Abstract
Elevated choline (Cho) level has been documented on proton magnetic resonance spectroscopy (1H MRS) in head and neck squamous cell carcinoma and therefore percentage changes in Cho levels after chemoradiotherapy may serve as a marker of residual cancer in a post-treatment mass (PTM). Forty-six patients underwent 1H MRS before treatment and the 30 patients with a PTM underwent repeat 1H MRS at 6 weeks post-treatment. The percentage change in Cho/creatine and Cho/water ratios were correlated with residual cancer. The mean pretreatment Cho/creatine and Cho/water ratios were 2.24 and 1.20 × 10−3, respectively. Cho persisted in four out of nine PTMs with residual cancer. Cho was absent in five out of nine PTMs with residual cancer and 21/21 PTMs without cancer. The number of PTMs with persistent Cho was too small to allow analysis of percentage change in ratios but the presence of Cho in a PTM showed significant correlation with residual cancer (p = 0.0046), producing a sensitivity, specificity, positive predictive value and negative predictive value of 44%, 100%, 100% and 81%, respectively. Therefore, the presence of Cho in a PTM may serve as a marker of residual cancer. Furthermore since so few PTMs contain Cho, a percentage change in Cho ratios may not be a useful method for monitoring treatment response.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chemoradiotherapy (CRT) and radiotherapy (RT) have been gaining acceptance as alternative therapies to radical surgery for advanced stage head and neck cancer such as squamous cell carcinoma (HNSCC) [1]. Following a course of CRT/RT a post-treatment mass (PTM) is frequently left behind at the site of the primary cancer or cervical nodal metastasis. In these patients it is important to determine whether the PTM is caused by a benign mass of inflammation/early fibrosis or by residual cancer. Surgical resection should be avoided in the noncancerous PTM to reduce morbidity, retain function and preserve the normal lymphatic barriers. On the other hand salvage surgery is required to improve locoregional control for residual cancers. In these patients surgery should be undertaken early after treatment, preferably within the first few months, before late radiation fibrosis becomes established.
Unfortunately, the early distinction of a residual cancer from a noncancerous PTM is problematic by both clinical and radiological assessment. As a result of these limitations there is great interest in the potential role of in vivo functional MR techniques that can be added to the conventional MR imaging assessment of tumour control [2, 3]. In particular, in vivo proton (1H) magnetic resonance spectroscopy (MRS) has demonstrated an elevated level of choline (Cho)-containing compounds in primary and nodal HNSCC [4–7]. The observed increase in Cho concentration has been attributed to increased cell membrane synthesis found in replicating cancers. Therefore Cho provides a potential marker for assessing tumour control after CRT/RT, the hypothesis being that successful treatment leads to a decrease in Cho. The aim of this study was to determine whether a change in the level of Cho between the pre- and post-treatment measured by 1H MRS could be used to identify residual cancer after treatment.
Materials and methods
Patients
The local ethics committee granted ethical approval for the study and informed consent was obtained from all patients. Consecutive patients who presented for the first time with histologically confirmed HNSCC undergoing nonsurgical primary treatment with CRT or RT were enrolled in this prospective study. 1H MRS was performed on the primary tumour or on a cervical lymph node metastasis greater than 1 cm3. The site was chosen on the basis that it was most likely to result in a successful 1H MRS spectrum, which in practice usually equated to the largest tumour, although other factors such as the ratio of the solid to the necrotic components, and the potential influence of artefact caused by susceptibility effects and movement were also taken into consideration. Histology of the biopsy specimen was the reference standard for the primary tumour, while the reference standard for a metastatic node was either histology or imaging criteria (shortest axial diameter ≥ 11 mm in the jugulodigastric region and ≥ 10 mm in all other regions of the neck). The minimum diameter of any metastatic node was measured and recorded. Patients recruited for the study underwent an early post-treatment examination by MRI and 1H MRS during the sixth or seventh week after the end of treatment.
