembolism: diagnosis with spiral volumetric CT with the single-breath-hold technique–comparison with pulmonary angiography. Radiology 185:381–387
Kreitner KF, Kunz RP, Ley S et al (2007) Chronic thromboembolic pulmonary hypertension—assessment by magnetic resonance imaging. Eur Radiol 17:11–21
Kreitner KF, Ley S, Kauczor HU et al (2004) Chronic thromboembolic pulmonary hypertension: pre- and postoperative assessment with breath-hold MR imaging techniques. Radiology 232:535–543
Lisbona R, Kreisman H, Novales-Diaz J, Derbekyan V (1985) Perfusion lung scanning: differentiation of primary from thromboembolic pulmonary hypertension. AJR Am J Roentgenol 144:27–30
Ryan KL, Fedullo PF, Davis GB, Vasquez TE, Moser KM (1988) Perfusion scan findings understate the severity of angiographic and hemodynamic compromise in chronic thromboembolic pulmonary hypertension. Chest 93:1180–1185
Stein PD, Athanasoulis C, Alavi A et al (1992) Complications and validity of pulmonary angiography in acute pulmonary embolism. Circulation 85:462–468
Bergin CJ, Sirlin CB, Hauschildt JP et al (1997) Chronic thromboembolism: diagnosis with helical CT and MR imaging with angiographic and surgical correlation. Radiology 204:695–702
Schwickert HC, Schweden F, Schild HH et al (1994) Pulmonary arteries and lung parenchyma in chronic pulmonary embolism: preoperative and postoperative CT findings. Radiology 191:351–357
Ley S, Grunig E, Kiely DG, van Beek E, Wild J (2010) Computed tomography and magnetic resonance imaging of pulmonary hypertension: pulmonary vessels and right ventricle. J Magn Reson Imaging 32:1313–1324
Sergiacomi G, Bolacchi F, Cadioli M et al (2010) Combined pulmonary fibrosis and emphysema: 3D time-resolved MR angiographic evaluation of pulmonary arterial mean transit time and time to peak enhancement. Radiology 254:601–608
Ohno Y, Hatabu H, Murase K et al (2007) Primary pulmonary hypertension: 3D dynamic perfusion MRI for quantitative analysis of regional pulmonary perfusion. AJR Am J Roentgenol 188:48–56
Ley S, Kreitner KF, Morgenstern I, Thelen M, Kauczor HU (2002) Bronchopulmonary shunts in patients with chronic thromboembolic pulmonary hypertension: evaluation with helical CT and MR imaging. AJR Am J Roentgenol 179:1209–1215
Jahnke C, Paetsch I, Schnackenburg B et al (2004) Coronary MR angiography with steady-state free precession: individually adapted breath-hold technique versus free-breathing technique. Radiology 232:669–676
Deshpande VS, Shea SM, Laub G, Simonetti OP, Finn JP, Li D (2001) 3D magnetization-prepared true-FISP: a new technique for imaging coronary arteries. Magn Reson Med 46:494–502
Hui BK, Noga ML, Gan KD, Wilman AH (2005) Navigator-gated three-dimensional MR angiography of the pulmonary arteries using steady-state free precession. J Magn Reson Imaging 21:831–835
Ley S, Kauczor HU, Heussel CP et al (2003) Value of contrast-enhanced MR angiography and helical CT angiography in chronic thromboembolic pulmonary hypertension. Eur Radiol 13:2365–2371
Acknowledgements
The authors S.R. and D.C. are funded by Pfizer and Bayer respectively through unrestricted research grants. The UK EPSRC (J.M.W.) and the Sheffield Cardio Vascular Biomedical Research Unit (A.J.S.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rajaram, S., Swift, A.J., Capener, D. et al. Diagnostic accuracy of contrast-enhanced MR angiography and unenhanced proton MR imaging compared with CT pulmonary angiography in chronic thromboembolic pulmonary hypertension. Eur Radiol 22, 310–317 (2012). https://doi.org/10.1007/s00330-011-2252-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-011-2252-x