Abstract
Objective:
To explore the clinical value of low-dose prospective ECG-triggering dual-source CT (DSCT) angiography in infants and children with complex congenital heart disease (CHD) compared with transthoracic echocardiography (TTE).
Methods:
Thirty-five patients (mean age: 16 months, range: 2 months to 6 years; male 15; mean weight: 12 kg) underwent low-dose prospective ECG-triggering DSCT angiography and TTE. Surgeries were performed in 29 patients, and conventional cardiac angiography (CCA) was performed in 8 patients. The accuracy was calculated based on the surgical and/or CCA findings. The overall imaging quality was evaluated on a five-point scale.
Results:
A total of 146 separate cardiovascular deformities were confirmed. DSCT missed three atrial septal defects and a patent ductus arteriosus. The accuracy of DSCT angiography and TTE was 97.3% (142/146) and 92.5% (135/146), respectively. Overall test parameters for DSCT angiography and TTE were similar (sensitivity, 97.3% vs 92.5%; specificity, 99.8% vs 99.8%). The average subjective image quality score was 4.3 ± 0.7. The mean effective dose was 0.38 ± 0.09 mSv.
Conclusions:
Prospective ECG-triggering DSCT angiography with a very low effective radiation dose allows the accurate diagnosis of anomalies in infants and children with complex CHD compared with TTE. It has great promise to become a commonly used second-line technique for complex CHD.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Conventional cardiac angiography (CCA) is the recognised gold standard method for assessment of complex congenital heart disease (CHD) in infants and children, but it is an invasive procedure with a potential procedure-related mortality of around 1% [1] and has the potential to impart high radiation doses [2]. Because of the risk, several non-invasive examination methods have been proposed as the major techniques for investigating complex CHD in children with the aim of decreasing the use of CCA. Although transthoracic echocardiography (TTE) is usually the initial choice because of the availability and the logistic simplicity, it may not be the perfect diagnostic tool because it is limited by the acoustic window, spatial resolution and the subjective interpretation of the operator [3].
Recently, multi-detector CT with improved spatial and temporal resolution has been used more frequently in children with CHD [4–8]. Non-ECG-gated imaging is usually used for the evaluation of extracardiac structural abnormalities [5, 9]. Retrospectively ECG-gated CT was performed when morphological evaluation of rapidly moving intracardiac or paracardiac structures, including the ascending aorta, cardiac valves and coronary artery, was required [6, 10]. However, the use of a low pitch or overlapping spiral CT acquisition results in the relatively high radiation dose of a retrospective ECG-gated examination [7, 8]. Although the combined use of dose-saving methods including a body-size-adaptive CT protocol, low tube voltage and tube current modulation [11, 12] can reduce the CT dose to 3–6 mSv for the patients with CHD [13], high radiation is still the major inherent limitation for retrospective ECG-gated CT.
Recently, prospective ECG-triggering or the step-and-shoot (SAS) technique, has been proposed to decrease radiation exposure in cardiac CT [14]. With this technique, radiation is only administered at predefined time points of the cardiac cycle, which is likely to be associated with a substantial reduction of the radiation dose. Recent studies have found that low-dose prospective ECG-triggering CT angiography is feasible in adults with mean heart rates below 70 bpm with a low effective dose of 1–4 mSv [15, 16]. The upper mean heart rates were not evaluated in terms of the feasibility of prospective ECG-triggering CT. With the combination of 83-ms temporal resolution of dual-source CT (DSCT), potential application in patients with higher heart rates has become possible [17].
To our knowledge, so far there have been no studies demonstrating the feasibility of prospective ECG-triggering DSCT angiography in infants and children. The purpose of this study is to explore the clinical value of low-dose prospective ECG-triggering DSCT angiography in infants and children with complex CHD.
Materials and methods
Patients
Our study was approved by the local ethics board; written informed consent was obtained from all patients (their parents). In our institute, CT angiography is part of the cardiovascular evaluation in patients with complex CHD according to the clinical indications. As no precise definition exists, “complex” CHD is defined as congenital heart disease with more than one separate cardiovascular anomaly by their transthoracic echocardiography findings in this study.
