Abstract
Objectives
Three-dimensional (3-D) selective- and relative-scale texture analysis (TA) was applied to structural magnetic resonance (MR) brain images to quantify the presence of grey-matter (GM) and white-matter (WM) textural abnormalities associated with schizophrenia.
Materials and methods
Brain TA comprised volume filtration using the Laplacian of Gaussian filter to highlight fine, medium and coarse textures within GM and WM, followed by texture quantification. Relative TA (e.g. ratio of fine to medium) was also computed. T1-weighted MR whole-brain images from 32 participants with diagnosis of schizophrenia (n = 10) and healthy controls (n = 22) were examined. Five patients possessed marker alleles (SZ8) associated with schizophrenia on chromosome 8 in the pericentriolar material 1 gene while the remaining five had not inherited any of the alleles (SZ0).
Results
Filtered fine GM texture (mean grey-level intensity; MGI) most significantly differentiated schizophrenic patients from controls (P = 0.0058; area under the receiver-operating characteristic curve = 0.809, sensitivity = 90%, specificity = 70%). WM measurements did not distinguish the two groups. Filtered GM and WM textures (MGI) correlated with total GM and WM volume respectively. Medium-to-coarse GM entropy distinguished SZ0 from controls (P = 0.0069) while measures from SZ8 were intermediate between the two.
Conclusions
3-D TA of brain MR enables detection of subtle distributed morphological features associated with schizophrenia, determined partly by susceptibility genes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Magnetic resonance imaging (MRI) of brain may lead to the identification of imaging biomarkers to permit early confident diagnosis, to inform treatment and to track illness progression in neurological and psychiatric disorders. However, in common psychiatric conditions such as schizophrenia, neuroimaging largely remains a research tool, rather than a clinically useful tool [1]. Nevertheless, depletion of grey matter (with some regions affected more than others) is reliably observed in brains of schizophrenic patients [2–9]; these changes lead to ventricular enlargement and an overall general reduction in whole brain tissue [10]. Different patterns of grey-matter deficits are associated with different schizophrenic symptom clusters, thereby linking phenotypical expressions of mental phenomena to distinct neuroanatomical substrates [11]. In other neuropsychiatric disorders, unbiased computer-driven approaches have been applied to MR image analysis to quantify, detect and characterize clinical groups [12–17]. Among these, uninformed (black-box) classification methods show promise in distinguishing diagnostic subgroups by combinations of measured parameters, but the abstract nature of the classification relative to biologically plausible features is often dissatisfying for understanding the underlying patho-aetiology.
In this preliminary study, we employ a novel volumetric selective- and relative-scale texture analysis (TA) for assessment of MR images to define abnormalities in brain texture (that may reflect distributed or diffuse neuropathology) in schizophrenic patients relative to controls. We also make an initial assessment of whether the presence of genetic markers of schizophrenia influences these textural measures.
Materials and methods
Participants
We studied MR examinations of 10 patients with clinical diagnosis (DSMIV-R 295.30) of paranoid schizophrenia (SZ) and 22 matched healthy controls, representing a subset of participants in an earlier structural neuroimaging study [18] whose data were available for reanalysis. This study was approved by the local Research Ethics Committee with each participant providing informed written consent that allowed for retrospective data analysis. Five of the schizophrenic patients also were shown to possess marker alleles associated with schizophrenia on chromosome 8 in the region of the PMC1 gene (SZ8; mean ± SD age, 38.8 ± 5.5 years; 2 M and 3 F), while the remaining five patients had not inherited any of these chromosome 8 alleles (SZ0; 40.6 ± 10.5 years; 4 M and 1 F). Schizophrenic symptoms were controlled by antipsychotic drugs, permitting full capacity to participate in consent and study procedures. The mean duration of illness was 20 ± 6.0 years. Each patient was unemployed at the time of MRI, and there was no significant difference in drug history between SZ8 and SZ0 patients in this study. Healthy controls were broadly matched to the patients by age, gender, social class and ethnicity. Controls were screened to exclude medication use and psychological, neurological or systemic illness but were not genotyped (40.3 ± 10.8 years; 14 M and 8 F).