MR technique
MR imaging and 1H MRS were performed on a 1.5-T whole-body system (Intera-NT, Best, the Netherlands) with a 30 mT/m maximum gradient capability. A standard volume head and neck coil was used to localise the lesion for 1H MRS and conventional MRI was performed for clinical staging and follow-up examinations. Signal reception for 1H MRS was achieved using a 20-cm-diameter circular receive-only surface coil placed over the lesion of interest to optimise the signal-to-noise ratio. With MR image guidance, the volume of interest (VOI) for 1H MRS was positioned by a radiologist with more than 10 years’ experience in head and neck radiology. Air, bone, muscle, fat and obviously necrotic areas of tumour were excluded. Water-suppressed spectra were acquired from each VOI by using the point-resolved spectroscopic (PRESS) sequence at medium (136 ms) TE and fixed (2,000 ms) pulse repetition time (TR). Optimisation procedures for spectroscopy consisted of automated receiver gain frequency adjustment, shimming and gradient tuning. Water suppression was achieved using a selective inversion recovery technique, starting the measurement at the zero crossing of the water signal. From each lesion 64 water-suppressed signals were acquired at a spectral bandwidth of 1,000 Hz. An unsuppressed water signal with 16 averages was also acquired as a reference spectrum. The line width of the water peak acquired without water suppression was measured to access the quality of magnet shim achievable under examination conditions. The averaged signals were exported and processed on an off-line computer (Dell Precision 650 Workstation).
Analysis of the spectra was performed by a physicist with more than 10 years’ experience in spectroscopy who was blinded to the clinical results. Metabolite peak amplitudes and line widths displayed in the frequency domain were determined using a time-domain fitting routine that employs prior knowledge known as the advanced method for accurate, robust and efficient spectral (AMARES), implemented in the MRUI software package [8]. The head and neck is a difficult region in which to perform MRS and noise is a problem that can produce a more irregular baseline compared with regions such as the brain. Therefore for resonances to be considered significant their amplitudes had to be at least twice the standard deviation of the noise as determined by MRUI. After the removal of residual water (4.65 ppm) and lipid peaks in the chemical shift range of 0.90–2.02 ppm from the free induction decay by means of the time domain Hankel–Lanczos singular value decomposition filtering [9], Cho and creatine (Cr) peak amplitudes were determined. As the starting values in the nonlinear least squares fitting algorithm, manually selected resonance frequency and line widths of Cho and Cr peaks were used. Prior knowledge incorporated into the fitting procedure consisted of the following: line width of Cr equal to that of Cho; resonance frequencies were constrained to lie within the range plus or minus 0.05 ppm of the known resonance frequencies of Cho and Cr; the zero-order phase correction was estimated by AMARES and the first-order phase was fixed at zero. For the measurement of water peaks in the reference spectra, manually selected starting values for resonance frequency and line width were used. A Gaussian model function was assumed in the estimation of Cho, Cr and water peak amplitudes. The calculated Cho, Cr and water peak amplitudes were used to determine the Cho/Cr and Cho/water ratio for each lesion.
Tumour control: criteria for diagnosis of residual cancer in a PTM
A diagnosis of residual cancer was made if at any time during follow-up there was (a) histological proof of carcinoma from a biopsy or surgical specimen or (b) increase in size of the PTM radiologically or clinically. The diagnosis of a noncancerous PTM was made on elective neck dissection or clinical and radiological follow-up where the PTM remained static or decreased in size over a follow-up time period of at least 6 months post-treatment.
Statistical analysis
The percentage change in Cho/Cr and Cho/water ratios, between the pretreatment and post-treatment 1H MRS, were correlated with a residual cancer by using logistic regression. The presence of Cho in the PTM was correlated with residual cancer by using a Pearson correlation and the two-sided Fisher’s exact test. A p value of less than 0.05 was considered statistically significant. The corresponding sensitivity, specificity, positive predictive value and negative predictive value were calculated for Cho as a marker for residual cancer.
Results
1H MRS
Pretreatment 1H MRS was performed in 46 patients with HNSCC at 46 cancer sites (19 primary squamous cell carcinomas and 27 cervical nodal metastases). The pretreatment imaging was performed up to 4 weeks (mean 18 days) before the start of treatment, except for two patients in whom the imaging was performed 5 weeks before treatment. The mean VOI selected for spectroscopy was 3.7 ± 2.5 (SD) cm3 and 44 out of the 46 spectra obtained were judged successful. The mean line width of the water peak was 6.2 ± 2.2 Hz and a Cho peak (3.2 ppm) was positively identified in 43 of these 44 cancers.