We prospectively enrolled 45 patients with complex CHD between January and May 2009 in our institute. General exclusion criteria for contrast-enhanced CT included nephropathy (serum creatinine level >150 mmol/l) (n = 1) and known hypersensitivity to iodine-containing contrast medium (n = 1). Patients with non-sinus rhythm were excluded from the study (n = 1). Patients who had undergone palliative or corrective surgeries were also excluded (n = 7).
A total of thirty-five patients (mean age: 16 months, range: 2 months to 6 years; male 15; mean weight: 12 kg; mean heart rate: 115 bmp, range: 90–142 bmp) with complex CHD could be included in this study. All patients underwent low-dose prospective ECG-triggering DSCT angiography and TTE. Palliative or corrective surgeries were performed in 29 patients, and CCAs were performed in 8 patients. Prospective ECG-triggering DSCT angiography was performed without complications in all patients. There was no extravasation of contrast material at the injection site. There was no systemic reaction to the contrast material.
Prospective dual-source CT protocol
All prospective ECG-triggering DSCT angiography examinations were performed using a DSCT (Somatom Definition, Siemens, Forchheim, Germany) during free-breathing. Sedation was achieved with peroral chloral hydrate and administered according to the patient’s body weight and clinical condition. The anatomical region covered by the CT data acquisition was from the thoracic inlet level to L1–2.
The following acquisition parameters were used: 2 × 32 × 0.6 mm detector collimation, a slice collimation of 2 × 64 × 0.6 mm enabled by Z-Sharp technology and a gantry rotation time of 0.33 s. Body weight-based adjustments of tube voltage and tube current were performed: <5 kg, tube voltage 80 kV, tube current 40–59 mAs; 5–10 kg, tube voltage 80 kV, tube current 60–79 mAs; >10 kg, tube voltage 80 kV, tube current 80–120 mAs. Prospective ECG-triggering technology necessitated a cycle time of 1.25 s for one acquisition and the subsequent table feed. The data acquisition window was 380 ms with padding technique. The centre of the data acquisition window was set at 40% of the R-R interval. The duration of the CT data acquisition was 5.39–9.14 s.
A dual-head power injector (Stellant; Medrad, Indianola, PA) was used. The saline flush technique was applied for all injections to reduce artefacts caused by undiluted intravascular contrast agent. The high concentration contrast material (Schering Ultravist, Iopromide, 350 mg I/ml, Berlin, Germany) was injected via peripheral veins in the elbow or back of the hand. We used 2 ml/kg of contrast medium plus the saline flush with half volume of the total contrast medium. The delay time was set to 25 s. The contrast volume was divided by 30 (the delay time + 5 s) to produce the flow rate for both contrast medium and saline chaser. For example, a 5-kg baby with peripheral access would be injected with 10 ml of iopromide 350 and 5 ml saline flush at 0.5 ml/s [(10 + 5)/30].
Image post-processing and analysis
Dual-source CT images were reconstructed in a monosegment mode by using a section thickness of 0.75 mm and a medium smooth-tissue convolution kernel (B26f). Images were reconstructed from 32% to 48% of the R-R interval in 2% increments, and the best reconstruction phase was used for evaluations. All images were transferred to an external workstation (Multi Modality Workplace, Siemens, Forchheim, Germany ) for further analysis. In addition to the CT axial slices, multiple planar reformation (MPR), volume rendering (VR) and maximum intensity projection (MIP) were used to visualise cardiac abnormalities.
The interpretation of prospective ECG-triggering DSCT angiography was performed without knowing the result of surgical, CCA or TTE findings. Two radiologists with more than 5 years’ experience in cardiac radiology semi-quantitatively assessed the overall image quality independently on a five-point scoring system [18] as follows:
-
5: excellent anatomical clarity; excellent image quality.
-
4: good anatomical clarity; all structures are clearly interpretable.
-
3: fair anatomical clarity; the anatomical relationships required clinically could be defined with confidence.
-
2: poor image quality or anatomical detail; incomplete demonstration of anatomical structures.
-
1: no useful information obtained.
For any disagreement in data analysis between the two observers, consensus agreement was achieved. Examinations graded 3, 4 or 5 were considered sufficient for complete diagnosis.
