Abstract
Objective
To determine the diagnostic value of contrast-enhanced ultrasound (CEUS) in the assessment of acute pancreatitis, with computed tomography (CT) as the reference standard.
Methods
Fifty consecutive patients (mean age 58.4 years; range 23–86 years) with acute pancreatitis underwent prospectively both CT and ultrasonography, including CEUS, within a 24-h interval. Pancreatic vascularisation was evaluated with CEUS after injection of a second-generation US contrast-enhancing agent. Acute pancreatitis severity was graded according to the Balthazar index. The results were compared with CT severity index and clinical outcome by using Spearman’s correlation coefficient.
Results
A significant correlation between CT and CEUS was found for the CT severity index (r = 0.926), extent of necrosis (r = 0.893) and Balthazar grade (r = 0.884). The sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) for detecting severe acute pancreatitis based on CT findings (severity index greater than 3 and/or presence of necrosis) were respectively 91%, 100%, 100% and 83%. A significant correlation between CEUS severity index and clinical variables was found: Ranson score (r = 0.442), C-reactive protein (CRP) levels 48 h after admission (r = 0.385) and length of hospital stay (r = 0.362).
Conclusion
CEUS is comparable to CT in detecting pancreatic necrosis as well as predicting its clinical course. Therefore, when CT is contraindicated CEUS may be a valid alternative.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Acute pancreatitis is an acute inflammatory process of the pancreas with a clinical course that varies from mild to severe. Mild pancreatitis is a self-limiting disease with low morbidity and mortality, while severe pancreatitis, also referred to as necrotising pancreatitis, is a potentially life-threatening disease in 10–25% of patients [1–3]. The detection of severe cases is important, because it can provide prognostic information and it may have therapeutic implications [1–3]. Early treatment of severe cases of necrosis can reduce morbidity and mortality.
At present computed tomography (CT) is considered the reference standard for diagnosis and staging of acute pancreatitis [4]. CT allows the detection of pancreatic necrosis (PN) and fluid collections, and in previous reports imaging parameters correlated with the outcome of the disease [5–8]. Others grading systems and laboratory tests have been developed to identify severe acute pancreatitis: the Ranson or Glasgow criteria, the acute physiology and chronic health evaluation II (APACHE II) scoring system, and C-reactive protein levels (CRP) [3, 9]. Other imaging techniques with a safer profile than CT, such as magnetic resonance imaging (MRI) and scintigraphy, have also been used to detect severe cases [8, 10–13]. It has been reported that these imaging techniques are as accurate as CT in diagnosing pancreatic necrosis and staging acute pancreatitis severity, avoiding radiation exposure and iodinated contrast media. However, MRI and scintigraphy are probably less readily available in hospitals and are difficult to apply in intensive care unit (ICU) patients [14].
Recently it has been shown that the use of US contrast agents allows examination of the vascularisation of abdominal organs, such as the liver, kidney or pancreas. Both second-generation US contrast-enhancing agents and low-mechanical index (MI) real-time harmonic US are required to examine the abdominal organs. It is known that the perfusion of the pancreas is well correlated with the enhancement of the gland parenchyma at contrast-enhanced ultrasound (CEUS) [15]. The blood supply of the pancreas is entirely arterial; thus, the enhancement of the gland begins almost together with the aortic enhancement (peak between 12 and 20 s after injection). Afterwards there is a progressive washout of contrast medium with loss of gland echogenicity [15]. A recent report showed that acute pancreatitis may be graded with CEUS with comparable results to contrast-enhanced CT, avoiding the drawbacks of iodinated contrast materials such as nephrotoxicity and idiosyncratic reactions [16]. Those authors proposed that CEUS could be an alternative imaging technique to CT, especially in cases when iodinated contrast medium injection is contraindicated.
The aim of this study was to determine the diagnostic value of CEUS in the assessment of the severity of acute pancreatitis using CT as the reference standard, and to correlate US findings with clinical outcome.
Materials and methods
This prospective study was performed in 54 consecutive patients with acute pancreatitis admitted to our hospital between August 2006 and June 2009. Ethics committee approval and written informed consent of the patients were obtained before CEUS. Inclusion criteria were: diagnosis of acute pancreatitis (patient’s symptoms and elevation of serum levels of amylase and lipase), clinical CT indication (persisting organ failure or new organ failure developing, signs of sepsis, deterioration in clinical status after admission or persisting pain) and patients older than 18 years. Exclusion criteria were: chronic pancreatitis, contraindication to administration of contrast agent (ultrasound or iodinated) or poor visualisation of the pancreas on ultrasound.
