Abstract
Objective
To evaluate the role of mean diffusivity (MD) as a predictive index of the response to chemotherapy in locally advanced breast cancer.
Methods
Twenty-one women referred to our institution with a diagnosis of locally advanced breast cancer underwent magnetic resonance imaging (MRI) studies at 1.5 T before beginning and after completing combined neoadjuvant chemotherapy. The examination protocol included an EPI sequence sensitised to diffusion (b-value 1,000 s/mm2) and three-dimensional (3D) coronal T1 sequences before and after intravenous contrast medium. Tumours were delineated by using dynamic MR acquisition before and after chemotherapy. The percentage of tumour volume reduction (PVR) and pre-(MDpre) and post-therapy (MDpost) MD values were computed for each lesion.
Results
PVR ≥ 65% was observed in 17/21 patients (responders). MDpre of responders (0.99 ± 0.27 10−3 mm2/s) was significantly (p = 0.025) lower than MDpre of non-responders (1.46 ± 0.33 10−3 mm2/s). Moreover, in patients as a whole PVR significantly correlated (p = 0.01, r = −0.54) with MDpre. MDpost (1.26 ± 0.39 10−3 mm2/s) of responders was significantly(p = 0.024) higher than MDpre (0.99 ± 0.27 mm2 10−3 mm2/s), whereas non-responders MDpost (1.00 ± 0.14 10−3 mm2/s)did not increase compared with MDpre (1.46 ± 0.33 10−3 mm2/s).
Conclusions
This preliminary study seems to indicate that low values of pre-chemotherapy MD may identify, before starting treatment, the patients with higher probability of response in terms of percentage of volume reduction of the lesion. MD may represent a complementary parameter useful to correctly select patients for neoadjuvant chemotherapy.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Locally advanced breast cancer includes large primary tumours (>5 cm in diameter), tumours of any size involving the skin and/or the chest wall, tumours with fixed or matted axillary lymph nodes (T3/T4, N2) and those that involve the ipsilateral subclavicular and supraclavicular lymph nodes [1]. These patients usually require neoadjuvant combined chemotherapy to down-stage the disease before surgical treatment. The early knowledge of response to neoadjuvant therapy has important consequences for optimal management, avoiding the continuation of toxic therapy in non-responding patients. In this regard, magnetic resonance imaging (MRI) is considered the best choice in evaluating the tumour and its response to the administered treatment, because of its higher accuracy compared with traditional methods of physical examination and mammography [2].
Diffusion-weighted (DW)-MRI is a technique sensitised to the diffusive properties of water molecules and it allows non-invasive in vivo measurements of diffusion [3]. Clinical applications of DW imaging arise from the principle that, during their random diffusion-driven displacements, water molecules probe tissue structures at a microscopic scale that is well beyond the usual image resolution [4]. Thus, DW-MRI can be used to characterise the ultra-structural properties and integrity of tissues [5]. DW-MRI has also proved to be useful in studying breast lesions with a potential application to distinguish benign lesions from malignant lesions [6–8]. Indeed, previous studies have shown that lower diffusion values of malignant lesions compared with benign ones and normal breast tissue are because of the increased cellularity of the malignant lesions [9, 10].
Recent investigations have suggested there is a possible role of quantitative DW-MRI in assessing the early response of cancers to neoadjuvant chemotherapy [11, 12]. In particular, DW-MRI seems to play a role in detecting early responses to chemotherapy in locally advanced breast cancer. DW-MRI, in fact, shows increased diffusion values due to cellular necrosis just after the first or second cycle of therapy treatment, before observing any reduction in the volume of the tumour [13–17].
Based on the close dependence of diffusion imaging on the organisation of tissues at microscopic level and, considering that the distribution of chemotherapeutic agents used is likely to be more effective in highly cellulated lesions than in tumours with large necrotic areas, we hypothesised that DW-MRI could be employed also to collect potential information in predicting the response to chemotherapy. Thus, in this preliminary study we enrolled a group of patients with locally advanced breast cancer to investigate the role of quantitative diffusion measurements as a predictive index of response to chemotherapy.