Transthoracic echocardiography
Two-dimensional TTE with Doppler ultrasound was performed from the apical, parasternal, subxiphoid and suprasternal approaches using a SONOS 7500 ultrasound system (Philips Medical Systems, Bothell, WA). All studies were performed by an echocardographic technician with more than 7 years’ experience and were saved digitally. All echocardiographic studies were analysed by a paediatric cardiologist with more than 10 years’ experience blinded to other studies’ results.
Radiation dose estimations
The parameters for imaging range, dose-length product and volume CT dose index were obtained from the protocol that summarised the relevant individual radiation exposure parameters of each CT angiography examination [19]. The effective dose delivered at CT cardiac angiography was derived from the dose-length product and conversion coefficients [20]. Infant-specific dose-length product conversion coefficients were given for a 16-cm phantom: a coefficient of 0.039 mSv/[mGy·cm] until 4 months of age, 0.026 mSv/[mGy·cm] between 4 months and 1 year of age, and 0.018 mSv/[mGy·cm] between 1 year and 6 years of age [21].
Statistics
The results of surgical or CCA findings were utilised as the reference standard. Test parameters (sensitivity, specificity, positive and negative predictive values) were calculated for separate cardiovascular anomalies, and the 95% CIs were calculated. Quantitative data were expressed as means ± standard deviations, and categorical data were given in proportions and percentages. Interobserver agreement in subjective image quality grading was assessed by using kappa statistics, and к-values of 0.61–0.80 corresponded to good agreement. The accuracy of DSCT angiography and TTE was compared by using the non-parametric chi-squared test. A p value < 0.05 was considered to indicate a significant difference.
Results
The final diagnoses of the 35 patients were pulmonary artery atresia with ventricle septal defect (n = 5, Fig. 1), tetralogy of Fallot (n = 10, Figs. 2 and 3), double outlet right ventricle (n = 5), interrupted aortic arch (n = 2), coarctation of the aorta (n = 3 Fig. 4), anomalous origin of one pulmonary artery (n = 1), total anomalous pulmonary venous return (n = 2), anomalous systemic venous return (n = 1) and transposition of great arteries (n = 6) by surgical and/or CCA findings as the reference standard.
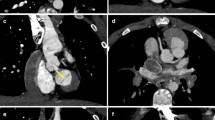
A 16-month-old boy with the diagnosis of pulmonary atresia with the fistula between the proximal RCA and MPA. Prospective ECG-triggering DSCT was performed at 80 kV and 80 mAs (effective dose, 0.43 mSv). (a) Multiplanar reformatted image and (b) volume-rendered image (anterior view) show the fistula (thick arrow) between the proximal RCA and MPA. (c) Thick-section oblique sagittal MIP image shows the ventricular septal defect (VSD) and overriding aorta. (d) Volume-rendered image (posterior view) shows two MAPCAs (slim arrows) arising from the DA. AA = ascending aorta, RCA = right coronary artery, MPA = main pulmonary artery, RPA = right pulmonary artery, RV = right ventricle, LV = left ventricle, MAPCA = main aortopulmonary collateral artery, DA = descending aorta
An 8-month-old boy with tetralogy of Fallot. Prospective ECG-triggering DSCT was performed at 80 kV and 60 mAs (effective dose, 0.29 mSv). (a) Ventricular septal defect (VSD) and overriding aorta on the reformatted images. (b) Thick-section oblique sagittal MIP image during systole shows critical stenosis of the right ventricular outflow tract (RVOT). RCA = right coronary artery, RV = right ventricle, LV = left ventricle, Ao = aorta, MPA = main pulmonary artery, LPA = right pulmonary artery
A 1-year-old boy with tetralogy of Fallot. Prospective ECG-triggering DSCT was performed at 80 kV and 70 mAs (effective dose, 0.38 mSv). (a) Thick-section oblique sagittal MIP image shows critical stenosis of the pulmonary valve (white arrow). (b) Oblique coronal multiplanar reformatted image shows patent ductus arteriosus (PDA) between aortic arch (Ar) and left pulmonary artery (LPA). Note stenosis of the proximal LPA (black arrow). RV = right ventricle, MPA = main pulmonary artery, RPA = right pulmonary artery
