Abstract
Objective
To prospectively determine the upgrade rate following surgery in benign papilloma initially diagnosed at ultrasound (US)-guided 14-gauge gun biopsy.
Methods
A total of 128 benign papillomas were diagnosed in 114 patients after a US-guided biopsy. Surgical excision was recommended where the biopsy indicated benign papilloma, regardless of imaging findings. The upgrade rate to ‘atypical’ and ‘malignancy’ was measured on a per-lesion basis. We analysed potential associations between clinical presentation, lesion variables and the results of surgical excision (using logistic regression).
Results
Of the 114 patients, 87 eventually underwent surgery: among the 100 supposed benign papillomas, surgical excision revealed fibrocystic change or no residual lesion in nine cases, intraductal papilloma in 74, atypical papilloma in 13, papillary ductal carcinoma in situ (DCIS) in three and one invasive papillary carcinoma. The upgrade rate for an atypical papilloma or papilloma with adjacent foci of atypical ductal hyperplasia (ADH) and malignancy was 13% (95% CI = 7.1–21.2%) and 4% (95% CI = 1.1–9.9%), respectively. The mean lesion size (P = 0.041) was significantly larger when lesions were upgraded to malignancy. Other features were not significantly associated with pathological underestimation (P > 0.05).
Conclusion
Surgical excision should be considered for benign intraductal papillomas above 1.5 cm in size.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Papillary lesions of the breast account for 1–2% of breast neoplasia and 10% of benign breast tumours [1–3]. Papillary lesions comprise a wide spectrum of lesions ranging from benign papilloma to papilloma with foci of atypia or carcinoma in situ to intraductal adenocarcinoma with a papillary growth pattern and papillary adenocarcinomas [3]. Typically, papillary lesions are composed of branching papillae with a fibrovascular stalk covered by epithelial and myoepithelial cells [2], and the presence or absence of a myoepithelial cell layer in the papillary component of the lesion is the most important feature that helps to differentiate a benign papilloma from a papillary carcinoma [3].
Recent research has suggested that benign papillary lesions can be diagnosed accurately by the use of a core needle biopsy [4] and a subsequent decision regarding clinical treatment of papillary breast lesions can be based on a core needle biopsy diagnosis. Percutaneous core needle biopsies have been increasingly utilised for the diagnosis of clinically occult and palpable breast lesions, replacing both fine needle aspiration (FNA) and open surgical biopsy for the initial diagnosis [5–7].
However, the management of benign papillary lesions remains controversial and concerns remain over the generalisation of such results with patient management because of the retrospective nature of studies with selection bias. In addition, most studies have focused on the use of stereotactic biopsies and management of benign papilloma diagnosed after an ultrasound (US)-guided core needle biopsy has not been well evaluated.
The objective of this study was to survey prospectively the potential risk of associated ductal carcinoma in situ (DCIS) and invasive carcinoma in follow-up surgical excision specimens of benign intraductal papilloma diagnosed after a US-guided core needle biopsy and to evaluate the clinical presentation and lesion variables that affect upgrade to malignancy.
Materials and methods
Patients
The institutional review board at our institution approved this prospective study and informed patient consent was obtained. From May 2007 to August 2008, US-guided 14-gauge core needle biopsies were performed in 2,699 patients. Benign intraductal papillomas were diagnosed after biopsies in 128 lesions in 114 (4.7%) patients. The mean patient age was 43.6 years (age range 22–69 years). For the diagnosis of benign papilloma, proliferation of ductal epithelium with fibrovascular core formation, presence of myoepithelial cells and absence of pleomorphism or cellular monotony were required. The presence of focal cytological or architectural atypia, the focal absence of myoepithelial cells and monotonous epithelial proliferation were considered as atypical papilloma, and they were not included in this study population. Among the 128 lesions in 114 patients, one patient who had a history of breast cancer in the ipsilateral breast and three patients with four lesions who had atypical papilloma in the ipsilateral breast were excluded from the study population.