Materials and methods
Patients
From December 2005 to September 2006 the breast oncology team (breast imaging radiologists, oncologists and/or breast surgeons) in our institution enrolled 45 consecutive women with a microhistologically proven diagnosis of locally advanced breast cancer for breast MRI at baseline and at the end of neoadjuvant chemotherapy. None of the patients had been treated with either hormone, chemotherapy or radiotherapy before the first MR examination. Written informed consent was obtained from all patients. The women underwent MRI studies both before the beginning and after completing the same cycles of combined neoadjuvant therapy: three cycles of FEC (5FU, epirubicin, cyclophosphamide) all drugs given on the 1st and 8th day of a 28-day cycle before surgery.
The patients underwent also clinical evaluation, mammography and ultrasonography before, during treatment and at the end of neoadjuvant chemotherapy.
MRI study and image processing
All acquisitions were performed by using a 1.5-T MR system (Magnetom Symphony, Siemens Medical Systems, Erlangen, Germany) with a maximum gradient strength of 30 mT/m and a slew rate of 75 (T·m−1)/s. A dedicated breast coil was used for radiofrequency reception of the MR signal. The conventional MRI protocol in our institution included a fast spin echo T2-weighted sequence in the sagittal plane (repetition time/echo time, 2,690 ms/79 ms; field of view, 300 × 177 mm; section thickness, 4 mm; intersection gap, 0.4 mm; matrix, 256 × 134; number of signals acquired, 1) and a pre-contrast and post-contrast fat-saturated three-dimensional (3D) T1-weighted sequence in the coronal plane (repetition time/echo time, 11 ms/4.8 ms; field of view, 350 × 203 mm; slab thickness, 151 mm; section thickness, 2.1 mm; intersection gap, 0.4 mm; matrix, 384 × 180; number of signals acquired, 1). A gadolinium chelate contrast medium was injected intravenously at a dose of 0.1 mmol/kg at a velocity rate of 2 ml/s using a power injector followed by a flush of saline solution.
DW images with b-value = 1,000 s/mm2 and one diffusion un-weighted image (b-value = 0 s/mm2) were obtained before conventional MRI with a spin echo EPI sequence in the coronal and axial plane (repetition time/echo time, 2,690 ms/79 ms; field of view, 300 × 177 mm; section thickness, 4 mm; intersection gap, 0.4 mm; matrix, 256 × 134; number of signals acquired, 1). The diffusion gradient was applied along the principal directions (x, y and z). Apparent diffusion coefficient (ADC) values along the principal directions were estimated voxelwise as:
where Sb=1000 and Sb=0 are respectively the signal of diffusion weighted and diffusion un-weighted image. Then, mean diffusivity (MD) maps were computed as:
The diffusion in normal breast tissue is isotropic and there is not evidence that the diffusion of breast lesion is anisotropic. However, in our study we preferred to quantify diffusion by means of MD instead of ADC. Indeed, in isotropic tissue MD and ADC values are the same but ADC is not suitable for characterising diffusion in anisotropic tissue since it depends on direction. On the other hand, MD, which is proportional to the trace of diffusion tensor when cross terms of diffusion gradients can be neglected, can quantify diffusion in isotropic and anisotropic tissue independently on direction.
All MR images were analysed by a radiologist with 10 years’ experience in breast imaging. Tumour volume was calculated by means of the dynamic MR study, summing the area of suspicious contrast uptake [18] in each image multiplied by slice thickness. In MD map a region of interest (ROI) was traced and the average ROI value of the breast lesion was measured. In order to improve the reliability and reproducibility of diffusion measurements, the ROI was manually drawn within the tumour reducing partial volume effects. This procedure was performed for each slice where the tumour was present and the mean value quantified the MD of the entire lesion.