A 14-month-old girl with coarctation of the aorta. Prospective ECG-triggering DSCT was performed at 80 kV and 80 mAs (effective dose, 0.41 mSv). (a) Multiplanar reformatted image shows simultaneously the atrial septum defect (ASD) and muscular ventricular septal defect (VSD) accompanied by coarctation of the aorta. (b) Volume-rendered image (posterior view) demonstrates narrowing of the aortic isthmus (arrow). RA = right atrium, LA = left atrium, RV = right ventricle, LV = left ventricle, AA = ascending aorta, Ar = aortic arch, DA = descending aorta, LSA = left subclavian artery, RSA = right subclavian artery
A total of 146 separate cardiovascular deformities were confirmed by surgical and/or CCA findings. The accuracy of low-dose prospective ECG-triggering DSCT angiography and TTE in diagnosing separate cardiovascular deformities was 97.3% (142/146) and 92.5% (135/146), respectively. DSCT was as accurate as TTE in revealing cardiovascular deformities (χ 2 = 3.48, P < 0.05). Details on separate cardiovascular deformities are given in Table 1. Out of the total 735 diagnoses made, 17(2.3%) differences between DSCT angiography and TTE were found. The overall deformity-based sensitivity, specificity, positive predictive value and negative predictive value were 97.3%, 99.8%, 99.3% and 99.3%, respectively, by DSCT angiography, and 92.5%, 99.8%, 99.3% and 98.2%, respectively, by TTE (Table 2).
In four patients, TTE missed all of the coronary artery anomalies. In one patient with tetralogy of Fallot (Fig. 5), the right coronary artery arose from the left coronary sinus of Valsalva, which distributed two larger branches crossing in front of the right ventricular outlet. In another patient with transposition of the great arteries (Fig. 6), both the right coronary artery and the left coronary artery arose from the non-coronary sinus of Valsalva with a common ostium. The left main coronary crossed between the main pulmonary artery and the left atrium. In the remaining two patients, one coronary-pulmonary fistula and one high-take off of the left coronary artery were missed by TTE. TTE missed two major aortopulmonary collateral arteries, three distal pulmonary artery stenoses and one anomalous systemic venous return. TTE misdiagnosed one patient as having coarctation of the aorta; operative and DSCT findings confirmed the actual diagnosis of interrupted aortic arch. On the other hand, DSCT misdiagnosed one normal atrium as one atrial septal defect and missed three atrial septal defects and a very small patent ductus arteriosus. One patient without atrial septal defect was misdiagnosed because of the streak artefacts produced by high-attenuation contrast material at the right atrium. In other patients, very small atrial septal defects (<3 mm) were missed.
Tetralogy of Fallot with coronary artery anomaly in a 2-year-old boy. Prospective ECG-triggering DSCT was performed at 80 kV and 80 mAs (effective dose, 0.43 mSv). (a) Volume-rendered image shows anomalous RCA arose from the left coronary sinus of Valsalva. (b) Thick-section oblique sagittal MIP image shows the large branch of the anomalous RCA crossing anteriorly the critical stenosis RVOT. RCA = right coronary artery, AA = ascending aorta, MPA = main pulmonary artery, RV = right ventricle, RVOT = right ventricular outlet
Completed transposition of the great arteries with a coronary artery anomaly in a 4-year-old girl. Prospective ECG-triggering DSCT was performed at 80 kV and 120 mAs (effective dose, 0.58 mSv). (a) Thick-section MIP image and (b) volume-rendered image (posterior view) show that both the right coronary artery (RCA) and left coronary artery (LCA) arose from the non-coronary sinus of Valsalva with a common ostium (arrow). AA = ascending aorta, MPA = main pulmonary, LA = left atrium
Subjective evaluation of image quality
Interobserver agreement was reached in all patients. The average subjective overall image quality score of prospective ECG-triggering DSCT was 4.3 ± 0.7 (range 3–5): score 3 (n = 4, 11%), score 4 (n = 15, 43%) and score 5 (n = 16, 46%). Diagnostic images (images graded 3 or more) were obtained in all examinations. There was good agreement (к = 0.78) for overall image quality scoring between the two reviewers.