Surgical excision was recommended for all patients with US-guided biopsy results of benign intraductal papilloma regardless of symptoms and imaging findings. Twenty-three patients refused to undergo surgical excision, and the results from surgical excision were available for 100 lesions in 87 patients (Fig. 1).
In this study, we included only patients in whom subsequent surgical excision was performed, because mammographic and US follow-up periods were less than 2 years in the remaining 23 lesions. Surgical excision was performed consecutively regardless of image findings or clinical symptoms. The mean patient age for patients undergoing surgery was 43.2 years (range 22–69 years). The mean interval between biopsy and surgical excision was 44.2 days (range 5–140 days). Among benign intraductal papillomas, 48% of the lesions presented with symptoms such as palpability or nipple discharge. Four patients had a history of contralateral breast cancer (Table 1).
Imaging techniques
Bilateral mammography was performed with a dedicated mammography unit (Senographe 800T, GE Medical Systems, Milwaukee, WI, USA or Lo-Rad MIV, Hologic, Bedford, MA, USA). Routine craniocaudal and mediolateral oblique views of the breast(s) and spot-magnification or open-magnification views over the area of the lesion were obtained. Ultrasonography was performed using a EUB-8500 (Hitachi Medical, Tokyo, Japan) with a 14–6 MHz linear transducer.
Biopsy
A core needle biopsy was performed with US guidance for all lesions. For US, all masses were sampled using a EUB-8500 (Hitachi Medical) with a 14–6 MHz linear transducer. Tissue samples were obtained using an automated biopsy gun with a 14-gauge needle (Pro-Mag 2.2, Manan Medical Products). The biopsy procedures were performed by one of five radiologists with 5–11 years’ experience in breast imaging. A mean of four core samples (range 3–5) were obtained per lesion when a biopsy was performed with US guidance.
Surgery
To identify exactly biopsy-proven intraductal papillomas, ultrasound-guided preoperative needle localisation was performed with a 21-gauge modified Kopans spring-hook localisation needle inserted into and through the lesion under ultrasound guidance with a freehand technique performed for 84 lesions. The hookwire was then inserted through the needle, and the needle was removed. Post-procedure craniocaudal and 90° mediolateral mammograms were obtained to ensure proper placement and to rule out displacement or retraction of the wire. For the remaining 16 lesions, ultrasound-guided preoperative skin marking was performed. The localisation method including needle localisation and skin marking was selected based on the palpability of the lesion or preference of the surgeon.
For excised lesions, the surgical pathological reports from the excision were reviewed (J.M.C.) and lesions were classified according to the following categories: (a) benign papilloma, (b) other benign lesions, (c) atypical papilloma or papilloma with adjacent foci of atypical ductal hyperplasia (ADH) and (d) ductal carcinoma in situ (DCIS) and invasive carcinoma. The image-guided biopsy results were compared with the final surgical pathological findings.
Data collection and statistical analysis
Radiologists who performed the procedures recorded the lesion size (maximal diameter as measured on US), lesion location and final assessment category according to the Breast Imaging Reporting and Data System (BI-RADS) [8, 9] and the number of core samples taken during the procedure. Pathological results of benign papilloma were compared with relevant imaging findings to determine concordances and discordances of biopsy results by two radiologists who specialise in breast imaging and who had no knowledge of the histological results.
To confirm that the lesions in the analysis set were not significantly different from eligible lesions, mean age, symptoms, history of breast cancer, initial BI-RADS assessment, lesion size, location and number of core specimens obtained were compared using the χ 2 test, Fisher’s exact test and Student’s t test. The significance level was set at P < 0.05. Statistical analyses were performed with the use of SPSS version 12.0 (SPSS, Chicago, IL, USA).