These measurements were performed both before and after the neoadjuvant chemotherapy.
Clinical and MRI data analysis
According to Pickles et al. [19] and Partridge et al. [20] we classified as “responders” (R) the patients with a percentage of tumour volume reduction at the end of chemotherapy (PVR) greater than 65% and as “non-responders” (NR) the patients with PVR less than 65%.
To assess the reliability of the MRI volume measurement, any linear correlation (Pearson test) between the largest diameter of the tumour measured by MRI at the end of chemotherapy and the largest diameter of residual tumour measured by pathology was evaluated [2].
Differences between MD values pre-chemotherapy (MDpre) between R and NR patients were assessed with a non-parametric two-tailed Mann-Whitney test. Any correlation between PVR and MDpre was investigated using Spearman’s rank correlation test in the group of patients as a whole. Moreover, MD values pre-chemotherapy and MD values post-chemotherapy (MDpost) in R and NR patients were compared using the two tailed Mann-Whitney test.
Results
Only 21 (age range: 39–68 years; mean age: 50 years) out of 45 women with locally advanced breast cancer completed the study. We were unable to follow-up on 24 patients: two patients decided immediately to undergo surgical treatment, while the other 22 patients reported logistic and technical problems. In particular, nine patients did not perform the planned MRI exam because they had difficulty getting to the exam centre; likewise, four patients performed the first MRI exam but they did not perform the second MRI exam. Five patients suffered from claustrophobia, so it was not possible to complete the MRI acquisition. Finally, in four women we observed important motion artefacts due to lack of compliance to the first MRI exam and they were excluded from the study.
Definitive histology resulted in invasive ductal cancer (IDC) in 15 patients, mucinous cancer in one patient, invasive lobular cancer (CLI) in two patients and IDC with in situ ductal cancer (DCIS) in three patients. The median initial tumour size on dynamic MRI was 4.1 cm (range 2.8–10 cm) in maximum diameter. The tumoural volume measured before neoadjuvant treatment ranged from 11 to 322 cm3 (mean volume 133 cm3, standard deviation 132 cm3). Nineteen out of 21 patients showed residual tumour after chemotherapy (range 0.3–160 cm3, mean volume 26 cm3, standard deviation 32 cm3). The largest diameter of tumour measured by MRI at the end of chemotherapy significantly (p < 0.001) correlated with the largest diameter of residual tumour measured by pathology.
PVR ≥ 65% was observed in 17/21 patients (responders), including two complete responses to treatment without any enhancement at dynamic MRI before surgery and without residual tumour at pathology. We were able to measure MD in all patients before and after treatment, excluding the measurements after chemotherapy in the two women having complete response.
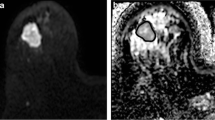
MDpre of responders (0.99 ± 0.27 10−3 mm2/s) was significantly (p = 0.025) lower than MDpre of non-responders (1.46 ± 0.33 10−3 mm2/s) (Figs. 1, 2, 3). Moreover, in the group of patients as a whole (responders and non-responders) PVR significantly correlated (p = 0.01, r = −0.54) with MDpre (Fig. 4). In responders (Fig. 5) MDpost (1.26 ± 0.39 10−3 mm2/s) was significantly (p = 0.024) higher than MDpre (0.99 ± 0.27 10−3 mm2/s) whereas in the small group of non-responders (Fig. 6) MDpost (1.00 ± 0.14 10−3 mm2/s) decreased with respect to MDpre (1.46 ± 0.33 10−3 mm2/s) but not significantly (p > 0.05).