Radiation dose estimations
The mean volume CT dose index was 1.39 ± 0.40 mGy (range: 0.83–1.92). The mean dose-length product was 19.86 ± 6.27 mGy·cm (range: 10–32), resulting in an estimated mean effective radiation dose of 0.38 ± 0.09 mSv (range: 0.25–0.58). Radiation dose estimates for each patient in prospective ECG-triggering DSCT angiography are shown in Table 3.
Discussion
Our study results indicate the technical and clinical feasibility of low-dose prospective ECG-triggering DSCT angiography in infants and children with complex CHD. The accuracy of prospective ECG-triggering DSCT angiography in diagnosing cardiovascular deformities was 97.3% (142/146). Prospective ECG-triggering DSCT angiography performed with tube current and voltage modulation resulted in mean effective doses of 0.38 ± 0.09 mSv.
Any diagnostic test that utilises ionising radiation should be performed in accordance with the ALARA (As Low As Reasonably Achievable) principle, especially in children. Therefore, dose exposure has become a major issue for CT cardiac angiography in children with congenital heart disease [22, 23]. One of the most important dose-saving strategies is patient-specific adaptation of mAs and kV in cardiac multislice CT [24]. Lee et al. [5] and Ben Saad et al. [18] indicated that the low-dose parameters were achieved through systematic use of the lower tube voltage (80 kV) and adaptation of the tube current to the infant’s weight. Further radiation dose reduction could be expected using new prospective sequential acquisition.
Prospective ECG-triggering sequential DSCT is one of the most efficient techniques for radiation dose reduction in CT cardiac angiography [25, 26]. Compared with the conventional sequential imaging mode, the imaging angle is extended from 260 degrees to 460 degrees, resulting in phase shifting of about ±8% after imaging, which allows more flexibility in retrospectively choosing different phases of the cardiac cycle for image reconstruction once the data have been acquired. With the advantage of 83-ms temporal resolution, adaptive sequential imaging allows for CT cardiac angiography in patients with high heart rates, especially in infants and children. Leschka et al. [27] recently found that at heart rates greater than 85.5 beats per minute, the best reconstruction time shifted to end systole. Based on his results, the centre of the data acquisition window was set at 40% of the R-R interval.
In our study, we combined prospective ECG-triggering sequential imaging, a tube voltage of 80 kV setting and adaptation of the tube current to the infant’s weight to reduce the radiation dose drastically without impairing image quality. Diagnostic images were obtained in all examinations in spite of their physiological status, and with good interobserver agreement. Low-dose prospective ECG-triggering DSCT angiography has no limitation to its valuable contributions to estimating the anatomical details and morphology of intracardiac deformities. DSCT angiography has no curb to providing evident visualisation of interatrial and interventricular septa and their defects. However, the diagnosis of atrial septal defect should be carefully made because the interatrial septum may be too thin to be delineated on CT images, especially in the region of the fossa ovalis [10]. TTE was found more suitable for septal defects because of the availability of flow imaging [28]. Compared with TTE, DSCT is more effective in demonstrating the extracardiac deformities present in complex CHD. It is very useful in demonstrating the pulmonary artery anatomy, along with the significant aortopulmonary collateral vessels. DSCT imaging also allows accurate identification of the aorta and systemic and pulmonary veins and their relationships.
In patients with complex CHD, such as tetralogy of Fallot or transposition of the great arteries, evaluation of the coronary artery anatomy is very important [29] because some variations can significantly contribute to morbidity and mortality during surgical correction and impact on management strategies [13, 30]. Our study confirms that a very fast heart rate does not impede coronary artery visualisation in children using DSCT. Prospective ECG-triggering DSCT angiography found all of the coronary artery anomalies, which demonstrates that DSCT is more sensitive than TTE in identifying the coronary arteries.
Comparisons with other imaging techniques
A major disadvantage of prospective ECG-triggering DSCT angiography compared with conventional cardiac angiography (CCA) is that it does not provide functional information. CAA is still the only means by which to obtain haemodynamic and physiological data (intracardiac and intravascular pressure curves, oxygen saturation data). On the other hand, procedure-related mortality in neonatal diagnostic cardiac catheterisation is still around 1% in advanced congenital heart centres despite the improvements in its techniques and catheters [9]. Furthermore, Bacher et al. [2] found that a high median effective dose of diagnostic cardiac catheterisations in children with CHD was 4.6 mSv.