Upgrade in diagnosis was determined when a patient had at least one lesion that was classified as DCIS or invasive cancer following surgical excision. Associations between patient clinical presentation, lesion variables and the results of surgical excision were examined by using logistic regression methods (SAS Software System, version 9.1, Cary, NC, USA) in consultation with a statistician, with a significance level of P < 0.05. Multiple lesions in the same breast were considered as independent lesions. Logistic regression in this study was fitted using penalised maximum likelihood. The significance tests and 95% confidence intervals for odds ratios were determined using the penalised likelihood ratio test and profile penalised likelihood confidence intervals, respectively [10, 11]. For the estimation of the odds ratio (OR), we used clinical presentations (age, symptoms and history of breast cancer) and lesion variables (imaging–pathology concordance/discordance, number of cores obtained, lesion size and lesion location) as the explanatory (independent) variables, and the histological diagnoses as the outcome (dependent) variables.
Results
Histological findings of surgical specimens
The histopathological findings of the 100 surgically excised lesions revealed benign papillomas in 74 cases, other benign lesions in nine cases, atypical papilloma or papilloma with adjacent foci of ADH in 13 cases, DCIS in three cases and an invasive carcinoma in one case. Individual characteristics of cases that were upgraded to malignancy are listed in Table 2.
Upgrade rates and predictors of histological upgrade
Four (4%, 95% CI = 1.1–9.9%) of the 100 lesions diagnosed as benign after a core needle biopsy were identified as malignant following surgical excision. Three lesions were DCIS and one lesion was an invasive cancer. Patient clinical presentations and lesion variables and correlations with histological upgrades are shown in Table 3.
Clinically, 22 lesions were palpable and 16 patients presented with nipple discharge. There were 60 asymptomatic patients. The presence or absence of symptoms did not correlate with histological upgrade (P = 0.590). In addition, a history of contralateral breast cancer did not correlate with histological upgrade (P = 0.630).
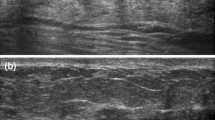
Of the four cancers identified after surgical excision, one cancer manifested as a mass and the other cancers were negative on mammography. All malignancies were evaluated with US and three cancers were depicted as ill-defined hypoechoic masses (Fig. 2) and one cancer was depicted as an intraluminal mass (Fig. 3). The initial BI-RADS final assessment category of all four cancers, recorded by the radiologist who performed the biopsy, was category 4, based on an initial assessment with mammography and US. Imaging–pathology concordance or discordance was assessed, and two of the four cancers were considered to show discordant image findings for biopsy results. Initial BI-RADS assessment and radiology–pathology concordance or discordance did not correlate with histological upgrade either (P = 0.076). There was no significant relationship between the number of cores obtained and the presence of malignancy at excision.
Papillary DCIS. a US demonstrates a diffuse, ill-defined, low echoic lesion in the left subareolar area. b A photomicrograph of a core needle biopsy specimen shows the intraductal papilloma with fibrovascular cores and mild ductal epithelial hyperplasia (hematoxylin and eosin (H&E) staining, ×400). c A photomicrograph of an excisional biopsy specimen shows atypical epithelial proliferation in papillary arrangements (arrow) with a focal micropapillary pattern, which is consistent with an intraductal papillary carcinoma (H&E staining, ×400)
Papillary DCIS. a US demonstrates a well-defined, low echoic intraductal lesion in the left subareolar area. b A photomicrograph of an excisional biopsy specimen shows intraductal papilloma with fibrovascular cores (arrow) and combined atypical epithelial proliferation in papillary arrangements (arrowhead) with a focal micropapillary pattern, which is consistent with an ductal carcinoma in situ (H&E staining, ×100)
The mean lesion size as measured on US for the malignancies was 18.0 ± 10.4 mm, larger than benign lesions and a significant relationship between lesion size and the presence of malignancy following surgical excision was noted (P = 0.041). In addition, upgrade to malignancy following surgical excision was significantly higher in lesions that had a size equal to or larger than 15 mm (P = 0.026). Mean lesion distance from the nipple in malignancies and benign lesions was 22.5 ± 17.1 mm and 16.6 ± 14.1 mm, respectively. However, significant differences were not noted between lesion location and the presence of malignancy following surgical excision.