Four patients were excluded from the study because of incomplete ultrasound imaging of the pancreas by meteorism (n = 2), contraindication to CT with contrast injection (previous reaction to iodinated contrast medium) (n = 1) and chronic pancreatitis (n = 1).
Imaging techniques
Ultrasound examinations were performed by using a Toshiba Aplio 80 (Toshiba, Tokyo, Japan) initially employing a 3- to 6-MHz convex-array transducer. Two radiologists (MJM, TR) with at least 10 years’ experience in abdominal US and 3 years’ experience in CEUS performed the examinations. The radiologists were unaware of the CT data, but had been informed about the diagnosis of acute pancreatitis. Each patient underwent abdominal US specifically for the pancreas and the peripancreatic tissue, beginning with an initial grey-scale examination to assess the echostructure of the glandule and the presence of abdominal fluid collections, ascites or pleural effusion. To evaluate pancreatic enhancement we selected, before contrast injection, the view at B-mode sonography in which the pancreatic parenchyma was best visualized. Then, patients were examined with a 3- to 4-MHz convex in the wideband contrast harmonic mode (pulse inversion-Toshiba Aplio) at low MI (MI < 0.10). The second-generation echo-signal enhancer SonoVue® (Bracco, Milan, Italy) was injected as a bolus in units of 2.4 ml through a three-way 20-gauge catheter into an antecubital vein, immediately followed by injection of 10 ml of normal saline solution (0.9% NaCl). We had to repeat the bolus injection in some cases in order to evaluate the whole pancreas. The range of total contrast used in each patient was between 2.4 and 7.2 ml, depending on the patient’s characteristics. For each examination a recording was begun a few seconds before the intravenous administration of the contrast agent, and continuous imaging was performed for 60 s. Video sequences were evaluated by consensus of the investigators immediately after the contrast-enhanced examination. The readers were blinded to the CT results.
Because the results of US depend strongly on the observer’s ability and subjectivity, we retrospectively reviewed video sequences to calculate the intra- and interobserver agreement in assessing the grade of pancreatic necrosis.
A venous contrast phase (60 s) using 4-line CT spiral (Somatom Volume Zoom, Siemens, Erlangen, Germany) was performed after intravenous injection of 1.5 ml/kg of contrast media (Ultravist®, Bayer Schering Pharma AG, Berlin, Germany) at a rate of 3 ml/s. CT parameters were a 5-mm slice thickness and 5-mm reconstruction interval.
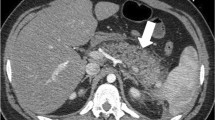
Interpretation and scoring of the CT images were retrospectively analysed by consensus of two experienced abdominal radiologists blinded to the CEUS results. Pancreatic necrosis was defined for both CT and CEUS as the detection of a non-enhanced area of pancreatic parenchyma (Figs. 1 and 2). Acute pancreatitis severity was graded according the Balthazar index [5]. The severity index was calculated with pancreatic inflammation plus pancreatic necrosis scores. A small peripancreatic hypoechoic halo that enhanced after contrast injection was considered in CEUS as peripancreatic inflammatory changes (Balthazar’s grade C), but if the halo was better seen after contrast injection it was evaluated as grade D (a small collection).
A 37-year-old man with necrosis of the tail of the pancreas due to acute pancreatitis. a Contrast-enhanced sonogram 15 s after contrast injection shows enhancement of an enlarged pancreas (small arrows) with absence of vascularisation in the tail of the pancreas, corresponding to necrosis (large arrow). b Corresponding CT reveals the same findings
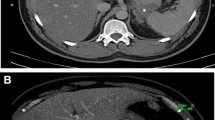
A 71-year-old woman with severe necrotising acute pancreatitis. a Transverse US image reveals decreased echogenicity of the body and tail of the pancreas (large arrows). Minimal peripancreatic fluid collection (*). b Enhanced image 13 s after contrast injection shows no enhancement of the pancreatic body and tail, representing greater than 50% necrosis, with normal enhancement of the pancreatic head. Peripancreatic fluid collection (*) is seen more clearly. c CT obtained the same day confirms pancreatic necrosis in the body and tail of the gland. Note the obese body habitus of the patient
Imaging techniques were performed within 72 h of admission. CEUS and CT were performed within a 24-h interval.
Clinical variables
The following variables were recorded in each patient: aetiology, levels of CRP 48–72 h after admission, Ranson score and the length of the hospital stay.
Statistical analysis
Basic descriptive statistics were obtained including mean, range and standard deviation and absolute frequency and percentage for discrete variables.