Transthoracic echocardiography (TTE) is the first-line option for children with congenital heart disease because it is non-invasive, safe, readily available and less expensive. The cost of an echocardiogram is approximately $120, whereas the cost of a DSCT angiography to a privately insured patient is approximately $300 in our institution. TTE is more effective in demonstrating the intracardiac deformities compared with other imaging modalities. However, compared with DSCT, TTE is more operator-dependent, offers less clarity in the display of the overall cardiac anatomy and has relative difficulties in visualising pulmonary veins, systemic veins and in general all anatomical structures obscured by overlying bone and aerated lung [3].
Magnetic resonance (MR) imaging provides anatomic and functional information that is superior to that provided by conventional cardiac imaging techniques such as CCA. Most importantly, there is no ionising radiation for this kind of examination. Furthermore, cine MR images can provide additional information about cardiac function, valve patency and the haemodynamic significance of vascular stenosis, whereas its long acquisition time may necessitate the sedation of young children and may present a problem in the examination of patients whose clinical condition is unstable. Moreover, MR imaging cannot be used to detect coronary artery anomalies [31].
Limitation
We acknowledge the following limitations of our study. First, we included a relatively small group of patients. Therefore, future studies on the diagnostic accuracy of low-dose prospective ECG-triggering DSCT angiography with larger patient populations are required. Second, DSCT angiography with prospective ECG-triggering does not allow functional assessment of the ventricle. Since the functional assessment can be done with either TTE or MR imaging, the benefit of obtaining functional information with ECG-gated CT must outweigh the increased radiation exposure to the patient. Finally, ionising radiation is the inherent limitation of DSCT angiography in infants and children.
In summary, prospective ECG-triggering allows low-dose DSCT angiography to be acquired with high diagnostic accuracy in the assessment of complex CHD in infants and children. Combining with transthoracic echocardiography, we believe that prospective ECG-triggering DSCT angiography has great promise to become a commonly used second-line technique for complex CHD.
References
Vitiello R, McCrindle BW, Nykanen D, Freedom RM, Benson LN (1998) Complications associated with pediatric cardiac catheterization. J Am Coll Cardiol 32:1433–1440
Bacher K, Bogaert E, Lapere R, De Wolf D, Thierens H (2005) Patient-specific dose and radiation risk estimation in pediatric cardiac catheterization. Circulation 111:83–89
Soongswang J, Nana A, Laohaprasitiporn D et al (2000) Limitation of transthoracic echocardiography in the diagnosis of congenital heart diseases. J Med Assoc Thai 83(Suppl 2):S111–S117
Westra SJ, Hill JA, Alejos JC, Galindo A, Boechat MI, Laks H (1999) Three-dimensional helical CT of pulmonary arteries in infants and children with congenital heart disease. AJR Am J Roentgenol 173:109–115
Lee T, Tsai IC, Fu YC et al (2006) Using multidetector-row CT in neonates with complex congenital heart disease to replace diagnostic cardiac catheterization for anatomical investigation: initial experiences in technical and clinical feasibility. Pediatr Radiol 36:1273–1282
Wang XM, Wu LB, Sun C et al (2007) Clinical application of 64-slice spiral CT in the diagnosis of the tetralogy of Fallot. Eur J Radiol 64:296–301
Leschka S, Oechslin E, Husmann L et al (2007) Pre- and postoperative evaluation of congenital heart disease in children and adults with 64-section CT. RadioGraphics 27:829–846
Khatri S, Varma SK, Khatri P, Kumar RS (2008) Sixty-four-slice multidetector-row computed tomographic angiography for evaluating congenital heart disease. Pediatr Cardiol 29:755–762
Tsai IC, Chen MC, Jan SL et al (2008) Neonatal cardiac multidetector row CT: why and how we do it. Pediatr Radiol 38:438–451