Image acquisition and pre-processing
All participants underwent MR at the Wellcome Department of Imaging Neuroscience (Wellcome Trust Centre for Neuroimaging) using a 3D-MPRAGE sequence based on linear and centric phase encoding with a 1-mm3 resolution for enhanced T1 tissue contrast at 2 Tesla (Magnetom, Siemens AG, Munich, Germany) [19]. Each MR examination lasted for around 45 min with the 3D-MPRAGE MR sequence amounting to 12 min, 32 s. MR images were pre-processed using components of the statistical parametric mapping software SPM2 (http://www.fil.ion.ucl.ac.uk/spm/) on a MATLAB platform (Mathworks, Natick, MA, USA), carried out as follows: The raw structural MRI images were re-orientated and re-sliced to obtain a final voxel size of 1 × 1 × 1 mm3. A fully automated brain extraction technique was employed to remove non-brain tissue from the original structural MRIs [20]. The re-sliced extracted volumes were optimally segmented into grey and white matter and CSF and non-CSF partitions. The segmented data were not smoothed in this study as part of image pre-processing. Global measures of grey- and white-matter volumes were calculated (in millilitres) from segmented images to test for differences between schizophrenic patients and controls.
Texture analysis
Segmented grey- and white-matter MRI images of each patient were analyzed individually using a dedicated program written in MATLAB. This specific application of 3D texture analysis extended methodology initially developed by Ganeshan et al. [21] to analyze textural properties of tissues examined by medical imaging techniques, e.g. in studies of pulmonary disorders using computed tomography pulmonary angiography (CTPA) lung images. The texture analysis (TA) comprised two stages:
-
1.
Volume filtration is accomplished by using three-dimensional (3-D) Laplacian of Gaussian (LoG) filter to process the MR image data differentially into fine to coarse textures.
-
2.
Quantification of texture using mean grey-level intensity, entropy and uniformity was performed on the filtered and unfiltered MRI segmented brain volumes.
Generating isometric volume
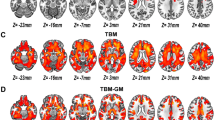
The 3-D LoG filter is a spherically symmetrical isotropic filter, the implementation of which required all the brain volume datasets to be isometric in physical size and sampling rate to highlight only those features in the brain that match the filter tuning parameter (σ). The voxel size of the segmented brain datasets was 1 × 1 × 1 mm3, i.e. with uniform isometric sampling in all the three axes. Each MRI brain image was acquired with 176 slices in the z direction and 256 × 256 pixels in x and y directions, i.e. equivalent to 256 × 256 × 176 voxels). Therefore zero-padding the brain volume only in the z plane achieved a uniform volume of 256 × 256 × 256 voxels necessary prior to 3-D LoG filtration. Texture extraction using 3-D LoG filtration carried out as described by Ganeshan et al. [21] resulted in deriving brain texture features of different scales or sizes corresponding to filter σ values: fine (σ = 0.5, 2 voxels, filter width = 2.0 mm), medium (σ = 1.0, 4 voxels, filter width = 4.0 mm) and coarse (σ = 1.5, 6 voxels, filter width = 6.0 mm). Figures 1 and 2 display the 3-D volume-rendered conventional and filtered (fine, medium and coarse) images of whole-brain grey and white matter respectively.
Quantification of texture
In evaluating the 3-D texture, with or without volume filtration, we considered the following statistical and spectral parameters: (1) mean grey-level intensity, (2) entropy, and (3) uniformity, which have been successfully employed previously in an earlier 3-D texture analysis study [21]. Entropy reflects irregularity, whereas uniformity indicates the distribution of grey levels within a volume. These parameters are defined below:
where N is the total number of voxels in the volume of interest V within the entire volume a(x,y,z), l is the grey level (for example l = 1 to k indicates grey level from 1 to k) in V and p(l) is the probability of the occurrence of the grey level l in V. Without filtration, the above three parameters are descriptors of intensity distribution, and with the application of the LoG filter, these parameters are descriptors for the distribution of gradient magnitudes (at different texture scales). These parameters represent quantitative metrics of features perceived visually as texture.
In addition to quantification of fine, medium and coarse textures using different band-pass regions of the filter, we computed ratios of texture parameters, i.e. fine-to-medium, fine-to-coarse and medium-to-coarse texture ratios. Texture ratios were used to minimize the effects of incidental variations in absolute MR signal intensity values occurring from one participant to another and also to reduce the effect of noise on texture quantification.
Statistical analysis
Statistical Mann Whitney test evaluated the changes in age, gender, brain texture and volume associated with schizophrenia (P < 0.05). The receiver-operating characteristics (ROC), including area under the curve, its significance, diagnostic threshold, sensitivity and specificity values were quantified for the parameter demonstrating the most significant discriminant value. For the above-identified significant parameters, differences among controls, SZ8 and SZ0 were also tested. Linear regression analyses were also used to assess relations between brain texture and volume.