The Spearman’s rank correlation coefficient was used to assess the relation between CEUS and CT findings and between CEUS findings and clinical parameters (Ranson score, CRP levels and length of hospital stay). Correlation between the severity of the pancreatitis based on the Ranson criteria and the presence of pancreatic necrosis in CT or CEUS was obtained with the Fisher’s exact test.
Based on CT findings as the gold standard, the sensitivity, specificity, positive predictive value and negative predictive value of CEUS were calculated for detecting both pancreatic necrosis and severe acute pancreatitis (defined as Balthazar severity index greater than 3 and/or presence of necrosis).
The inter- and intraobserver agreement for assessing the pancreatic necrosis was calculated by means of the exact percentage of agreement, along with the kappa statistic, which is used to estimate the proportion of agreement between two or more observers above that expected by chance. A kappa value between 0.41 and 0.60 was considered to indicate fair agreement, between 0.61 and 0.80 good agreement, and between 0.81 and 1.00 excellent agreement.
All data were analysed with Statistical Package for Social Sciences, version 15.0.1 (SPSS Inc., Chicago, IL, USA). For all tests, p values less than 0.05 were considered to indicate a statistically significant difference.
Results
A total of 50 patients were included in the study group (28 men and 22 women; mean age 58.4 years; range 23–86 years). The most frequent aetiology of acute pancreatitis was gallstones in 27 patients, followed by alcohol abuse in 9 patients. The mean Ranson criterion was 3 (range 0–6). The mean C-reactive protein (CRP) value and length of hospitalisation were 245 mg/l (range 16–500) and 21.5 days (range 4–105) respectively.
Table 1 shows the comparative findings of CEUS and CT. Twenty-one patients (42%) had pancreatic necrosis at CT: ten patients in the pancreatic body and tail, five cases in the body, three in the head, two in the tail and another one in the head and the body. All but three cases with necrosis were correctly diagnosed by using CEUS (r = 0.837, p < 0.01). In all of these three cases the grade of necrosis was less than 30%, and it was localised in one case in the head, in another case in the body, and in the last one in the tail (Fig. 3). The sensitivity, specificity, positive predictive value and negative predictive value for detecting pancreatic necrosis were 86%, 97%, 95% and 90% respectively. The comparative analysis of the grade of necrosis was similar in all patients, except in three cases where the grade of necrosis was underestimated by CEUS. In two patients with necrosis localised in the pancreatic body and tail, the CT percentage of necrosis was 30–50% while CEUS showed less than 30% necrosis. In another patient with necrosis located in the pancreatic head and body and considered more than 50% on CT, the estimation of necrosis with CEUS was 30–50% (r = 0.893, p < 0.01).
A 42-year-old man with acute pancreatitis and necrosis missed on CEUS. a Transverse US image of the pancreas 19 s after contrast injection depicts a normal enhancing pancreas (arrows). b Corresponding CT reveals ill-defined unenhanced area in the pancreatic body (arrow). Necrosis CT was graded as less than 30%
A significant correlation between CT and CEUS was found for the CT severity index (r = 0.926, p < 0.01) (Fig. 4). The sensitivity, specificity, positive predictive value and negative predictive value for detecting severe acute pancreatitis (severity index greater than 3 and/or presence of necrosis) were respectively 91% (95% CI 78–97), 100% (95% CI 80–100), 100% (95% CI 89–100) and 83% (95% CI 61–94).
A significant correlation between CT and CEUS inflammation grade was also found (r = 0.884, p < 0.01). CEUS underestimated the inflammation grade in five patients: in two patients a diffuse enlargement of the pancreas was seen on US while CT also showed inflammatory changes in the peripancreatic fat; ultrasound did not demonstrate a small ill-defined fluid collection localised in the left pararenal space in two patients and in the right pararenal space in one.
A significant correlation between CEUS severity index and clinical variables was found: Ranson score (r = 0.442, p < 0.01), CRP levels 48 h after admission (r = 0.385, p < 0.05) and length of hospital stay (r = 0.362, p < 0.05). A significant correlation was also found between the Balthazar CT severity index and clinical variables: Ranson score (r = 0.392, p < 0.05), CRP levels 48 h after admission (r = 0.457, p < 0.01) and length of hospital stay (r = 0.497, p < 0.01).
Four or more positive Ranson signs were considered clinically severe pancreatitis: this was seen in 21 patients. When using the Ranson criteria, no significant differences in the presence of pancreatic necrosis detected on CT or CEUS was seen between mild and severe pancreatitis (p = 0.093; p = 0.094, respectively).