Following a course of CRT (n = 26) or RT alone (n = 4) the post-treatment MRI scan was repeated 6–7 weeks (mean 43 days) after the end of treatment, except for two patients examined at 5 weeks and one patient at 9 weeks post-treatment. Post-treatment 1H MRS was repeated at the same site (mean VOI 3.0 ± 1.7 cm3) in the 38 (83%) patients who had a residual PTM of at least 1 cm3; 1H MRS was successful in 32/38, but two patients with successful 1H MRS were later withdrawn from the study because they died before the nature of their residual mass could be confirmed. This resulted in 30 patients with a PTM for analysis in this study (Fig. 1) comprising 27 men and 3 women (mean age 55 years, range 43–74 years) with a PTM at 10 primary sites and 20 nodal sites (the pretreatment size of the 20 nodal metastases ranged from 16 to 60 mm (mean 30 mm) in minimum diameter).
The pretreatment mean Cho/Cr and Cho/water ratios for the HNSCCs that left behind a PTM are shown in Table 1. The post-treatment mean Cho/Cr and Cho/water ratios for the four PTMs which showed a persistent Cho peak are shown in Table 1. Mean line width of the water peak obtained from these lesions was 5.4 ± 1.3 Hz.
Tumour control
In summary, following treatment 9/30 patients (33%) had a residual cancer (4 primary and 5 nodal sites) and 21 patients had a noncancerous PTM (6 primary and 15 nodal sites). The diagnosis of a noncancerous PTM was made after elective neck dissection in five patients and by clinical and radiological follow-up (range 8–53 months, mean 31 months, median 28 months) in 16 patients.
Correlation between 1H MRS and tumour control
The number of patients with Cho in the PTM was too small to perform a statistical analysis to correlate the relationship between the percentage change in the pre- and post-treatment Cho ratios (Cho/Cr or Cho/water) on 1H MRS and the presence of a cancerous PTM by using logistic regression. However, there was a significant correlation between residual cancer and the persistence of Cho (Fisher’s exact test p = 0.0034, Pearson’s correlation coefficient = 0.59).
Using the presence of Cho in the PTM as a marker for residual cancer produced 4 true positive (Fig. 2), 21 true negative (Fig. 3), 0 false positive and 5 false negative results. The four true positive results arose in patients in whom the residual cancer was diagnosed 6 months or less after the end of treatment (one nodal cancer which continued to grow post-treatment and three primary cancers diagnosed at 9, 19 and 22 weeks post-treatment). Two of the five false negative results arose in patients with residual cancer diagnosed 6 months or less (one necrotic maxillary sinus cancer where post-treatment 1H MRS was technically difficult to perform because the only solid portion abutted the sinus wall and the other was a small solid nodal cancer), while the remaining three arose in patients with residual cancer diagnosed more than 6 months after the end of treatment (all nodal cancers that showed an initial volume reduction followed by regrowth at 27, 32 and 77 weeks post-treatment and one was the only HNSCC that did not have a pretreatment Cho peak).
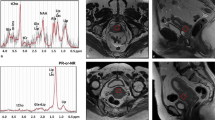
1H MRS spectrum after lipid removal at TE 136 ms from a hypopharyngeal carcinoma with residual cancer after treatment (arrows). a Presence of an intense Cho peak can be clearly identified before the patient underwent treatment. b Post-treatment spectroscopy performed at the same location shows persistence of the Cho in the PTM
1H MRS spectrum at TE 136 ms from a metastatic neck node which showed no residual cancer post-treatment (arrows). a The spectrum with lipids removed shows an intense Cho peak at 3.2 ppm before the patient underwent treatment. b Post-treatment spectroscopy performed at the same location shows no detectable Cho in the unprocessed spectrum from the PTM
Using Cho as a biomarker to identify the residual cancer (cancer diagnosed at any time after the end of treatment) produced a sensitivity of 44%, specificity of 100%, positive predictive value (PPV) of 100%, and negative predictive value (NPV) of 81%. Analysis of patients in whom the cancer was diagnosed 6 months or less after the end of treatment reduced the number of false negative results to two, increasing the sensitivity and NPV to 67% and 92%, respectively, while retaining a 100% specificity and PPV.