Results
Grey-matter texture
Patients with schizophrenia (SZ) showed significantly lower total grey-matter volume than controls (P = 0.0088). Furthermore patients were most significantly differentiated from controls by selective-scale filtered fine texture analysis (mean grey-level intensity, P = 0.0058, Table 1). Differences were also apparent in brain texture analysis without filtration (mean grey-level intensity, P = 0.0083) and in the ratio of medium-to-coarse textures, where group differences were observed for all the three texture parameters. For this ratio, entropy showed the greatest degree of differentiation (entropy, P = 0.0114) compared to mean grey-level intensity and uniformity (Table 1). A fine mean grey-level intensity value exceeding 154.42 predicted patients with schizophrenia (sensitivity = 90%, specificity = 70%, area under the ROC curve = 0.809, P = 0.0007; Table 2). Texture features showed some association with measures of total grey-matter volume; notably fine, medium and the ratio of medium-to-coarse grey-level intensities correlated with raw grey-matter volume. Texture without filtration showed no association with volume (Table 3).
Together the significant grey-matter texture measurements robustly distinguished the SZ0 patient group from controls (Table 4). Texture without filtration (mean grey-level intensity) and the ratio of medium-to-coarse texture (entropy) demonstrated greater degrees of differences than the rest. Differences from controls in these same measurements were attenuated in the SZ8 patients, such that there was no significant differences between the SZ8 group and either the SZ0 group or controls.
White-matter texture
None of the white-matter measurements (volume or texture) significantly differentiated patients with schizophrenia (SZ8 and/or SZ0) from controls. However, there was some correlation between volume and texture (intensity measurements), whereas texture without filtration showed no association (Table 3).
Discussion
Our study demonstrates the application of 3-D texture analysis of whole-brain MRI-T1 images to detect subtle differences in grey-matter texture between schizophrenic patients and healthy controls. Our data also provide tentative evidence for genetic influences on grey-matter texture, indicated by a relative attenuation of differences in those schizophrenic patients with vulnerability alleles on chromosome 8. Interestingly no effect was apparent for white-matter texture across the brain. Our 3-D whole-brain texture analysis represents a sensitive quantitative index of differences in neural integrity associated with schizophrenia, suggesting the potential of this technique as a diagnostic tool for the assessment of patients with psychiatric symptoms.
Technically, the accuracy of TA with filtration for discriminating between schizophrenic and control groups was slightly better than that provided by texture quantification without filtration and also measurement of total grey-matter volume. This filtering, similar to a non-orthogonal wavelet approach uses a circularly symmetric isotropic filter (LoG) to enhance features corresponding to a particular scale and variation in signal intensity within the MR volume. The biological validity of the TA approach is reflected in observed correlations between texture and raw grey- and white-matter volumes. By using volume filtration, we were able to adopt first-order statistical texture quantification which benefits from being directionally independent and has the advantage of being less computationally expensive. Additionally this selective- and relative-scale texture (using the ratio of different scales of textures is also likely to be generally robust across different centres using differen MR systems) quantification approach also benefits from the lack of a priori nature of the mathematical model and shape description of local structure boundaries.
Speculatively, intensity differences and inhomogeneity in fine grey-matter texture may reflect the consequences of the cortical thickness of histological perturbations, for example abnormal pyramidal cell clustering [22, 23], synaptic pruning [24] and cell migration [25], within cortical layers reported in the brains of schizophrenic patients. Similarly, textural measures over other distinct spatial frequencies may be tuned to abnormalities in myelination [26, 27] and gyrification. Gyrification arises from the neuro-developmental expansion of the cortical mantle and histological maturation of cortical layers and connecting white-matter tracts. The thickness of the human cerebral cortex (highly folded sheet of neurons) ranges from 1 to 4.5 mm, with an average of 2.5 mm [28, 29]. The degree of folding can be computed as a gyrification index (GI), i.e. the ratio of the entire cortical or inner contour of the brain to the superficially exposed or outer contour [30]. GI measurement is used in the assessment of both normal brain development and the progression of neurodegenerative and psychiatric disorders [31], particularly in SZ employed as risk-stratification [32], reflecting hypogyria [33, 34] or hypergyria [34–36]. However, the GI-assessing methodologies are often complex or labor intensive [30, 31]. Filter widths used in this study correspond to diametrically opposite boundary points of textural features and may also be sensitive to specific regional variations in gyral folding and cortical thickness (measured as the distance between grey/white matter and pial surfaces). Fine texture differences (2 mm width) may reflect differences within sensory areas, medium texture (4 mm width) may correspond to the fundi of sulci and/or less prominent crowns of gyri, while the coarse texture (filter width 6 mm) may correspond to prominent crowns of gyri [31]. Relative texture, in particular medium-to-coarse texture ratio (entropy), is likely to track indirectly the more established GI measure (see Figs. 1 and 2).