Thirteen (13%, 95% CI = 7.1–21.2%) of the 100 lesions were also upgraded to atypical papilloma or papilloma with adjacent foci of ADH. Nine cases showed negative findings on mammography and four cases showed a mass or asymmetry. On US examination, four lesions were depicted as intraluminal masses, six lesions were depicted as hypoechoic masses with adjacent ductal changes and three lesions were depicted as irregular hypoechoic masses. The mean lesion size measured on US was 10.5 ± 7.0 mm, and the mean distance from the lesion to the nipple was 15.8 ± 11.9 mm. Both of these findings showed no significant differences compared with values of lesions proven to be benign following surgical excision.
Discussion
With the widespread use of core needle biopsies for the initial diagnosis of ultrasound-detected lesions, papillary lesions of the breast are occasionally encountered. These lesions include a wide spectrum of entities that range from benign papilloma to invasive papillary adenocarcinoma [1, 12]. There continues to be a lack of agreement on the management of benign papillomas diagnosed after a core needle biopsy.
Our study is to the best of our knowledge one of the largest prospective studies to date to highlight the need for the surgical excision of benign papillomas diagnosed after a US-guided 14-gauge core needle biopsy, regardless of imaging findings and clinical presentation. In comparison with other studies that have determined variable false negative rates of 14-gauge core needle biopsy in benign intraductal papillomas, 0–14% [13–15], a 4% upgrade rate to DCIS or invasive ductal cancer was determined in our study.
In previous studies, the rate of surgical excision has been variable, as the studies included all types of papillary lesions, not only benign intraductal papilloma but also papilloma with atypia. Liberman et al. [14] and Mercado et al. [15] demonstrated high malignancy rates for papillary lesions. Liberman et al. [14] targeted concordant core biopsies in a recent series of 50 cases; only 50% of lesions were subjected to surgical excision. Although a short period of 2 years of mammographic follow-up was used to survey patients who did not undergo surgical excision, 14% of patients were found to have a subsequent malignancy (one infiltrating ductal carcinoma and four DCIS) and 17% of patients had high-risk lesions. Four of the cancers were found on interval follow-up because of interval growth detected on mammography, new discharge or development of a mass at the biopsy site. Mercado et al. showed similar findings for benign concordant lesions with a high risk of upgrade to ADH or DCIS [15]. Intraductal papillomas and papillary carcinomas have a considerable overlap in imaging features, and it is more difficult to differentiate them on ultrasound than other breast neoplasms. This is because malignant papillary lesions frequently show a round or oval shape and a circumscribed margin, which are findings suggestive of benign lesions in other breast neoplasm [16]. According to Lam et al. one lesion showing both benign radiological features and benign core biopsy results was found to be ductal carcinoma in situ at histology, and a significant proportion of malignant papillary lesions did not show any malignant features on mammography or on ultrasound [17].
However, recent studies have challenged this standard by suggesting that benign papillary lesions can be managed with clinical follow-up alone [5, 18], stating that these are the fault of sampling errors in clear cases of non-concordant radiology. Ivan et al. postulated that provided benign papilloma had concordant imaging findings, an excisional biopsy could be avoided [6]. Of 30 benign papillary lesions, none had subsequent malignant features. However, only six cases underwent surgical excision and the follow-up period ranged from 1 to 51 months. The nature of follow-up, whether clinical or mammographic, was not specified and recommendations regarding frequency of follow-up were not provided. In addition, Agoff and Lawton similarly advocated follow-up for benign core biopsies [7]. In this study, the mean length of follow-up was 35.6 months; the investigators acknowledged a lack of long-term follow-up. Similarly, Sydnor et al. reported high malignancy rates for atypical patients (67%) but only 3% of benign cores had a malignancy as identified in excision specimens [13]. These investigators concluded that the low malignancy rate supports the use of follow-up alone.