Complete resolution to normal happened in 23 patients (46%), 14 (28%) developed local complications and 13 (26%) had systemic complications with the need for intensive care admission in 9 of them. Six of the 13 patients with systemic complications also showed local complications. The presence of local complications was significantly correlated with high scores on both the CT (r = 0.532; p = 0.002) and the CEUS (r = 0.500; p = 0.004) severity index. However, the severity index score did not correlate significantly with the development of systemic complications (CT, p = 0.105; CEUS, p = 0.149).
Intraobserver agreements in the evaluation of pancreatic necrosis grade ranged from 86% to 93%. The kappa statistics were 0.770 and 0.876, indicating good to excellent agreement. Interobserver agreement on CEUS on the same evaluation ranged from 84% to 91%. The kappa statistic for the observers ranged from 0.719 to 0.845, indicating good to excellent agreement.
Discussion
Ultrasound pancreatic features of acute pancreatitis range from normal findings to focal or diffuse enlargement of the pancreas with a heterogeneous or hypoechoic gland. US can also detect the presence of peripancreatic or pararenal collections, ascites and pleural effusion [8, 14]. However, its role in the first days of this illness is limited because differentiation between necrotic and non-necrotic pancreatitis cannot be made. As a consequence, the current role of ultrasound in the early investigation of acute pancreatitis is the detection of gallstones or common bile duct stones as the cause of the pancreatic inflammation [4, 8, 17].
Since the advent of the use of ultrasound contrast agents, a new imaging approach to evaluating pancreatic vascularisation has been introduced. Our study confirms the value of CEUS in detecting pancreatic necroses and as a predictive indicator of the severity of an episode of acute pancreatitis. In our series a significant correlation between CT and CEUS was found for the CT severity index (r = 0.926), the extent of necrosis (r = 0.893) and Balthazar grade (r = 0.884), with similar results to those published by Rickes and colleagues [16].
For detecting necrosis there was discordance between our results and those of Rickes et al.’s study [16]. In their study, necrosis was detected with CEUS in all cases, while we missed three cases with mild necrosis (86% sensitivity). In our experience, the pancreatic tail is hard to assess because of the interposition of abdominal gas. This fact could explain the false-negative cases. However, it happened in only one of the missed necrosis cases. In the other two patients, necrosis was localised in the head and in the body of the pancreas respectively (the areas of the pancreas easier to evaluate with US in the absence of meteorism). The pancreatic borders are less precise on US than on CT, especially after contrast agent injection; therefore, small areas of necrosis localised in the periphery of the gland can be either missed or misinterpreted as a small peripancreatic collection in CEUS. However, it is known that CT specificity of necrosis falls from 100% to 50% when there are only small areas of no enhancement [18]. Possible causes of these false-positive cases are oedema of intrapancreatic fluid, zones that may prove to be reversible over time [8].
On the other hand, in our series, we had only one false-positive case of pancreatic necrosis. In this case a small collection localised anterior to the pancreatic body was erroneously considered as an area of necrosis in the pancreatic gland on CEUS.
As for the number of collections, CEUS missed three cases, all of them small. In our experience collections are better depicted with the use of CEUS (Fig. 2b) because contrast agent increases the differences in the echogenicity between the pancreatic parenchyma and the collections, which do not enhance.
On the other hand, there was excellent agreement (94%) between CT and CEUS for detecting severe acute pancreatitis (severity index greater than 3 and/or presence of necrosis). This result differs from that of Rickes et al. who found discordance between the two techniques in five patients (15%).
The main limitation of our study is the relatively small sample size; larger studies should be performed in order to determine the actual sensitivity of CEUS in staging acute pancreatitis. Moreover, because the examinations were performed by experts in US and CEUS, the results could be different with trainees or non-expert radiologists.
Ultrasound evaluation of the pancreas is less effective in obese patients; moreover, in the early phase of pancreatitis the presence of meteorism can preclude the visualisation of part or all of the pancreas (this happened in only two cases (5%) in our study, a percentage similar (8%) to that in Rickes’ study). It is worth mentioning that US evaluation for the detection of pancreatic parenchymal necrosis, similar to CT evaluation, should be made 48 h after the onset of symptoms to avoid underestimating the amount of necrosis, when meteorism due to adynamic ileus has decreased in many cases. Larger prospective studies are needed to evaluate whether adequate pancreas visualisation is possible with state-of-the-art machines in cases of acute pancreatitis. Another limitation is that video sequences of CEUS are more difficult to handle than the static images of CT.