Goo HW, Park IS, Ko JK, Kim YH, Seo DM, Park JJ (2005) Computed tomography for the diagnosis of congenital heart disease in pediatric and adult patients. Int J Cardiovasc Imaging 21:347–365
Kalra MK, Maher MM, Toth TL et al (2004) Techniques and applications of automatic tube current modulation for CT. Radiology 233:649–657
Herzog C, Mulvihill DM, Nguyen SA et al (2008) Pediatric cardiovascular CT angiography: radiation dose reduction using automatic anatomic tube current modulation. AJR Am J Roentgenol 190:1232–1240
Goo HW, Seo DM, Yun TJ et al (2009) Coronary artery anomalies and clinically important anatomy in patients with congenital heart disease: multislice CT findings. Pediatr Radiol 39:265–273
Hsieh J, Londt J, Vass M, Li J, Tang X, Okerlund D (2006) Step-and-shoot data acquisition and reconstruction for cardiac x-ray computed tomography. Med Phys 33:4236–4248
Husmann L, Valenta I, Gaemperli O et al (2008) Feasibility of low-dose coronary CT angiography: first experience with prospective ECG-gating. Eur Heart J 29:191–197
Stolzmann P, Leschka S, Scheffel H et al (2008) Dual-source CT in step-and-shoot mode: noninvasive coronary angiography with low radiation dose. Radiology 249:71–80
Matt D, Scheffel H, Leschka S et al (2007) Dual-source CT coronary angiography: image quality, mean heart rate, and heart rate variability. AJR Am J Roentgenol 189:567–573
Ben Saad M, Rohnean A, Sigal-Cinqualbre A, Adler G, Paul JF (2009) Evaluation of image quality and radiation dose of thoracic and coronary dual-source CT in 110 infants with congenital heart disease. Pediatr Radiol 39:668–676
Stolzmann P, Scheffel H, Schertler T et al (2008) Radiation dose estimates in dual-source computed tomography coronary angiography. Eur Radiol 18:592–599
Einstein AJ, Moser KW, Thompson RC, Cerqueira MD, Henzlova MJ (2007) Radiation dose to patients from cardiac diagnostic imaging. Circulation 116:1290–1305
Shrimpton PC (2004) Assessment of patient dose in CT. NRPBPE/1/2004
Goo HW, Suh DS (2006) Tube current reduction in pediatric non-ECG-gated heart CT by combined tube current modulation. Pediatr Radiol 36:344–351
Linton OW, Mettler FA Jr (2003) National Council on Radiation Protection and Measurements National conference on dose reduction in CT, with an emphasis on pediatric patients. AJR Am J Roentgenol 181:321–329
Paul JF, Abada HT (2007) Strategies for reduction of radiation dose in cardiac multislice CT. Eur Radiol 17:2028–2037
Schoenhagen P (2008) Back to the future: coronary CT angiography using prospective ECG triggering. Eur Heart J 29:153–154
Hausleiter J, Meyer T, Hermann F et al (2009) Estimated radiation dose associated with cardiac CT angiography. JAMA 301:500–507
Leschka S, Wildermuth S, Boehm T et al (2006) Noninvasive coronary angiography with 64-section CT: effect of average heart rate and heart rate variability on image quality. Radiology 241:378–385
Beier UH, Jelnin V, Jain S, Ruiz CE (2006) Cardiac computed tomography compared to transthoracic echocardiography in the management of congenital heart disease. Catheter Cardiovasc Interv 68:441–449
Ruzmetov M, Jimenez MA, Pruitt A, Turrentine MW, Brown JW (2005) Repair of tetralogy of Fallot with anomalous coronary arteries coursing across the obstructed right ventricular outflow tract. Pediatr Cardiol 26:537–542
Massoudy P, Baltalarli A, de Leval MR et al (2002) Anatomic variability in coronary arterial distribution with regard to the arterial switch procedure. Circulation 106:1980–1984
Boechat MI, Ratib O, Williams PL, Gomes AS, Child JS, Allada V (2005) Cardiac MR imaging and MR angiography for assessment of complex tetralogy of Fallot and pulmonary atresia. Radiographics 25:1535–1546
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cheng, Z., Wang, X., Duan, Y. et al. Low-dose prospective ECG-triggering dual-source CT angiography in infants and children with complex congenital heart disease: first experience. Eur Radiol 20, 2503–2511 (2010). https://doi.org/10.1007/s00330-010-1822-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-010-1822-7