The results of our study revealed a considerable upgrade rate to DCIS or invasive cancer for benign papillary lesions of the breast after a core needle biopsy. In addition, one patient refused to undergo surgical excision at the time of diagnosis of a benign papilloma after a core needle biopsy, and the patient presented with interval growth of the lesion as detected on US after 1 year, even though the patient was not included in the analysis set. The final pathological report of the surgical excision sample revealed the presence of DCIS.
The different upgrade rate determined for malignancy in the present study from rates determined in previous reports can be explained in two ways. The present study is a prospective study, which is a strength of this study and we did not consider patient symptoms, clinical manifestations, image findings and risk of breast cancer for surgical excision. In retrospective studies, clinical features of patients may influence management, which suggests that a benign-appearing lesion tends to undergo follow-up and a lesion that presents with clinical symptoms or suspicious findings on images or with a large size tends to be treated surgically. In addition, the follow-up period is not sufficient in many cases and follow-up loss occurs frequently. Thus, the upgrade rate might be estimated to be higher or lower by selection bias. Accordingly, if we reselect the benign papillomas with concordant image findings in retrospective analysis, our upgrade rate would be lower than 4%. Even though the upgrade rate is lower in cases of concordant imaging–pathology features, intraductal papillomas and papillary carcinomas have a considerable overlap in imaging features as mentioned above and the assessment of imaging–pathology concordance is still subjective.
A second reason for the difference in the upgrade rate is that we only included lesions detectable by ultrasound that were diagnosed by a US-guided core needle biopsy. Upgrade rates in previous studies have included mammographic abnormalities that have also been proven after a stereotactic biopsy. Lesions with suspicious microcalcifications without visualisation of a definite mass on a US examination might be excluded from our study, which could lead to the difference in the upgrade rate in our study.
The prospective analysis of variables showed that the distinguishing radiological feature of upgraded cases is size as measured on US (P = 0.042), especially for a lesion size greater than 15 mm (P = 0.026). Three of the four malignancies measured more than 15 mm. Underestimation of malignancy was secondary to undersampling of large ill-defined lesions or the surrounding tissue, which may contain foci of malignancy, as some studies have suggested [19], and no other factor was associated with histological upgrade. Imaging findings agreed with the final pathological report in only 50% of the malignancies in our study, thus imaging–pathology discordance could not be a predictive factor for the decision to perform surgical excision.
There are still several limitations to our study. One limitation is that we did not include lesions that were subjected to a stereotactic biopsy, as mentioned above. A second limitation is that the biopsy was a 14-gauge core needle biopsy in all cases. Liberman et al. reviewed this issue and found no statistical difference between the use of 11-gauge and 14-gauge core biopsies [14], and Jackman et al. also demonstrated that the size of a core biopsy could not predict benignity [20]. However, a prospective analysis of large-core biopsies is still needed.
In conclusion, 4% of benign papillomas diagnosed after a core needle biopsy were associated with malignancy following surgical excision. The mean lesion size was significantly larger for lesions upgraded to malignancy, and we recommend excision of 14-gauge core needle biopsy-proven benign papillary lesions of the breast, especially for lesions larger than 1.5 cm.