Discussion
In the present study we investigated whether quantitative diffusion measurements of MD could represent a predictive index of the response to chemotherapy in locally advanced breast cancer. The main finding of our study shows that MD pre-chemotherapy values were significantly lower in responders than in non-responders. The lower baseline MD values of responders could be due to the more packed cellularity of these neoplasms with less micro- and macroscopic necrosis. Thus, chemotherapy could reach a large number of cells and actively affect them, suggesting a better response to chemotherapy in patients with lower MDpre values. We emphasise that this hypothesis is corroborated by the significant negative correlation between MD pre-chemotherapy and the percentage of tumour volume reduction in patients as a whole.
The early knowledge of response to neoadjuvant chemotherapy in patients with locally advanced breast cancer is a fundamental element. To the best of our knowledge, this is the first study which indicates that MD measurements could represent, besides a way for monitoring early response during treatment as previously reported [13], also a predictive index of response to chemotherapy in locally advanced breast cancer. Therefore, the measurement of MDpre could help clinicians to identify patients potentially suitable for neoadjuvant chemotherapy.
Since any changes in tumour size represent the parameter usually employed for monitoring tumour response from the oncological point of view, in the present study the PVR was used to evaluate the response to neoadjuvant chemotherapy. The reliability of volume measurements on MRI was confirmed by the significant linear correlation between the largest diameter of tumour measured by MRI at the end of chemotherapy and the largest diameter of residual tumour measured by pathology.
The accuracy of volume and diffusion measurements may become difficult to assess heterogeneous tumours without a clear delineation, such as that depicted in Fig. 2, or tumours with linear/dendritic neoplastic distribution and multicentricity with lesions smaller than resolution of MD maps, although they were not observed in our patients. This could be a limit of MD measurements, especially in monitoring treatment in cases of irregular tumour fragmentation after chemotherapy with small and multifocal neoplastic residuals. Besides, we recognise as a limitation of our study the relatively low number of patients due to the high rate of incomplete MRI examinations. Hence, our results have to be considered as precursors to future studies in larger samples of patients. In this regard, we tentatively tried to infer further analysis from the data reported by Sharma et al. [17] and Pickles et al. [13]. It should be noticed that the study of Sharma and co-workers includes a number of patients (29) comparable with the number of patients included in our study (21). On the other hand, Pickles and co-workers enrolled a smaller number of patients. The data of Sharma and co-workers seem to indicate that in responder patients (PVR > 65%) the mean ADC before treatment is lower compared with non-responder patients (PVR < 65%) but not significantly (p > 0.05, Mann Whitney test). Pickles and co-workers did not measure tumour volume. However, the ADC pre-chemotherapy does not significantly correlate (p > 0.05, Spearman test) with the percentage variation of the longest diameter of the tumour. We think that a comparison between different studies should be performed with caution. Indeed, in our study we measured MD using b-values of 0, 1,000 s/mm2, whereas Sharma et al. [17] and Pickles et al. [13] measured ADC using b-values of 0, 500, 1,000 s/mm2 and 0, 700 s/mm2, respectively. Moreover, for the three studies different MR scanners and acquisition sequences were employed.
In agreement with previous studies concerning diffusion measurements in different types of tumour [21, 12], even if we did not perform an early evaluation of tumour response, our results confirmed in responders increased MD values after chemotherapy compared with MD values before chemotherapy. This could be due to a reduction of neoplasm cellularity owing to necrosis with increased water diffusion and the possible influence on patient prognosis [22]. Ogston et al. [23], in fact, in their study showed that a cellular reduction after chemotherapy seemed to correlate with a longer survival rate and thus a better prognosis. In our study we were not able to consider the prognosis of the responders because of the short-term follow-up of patients. On the other hand, in non-responders MDpost decreased with respect to MDpre, although not significantly. The pattern of MD variation observed in non-responders is similar to that reported in the article of Ross et al. [24]. It could be interpreted as a reduced cell-killing effect during treatment and further proliferation of tumour cells with increasing cellular density, resulting in reduced diffusion. In this regard, Ross et al. [24] and Roth et al. [25] have suggested that MD may decrease in non-responders due to absent cellular lysis and also neoplastic cells proliferation.