An intriguing aspect of our study was the preliminary evidence for genetic influences on brain texture. A preliminary genetic-neuroimaging relationship has also been observed in small samples of individuals with intermediate phenotypes whose neuropsychological profile does not necessarily differ from those of healthy controls [37]. In our study, texture differences were amplified in SZ0 patients (particularly for entropy of medium-to-coarse texture) relative to SZ8 patients. A previous study has shown enhanced grey-matter depletion across temporal lobe sensory and association cortices in SZ0 patients, whereas SZ8 patients showed greater medial orbitofrontal cortex involvement [18]. Thus differences in local expression of grey-matter involvement may be expressed regionally as differences in textural features. Clinically the expression of schizophrenia in SZ8 patients is characterized by affective deterioration, poor prognosis, increased thought disorder and less depressive symptoms compared to SZ0 [38]. It is possible that these texture results may be employed within clinical practice to identify sub-groups of SZ for intensive follow-up or tailored treatment.
Nevertheless, further work is needed to determine the precise relationship between quantifiable grey-matter texture features from MR data and tissue-level and genetic signatures of neuro-developmental neuropathology. Also the effects of pre-processing techniques (smoothing, normalisation) on TA and the requirement for further refinement of the methodology need to be ascertained. This study lacked a repeatability analysis to test the preciseness of our texture features due to the availability of only image data from part of an earlier neuroimaging study. Future work will also focus on generating statistical parametric texture maps to identify brain areas responsible for texture alterations observed in our study, since this may add further power in the characterization of neuropsychiatric disorders.
Conclusions
This study provides initial evidence about the use of selective- and relative-scale texture analysis of structural MRI data in detecting schizophrenic patients from controls and indicates a texture-genetic relationship. Our findings naturally require replication in larger groups of patients with schizophrenia to establish further the predictive value of our methodology.
References
Woolley J, McGuire P (2005) Neuroimaging in schizophrenia: what does it tell the clinician? Adv Psychiatr Treat 11:195–202
Weinberger DR, Torrey EF, Neophytides AN, Wyatt RJ (1979) Structural abnormalities in the cerebral cortex of chronic schizophrenic patients. Arch Gen Psychiatr 36:935–939
Johnstone EC, Crow TJ, Frith CD et al (1976) Cerebral ventricular size and cognitive impairment in chronic schizophrenia. Lancet 2:924–926
Harvey I, Ron MA, Du Boulay G et al (1993) Reduction of cortical volume in schizophrenia on magnetic resonance imaging. Psychol Med 23:591–604
Lim KO, Tew W, Kushner M et al (1996) Cortical grey matter volume deficit in patients with first-episode schizophrenia. Am J Psychiatr 153:1548–1553
Wright IC, Rabe-Hesketh S, Woodruff PW et al (2000) Meta-analysis of regional brain volumes in schizophrenia. Am J Psychiatr 157:16–25
Nelson MD, Saykin AJ, Flashman LA et al (1998) Hippocampal volume reduction in schizophrenia as assessed by magnetic resonance imaging: a meta-analytic study. Arch Gen Psychiatr 55:433–440
Gur RE, Maany V, Mozley PD et al (1998) Subcortical MRI volumes in neuroleptic-naive and treated patients with schizophrenia. Am J Psychiatr 155:1711–1717
Lawrie SM, McIntosh AM, Hall J et al (2008) Brain structure and function changes during the development of schizophrenia: the evidence from studies of subjects at increased genetic risk. Schizophr Bull 34:330–340
Lawrie SM, Abukmeil SS (1998) Brain abnormality in schizophrenia. A systematic and quantitative review of volumetric magnetic resonance imaging studies. Br J Psychiatr 160:179–186
Liddle PF (1987) Schizophrenic syndromes, cognitive performance and neurological dysfunction. Psychol Med 17:49–57
Lerski R (2006) Clinical applications of texture analysis. In: Hajek M, Dezortova M, Materka A, Lerski R (eds) Texture analysis for magnetic resonance imaging. Med4publishing, Prague, pp 151–187
Kloppel S, Stonnington CM et al (2008) Automatic classification of MR scans in Alzheimer’s disease. Brain 131:681–689