References
Liberman L (2000) Clinical management issues in percutaneous core breast biopsy. Radiol Clin North Am 38:791–807
Tavassoli F (2003) Intraductal papillary neoplasms. In: Tavassoli FA, Young RH, Stratton MR (eds) WHO classification of tumours pathology and genetics of tumours of the breast and female genital organs. IARC, Lyon, pp 76–80
Tavassoli FA (1992) Papillary lesions. In: Tavassoli FA (ed) Pathology of the breast. Appleton & Lange, Norwalk, Conn, pp 193–227
Mercado CL, Hamele-Bena D, Singer C, Koenigsberg T, Pile-Spellman E, Higgins H, Smith SJ (2001) Papillary lesions of the breast: evaluation with stereotactic directional vacuum assisted biopsy. Radiology 221:650–655
Reynolds HE (2000) Core needle biopsy of challenging benign breast conditions: a comprehensive literature review. AJR Am J Roentgenol 174:1245–1250
Ivan D, Selinko V, Sahin AA, Sneige N, Middleton LP (2004) Accuracy of core needle biopsy diagnosis in assessing papillary breast lesions: histologic predictors of malignancy. Mod Pathol 17:165–171
Agoff SN, Lawton TJ (2004) Papillary lesions of the breast with and without atypical ductal hyperplasia: can we accurately predict benign behavior from core needle biopsy? Am J Clin Pathol 122:440–443
American College of Radiology (2003) Breast imaging reporting and data system—mammography (BI-RADS™), 4th edn. American College of Radiology, Reston
American College of Radiology (2003) Breast imaging reporting and data system—ultrasound (BI-RADS™), 4th edn. American College of Radiology, Reston
Heinze G, Schemper M (2002) A solution to the problem of separation in logistic regression. Stat Med 21:2409–2419
Heinze G, Ploner M (2003) Fixing the nonconvergence bug in logistic regression with SPLUS and SAS. Comput Methods Programs Biomed 71:181–187
Valdes EK, Feldman SM, Boolbol SK (2007) Papillary lesions: a review of the literature. Ann Surg Oncol 14:1009–1013
Sydnor MK, Wilson JD, Hijaz TA, Massey HD, Shaw de Paredes ES (2007) Underestimation of the presence of breast carcinoma in papillary lesions initially diagnosed at core-needle biopsy. Radiology 242:58–62
Liberman L, Tornos C, Huzjan R, Bartella L, Morris EA, Dershaw DD (2006) Is surgical excision warranted after benign, concordant diagnosis of papilloma at percutaneous breast biopsy? AJR Am J Roentgenol 186:1328–1334
Mercado CL, Hamele-Bena D, Oken SM, Singer CI, Cangiarella J (2006) Papillary lesions of the breast at percutaneous core-needle biopsy. Radiology 238:801–808
Ganesan S, Karthik G, Joshi M, Damodaran V (2006) Ultrasound spectrum in intraductal papillary neoplasms of breast. Br J Radiol 79:843–849
Lam W, Chu W, Tang A, Tse G, Ma T (2006) Role of radiologic features in the management of papillary lesions of the breast. AJR Am J Roentgenol 186:1322–1327
Jacobs TW, Connolly JL, Schnitt SJ (2002) Nonmalignant lesions in breast core needle biopsies: to excise or not to excise? Am J Surg Pathol 26:1095–1110
Page DL, Salhany KE, Jensen RA, Dupont WD (1996) Subsequent breast carcinoma risk after biopsy with atypia in breast papilloma. Cancer 78:258–266
Jackman RJ, Birdwell RL, Ikeda DM (2002) Atypical ductal hyperplasia: can some lesions be defined as probably benign after stereotactic 11-gauge vacuum-assisted biopsy, eliminating the recommendation for surgical excision? Radiology 224:548–554
Acknowledgements
This study was supported by a grant (A070001) from the Korea Healthcare Technology R and D Project, and a grant (A01185) from the National R&D Program for Cancer Control, Ministry for Health, Welfare and Family Affairs, Republic of Korea.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chang, J.M., Moon, W.K., Cho, N. et al. Risk of carcinoma after subsequent excision of benign papilloma initially diagnosed with an ultrasound (US)-guided 14-gauge core needle biopsy: a prospective observational study. Eur Radiol 20, 1093–1100 (2010). https://doi.org/10.1007/s00330-009-1649-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-009-1649-2