Conclusions
Quantitative DW-MRI could represent a further analysis in the evaluation of locally advanced breast cancer. Low values of pre-chemotherapy MD may identify, before starting treatment, the patients with a higher probability of response to treatment in terms of increased percentage of volume reduction of the lesion. Thus, this preliminary study suggests that MD measurements could represent, besides the previously showed capabilities in monitoring early response to therapy, a complementary parameter potentially useful to correctly select patients for neoadjuvant chemotherapy. The preliminary results of the present study and assessment of the clinical impact of MD pre-treatment measurements require further validation on a large number of cases.
References
Green FL, Page DL, Fleming ID et al (2002) Part VII breast. In: AJCC cancer staging handbook, 6th edn. Springer, Berlin, pp 255–281
Londero V, Bazzocchi M, Del Frate C, Puglisi F, Di Loreto C, Francescutti G, Zuiani C (2004) Locally advanced breast cancer: comparison of mammography, sonography and MR imaging in evaluation of residual disease in women receiving neoadjuvant chemotherapy. Eur Radiol 14:1371–1379
Basser PJ, Mattiello J, Le Bihan D (1994) Estimation of the effective self-diffusion tensor from the NMR spin-echo. J Magn Reson B 103:247–254
Le Bihan D, Mangin JF, Poupon C, Clark CA, Pappata S, Molko N, Chabriat H (2001) Diffusion tensor imaging: concepts and applications. J Magn Reson Imaging 13:534–546
Neil J, Miller J, Mukherjee P, Huppi S (2002) Diffusion tensor imaging of normal and injured developing human brain: a technical review. NMR Biomed 15:543–552
Woodhams R, Matsunaga K, Iwabuchi K, Kan S, Hata H, Kuranami M, Watanabe M, Hayakawa K (2005) Diffusion-weighted imaging of malignant breast tumors: the usefulness of apparent diffusion coefficient (ADC) value and ADC map for the detection of malignant breast tumors and evaluation of cancer extension. J Comput Assist Tomogr 29:644–649
Sinha S, Lucas-Quesada FA, Sinha U, DeBruhl N, Bassett LW (2002) In vivo diffusion-weighted MRI of the breast: potential for lesion characterization. J Magn Reson Imaging 15:693–704
Marini C, Iacconi C, Giannelli M, Cilotti A, Moretti M, Bartolozzi C (2007) Quantitative diffusion-weighted MR imaging in the differential diagnosis of breast lesion. Eur Radiol 17:2646–2655
Guo Y, Cai YQ, Cai ZL, Gao YG, An NY, Ma L, Mahankali S, Gao JH (2002) Differentiation of clinically benign and malignant breast lesions using diffusion-weighted imaging. J Magn Reson Imaging 16:172–178
Hatakenaka M, Soeda H, Yabuuchi H, Matsuo Y, Kamitani T, Oda Y, Tsuneyoshi M, Honda H (2008) Apparent diffusion coefficients of breast tumors: clinical application. Magn Reson Med Sci 7:23–29
Hamstra DA, Chenevert TL, Moffat BA, Johnson TD, Meyer CR, Mukherji SK, Quint DJ, Gebarski SS, Fan X, Tsien CI, Lawrence TS, Junck L, Rehemtulla A, Ross BD (2005) Evaluation of the functional diffusion map as an early biomarker of time-to-progression and overall survival in high-grade glioma. Proc Natl Acad Sci USA 102:16759–16764
Hayashida Y, Yakushiji T, Awai K, Katahira K, Nakayama Y, Shimomura O, Kitajima M, Hirai T, Yamashita Y, Mizuta H (2006) Monitoring therapeutic responses of primary bone tumors by diffusion-weighted image: initial results. Eur Radiol 16:2637–2643