Current clinical guidelines establish that CT is the imaging technique of choice for the diagnosis and evaluation of patients with acute pancreatitis, with a high degree of accuracy in detecting pancreatic necrosis and predicting the outcome of patients. Indeed, CT shows complete visualisation of the peripancreatic retroperitoneal region and readily conveys the extent of the abnormalities to the clinician. However, CEUS can be useful where CT is contraindicated, particularly in patients who cannot receive iodinated contrast material because of idiosyncratic reactions or renal insufficiency. Moreover, to avoid extra radiation dose, CEUS can also be used as a follow-up imaging method in patients with an initial CT staging. CEUS has other advantages such as: low cost, availability and above all mobility, allowing the assessment of ICU patients in their own beds.
In conclusion, our results indicate that CEUS is comparable to CT in the assessment of acute pancreatitis severity and therefore it can be an alternative when CT is contraindicated. CEUS can detect pancreatic necrosis and it can predict the clinical course of acute pancreatitis.
References
Bradley EL III (1993) A clinically based classification system for acute pancreatitis. Summary of the International Symposium on Acute Pancreatitis, Atlanta, GA, September 11 through 13, 1992. Arch Surg 128:586–590
Steinberg W, Tenner S (1994) Acute pancreatitis. N Engl J Med 330:1198–1210
Dervenis C, Johnson CD, Bassi C et al (1999) Diagnosis, objective assessment of severity, and management of acute pancreatitis. Santorini consensus conference. Int J Pancreatol 25:195–210
Working Party of the British Society of Gastroenterology (2005) UK guidelines for the management of acute pancreatitis. Gut 54:1–9
Balthazar EJ, Robinson DL, Megibow AJ, Ranson JHC (1990) Acute pancreatitis value of CT in establishing prognosis. Radiology 174:331–336
Balthazar EJ (2002) Acute pancreatitis: assessment of severity with clinical and CT evaluation. Radiology 223:603–613
Mortelé KJ, Wiesner W, Intriere L et al (2004) A modified CT severity index for evaluating acute pancreatitis: improved correlation with patient outcome. AJR Am J Roentgenol 183:1261–1265
Bollen TL, van Santvoort HC, Besselink MGH, van Es WH, Gooszen HG, van Leuwen MS (2007) Update of acute pancreatitis: ultrasound, computed tomography, and magnetic resonance imaging features. Semin Ultrasound CT MR :371–383
Lankisch PG, Blum T, Maisonneuve P (2003) Lowenfels AB (2001) Severe acute pancreatitis: When to be concerned? Pancreatology 3:102–110
Piironen A (2001) Severe acute pancreatitis: contrast-enhanced CT and MRI features. Abdom Imaging 26:225–233
Arvanitakis M, Delhaye M, De Maertelaere V (2004) Computed tomography and magnetic resonance imaging in the assessment of acute pancreatitis. Gastroenterology 126:715–723
Moreno-Osset E, Lopez A, de la Cueva L et al (2005) 99 m Tc-hexamethylpropylene amine oxime leukocyte scintigraphy in acute pancreatitis: an alternative to contrast-enhanced computed tomography? Am J Gastroenterol 100:153–161
López A, de la Cueva L, Martínez MJ et al (2007) Usefulness of technetium-99 m hexamethylpropylene amine oxime-labeled leukocyte scintigraphy to detect pancreatic necrosis in patients with acute pancreatitis. Prospective comparison with Ranson, Glasgow and APACHE-II scores and serum C-reactive protein. Pancreatology 7:470–478
Merkle EM, Görich J (2002) Imaging of acute pancreatitis. Eur Radiol 12:1979–1992
D'Onofrio M, Zamboni G, Faccioli N, Capelli P, Pozzi Mucelli R (2007) Ultrasonography of the pancreas. 4. Contrast-enhanced imaging. Abdom Imaging 32:171–181
Rickes S, Uhle C, Kahl S (2006) Echo enhanced ultrasound: a new valid initial imaging approach for severe acute pancreatitis. Gut 55:74–78
Elmas N (2001) The role of diagnostic radiology in pancreatitis. Eur J Radiol 38:120–132
Benziane K, Azais O, Gasquet C (1992) A new computed tomography classification of acute pancreatitis. Gastroenterol Clin Biol 16:721–722
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ripollés, T., Martínez, M.J., López, E. et al. Contrast-enhanced ultrasound in the staging of acute pancreatitis. Eur Radiol 20, 2518–2523 (2010). https://doi.org/10.1007/s00330-010-1824-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-010-1824-5