Freeborough PA, Fox NC (1998) MR image texture analysis applied to the diagnosis and tracking of Alzheimer’s disease. IEEE Trans Med Imag 17:475–479
Liu Y, Teverovskiy L, Carmichael O et al (2004) Discriminative MR image feature analysis for automatic schizophrenia and Alzheimer’s disease classification. Technical report CMU-RI-TR-04-15. The Robotics Institute, Carnegie Mellon University, Pittsburgh
Kovalev VA, Petrou M, Suckling J (2003) Detection of structural differences between the brains of schizophrenic patients and controls. Psychiatr Res 124:177–189
Im K, Lee JM et al (2006) Fractal dimension in human cortical surface: multiple regression analysis with cortical thickness, sulcal depth, and folding area. Hum Brain Mapp 27:994–1003
Gurling H, Critchley H, Datta SR et al (2006) Genetic association and brain morphology studies and the chromosome 8p22 pericentriolar material 1 (PCM1) gene in susceptibility to schizophrenia. Arch Gen Psychiatr 63:844–854
Deichmann R, Good CD, Josephs O, Ashburner J, Turner R (2000) Optimization of 3-D MP-RAGE sequences for structural brain imaging. NeuroImage 12:112–127
Ashburner J, Friston KJ (2000) Voxel-based morphometry: the methods. NeuroImage 11:805–821
Ganeshan B, Miles KA, Young RCD, Chatwin CR (2008) Three dimensional selective-scale texture analysis of CT pulmonary angiograms. Invest Radiol 43:382–394
Jonsson SA, Luts A, Guldberg-Kjaer N, Ohman R (1999) Pyramidal neuron size in the hippocampus of schizophrenics correlates with total cell count and degree of cell disarray. Eur Arch Psychiatr Clin Neurosci 249:169–173
Casanova MF, Rothberg B (2002) Shape distortion of the hippocampus: a possible explanation of the pyramidal cell disarray reported in schizophrenia. Schizophr Res 55:19–24
Roberts RC, Roche JK, Conley RR (2005) Synaptic differences in the postmortem striatum of subjects with schizophrenia: a stereological ultrastructural analysis. Synapse 56:185–197
Tabarés-Seisdedos R, Escámez T et al (2006) Variations in genes regulating neuronal migration predict reduced prefrontal cognition in schizophrenia and bipolar subjects from Mediterranean Spain: a preliminary study. Neuroscience 139:1289–1300
Haroutunian V, Davis KL (2007) Introduction to the special section: myelin and oligodendrocyte abnormalities in schizophrenia. Int J Neuropsychopharmacol 10:499–502
Konrad A, Winterer G (2008) Disturbed structural connectivity in schizophrenia sprimary factor in pathology or epiphenomenon? Schizophr Bull 34:72–92
Zilles K (1990) In: Paxinos G (ed) The human nervous system. Academic, San Diego, pp 757–802
Von Economo C (1929) The cytoarchitectonics of the human cerebral cortex. Oxford University Press, London
Moorhead TWJ, Harris JM et al (2006) Automated computation of the gyrification index in prefrontal lobes: methods and comparison with manual implementation. NeuroImage 31:1560–1566
Fischl B, Dale AM (2000) Measuring the thickness of the human cerebral cortex from magnetic resonance images. PNAS 97:11050–11055
Harris JM, Yates S et al (2004) Gyrification in first-episode schizophrenia: a morphometric study. Biol Psychiatr 55:141–147
Kulynych JJ, Luevano LF et al (1997) Cortical abnormality in schizophrenia: an in vivo application of the gyrification index. Biol Psychiatr 41:995–999
Sallet PC, Elkis H et al (2003) Reduced cortical folding in schizophrenia: an MRI morphometric study. Am J Psychiatr 160:1606–1613
Vogeley K, Tepest R et al (2001) Right frontal hypergyria differentiation in affected and unaffected siblings from families multiply affected with schizophrenia: a morphometric MRI study. Am J Psychiatr 158:494–496
Harris JM, Whalley H et al (2004) Abnormal cortical folding in high risk individuals: a predictor of the development of schizophrenia? Biol Psychiatr 56:182–189
Hariri AR, Weinberger DR (2003) Imaging genomics. Br Med Bull 65:259–270
Kendler KS, Myers JM et al (2000) Clinical features of schizophrenia and linkage to chromosomes 5q, 6p, 8p, and 10p in the Irish study of high density schizophrenia families. Am J Psychiatr 157:402–408
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ganeshan, B., Miles, K.A., Young, R.C.D. et al. Three-dimensional textural analysis of brain images reveals distributed grey-matter abnormalities in schizophrenia. Eur Radiol 20, 941–948 (2010). https://doi.org/10.1007/s00330-009-1605-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-009-1605-1