Pickles MD, Gibbs P, Lowry M, Turnbull LW (2006) Diffusion changes precede size reduction in neoadjuvant treatment of breast cancer. Magn Reson Imaging 24:843–847
Chenevert TL, Meyer CR, Moffat BA, Rehemtulla A, Mukherji SK, Gebarski SS, Quint DJ, Robertson PL, Lawrence TS, Junck L, Taylor JM, Johnson TD, Dong Q, Muraszko KM, Brunberg JA, Ross BD (2002) Diffusion MRI: a new strategy for assessment of cancer therapeutic efficacy. Mol Imaging 1:336–343
Theilmann RJ, Borders R, Trouard TP, Xia G, Outwater E, Ranger-Moore J, Gillies RJ, Stopeck A (2004) Changes in water mobility measured by diffusion MRI predict response of metastatic breast cancer to chemotherapy. Neoplasia 6:831–837
Manton DJ, Chaturvedi A, Hubbard A, Lind MJ, Lowry M, Maravejas A, Pickles MD, Tozer DJ, Turnbull LW (2006) Neoadjuvant chemotherapy in breast cancer: early response prediction with quantitative MR imaging and spectroscopy. Br J Cancer 94:427–435
Sharma U, Danishad K, Seenu V, Jagannathan N (2009) Longitudinal study of the assessment by MRI and diffusion-weighted imaging of tumor response in patients with locally advanced breast cancer undergoing neoadjuvant chemotherapy. NMR Biomed 22:104–113
Belli P, Costantini M, Malaspina C, Magistrelli A, LaTorre G, Bonomo L (2006) MRI accuracy in residual disease evaluation in breast cancer patients treated with neoadjuvant chemotherapy. Clinical Radiology 61:946–953
Pickles MD, Lowry M, Manton DJ, Gibbs P, Turnbull LW (2005) Role of dynamic contrast enhanced MRI in monitoring early response of locally advanced breast cancer to neoadjuvant chemotherapy. Breast Cancer Res Treat 91:1–10
Partridge SC, Gibbs JE, Lu Y, Esserman LJ, Tripathy D, Wolverton DS, Rugo HS, Hwang ES, Ewing CA, Hylton NM (2005) MRI measurements of breast tumor volume predict response to neoadjuvant chemotherapy and recurrence-free survival. AJR Am J Roentgenol 184:1774–1781
Dudeck O, Zeile M, Pink D, Pech M, Tunn PU, Reichardt P, Ludwig WD, Hamm B (2008) Diffusion-weighted magnetic resonance imaging allows monitoring of anticancer treatment effects in patients with soft-tissue sarcomas. J Magn Reson Imaging 27:1109–1113
Rajan R, Poniecka A, Smith TL, Yang Y, Frye D, Pusztai L, Fiterman DJ, Gal-Gombos E, Whitman G, Rouzier R, Green M, Kuerer H, Buzdar AU, Hortobagyi GN, Symmans WF (2004) Change in tumor cellularity of breast carcinoma after neoadjuvant chemotherapy as a variable in the pathologic assessment of response. Cancer 100:1365–1373
Ogston KN, Miller ID, Payne S et al (2003) A new histological grading system to assess response of breast cancers to primary chemotherapy: prognostic significance and survival. Breast 12:320–327
Ross BD, Moffat BA et al (2003) Evaluation of Cancer Therapy Using Diffusion Magnetic Resonance Imaging. Mol Cancer Ther 2:581–587
Roth Y, Tichler T, Kostenich G, Ruiz-Cabello J, Maier SE, Cohen JS, Orestein A, Mardor Y (2004) High-b-value diffusion-weighted MR imaging for pretreatment prediction and early monitoring of tumor response to therapy in mice. Radiology 232:685–692
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Iacconi, C., Giannelli, M., Marini, C. et al. The role of mean diffusivity (MD) as a predictive index of the response to chemotherapy in locally advanced breast cancer: a preliminary study. Eur Radiol 20, 303–308 (2010). https://doi.org/10.1007/s00330-009-1550-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-009-1550-z