Discussion
Determining whether a PTM contains viable tumour remains one of the greatest challenges in the management of HNSCC. The results of this study confirmed that PTMs are common following CRT/RT, being found in over 80% of patients in this study, most of which were benign. With the recognition that most PTMs are benign, the management of HNSCC in some centres has been shifting away from more aggressive elective surgical dissection of all PTMs to a more conservative “wait and see” approach, but early post-treatment clinical assessment of tumour control remains problematic. For those patients with clinically suspicious endoscopic findings at the primary site, direct visualisation and biopsy may need to be repeated under general anaesthesia, but the correct selection of patients is hampered by the early radiation mucositis. Furthermore, biopsy of a PTM at a primary or nodal site can miss residual cancer because of sampling error.
Radiological assessment of the PTM using standard morphological imaging techniques such as MR imaging depends heavily on a change in size to demonstrate residual cancer, but this technique can have major limitations in the first months after treatment. Squamous cell carcinomas at the primary site can be associated with significant post-treatment swelling in the adjacent mucous membranes of the aerodigestive tract on imaging. Post-treatment evaluation of nodal metastases is also problematic as it has been shown that 17% of nodes with residual cancer revert back to normal size while over 50% without residual cancer remain enlarged [10], some of these measuring up to 5 cm in size [11]. Over time there is regression or resolution of the benign PTM but this can take many months or years to achieve. Fluorine-18-fluorodeoxyglucose (FDG) PET-CT has improved the diagnostic accuracy of the early evaluation of the PTM but patients have to undergo an additional examination involving radiation, and in the early post-treatment period FDG PET this is not without the problem of false positive [12] and false negative results [13].
In cases where a clinical or radiological diagnosis of residual cancer cannot be made the diagnostic radiologist is often faced with the dilemma of deciding which patients to suggest for more aggressive intervention, in the form of examination under general anaesthesia, repeated biopsies or surgical resection, and which patients to suggest for close radiological and clinical surveillance. This diagnostic dilemma has led to the current interest in finding a marker for tumour control that can be obtained at the same time as the routine post-treatment MR imaging assessment.
The results of this study show that Cho from 1H MRS has the potential to assist in these difficult cases by identifying residual cancer in the early PTM, at a time that is optimal for performing salvage surgery and when other radiological techniques are suboptimal. Choline peaks have been identified in many in vivo human cancers including HNSCC [4–7]. The detectable Cho peak represents the total Cho and is composed of Cho-containing compounds such as phosphocholine, phosphatidylcholine and glycerophosphocholine. The exact cause of raised Cho level is controversial but has been attributed to the increased synthesis of cell membranes in replicating cancers. Creatine, a marker of energy metabolism, is another peak that is shown to be present in in vitro studies of HNSCC and is often detected in in vivo studies when good shimming is achieved [4, 6, 7], but the peak may often be too small to detect against the baseline in the head and neck region. Lactate is another peak described in HNSCC but this peak requires special lactate editing techniques which are not widely available [5, 14]. There are several reports describing the use of Cho in vivo for monitoring treatment response or detecting tumour recurrence in lymphoma, breast, brain, liver and prostate cancer [15–23]. In general these studies show that Cho ratios tend to decrease during treatment in responders and become elevated in recurrent tumours. Bisdas et al. [7] reported elevated Cho/Cr ratios in the head and neck of three of five patients with tumour recurrence in the head and neck, while Huang et al. [24] showed a reduction in the Cho/water ratios following nonsurgical treatment in five patients with HNSCC.
The original aim of the present study was to correlate the presence of residual cancer in a PTM with the percentage change in Cho ratios between the pre- and post-treatment 1H MRS. However, this study has shown that the presence of Cho in a PTM is uncommon and therefore the measurement of percentage change in Cho ratios is unlikely to be of value in monitoring treatment response. Nevertheless, it was found that after treatment the presence of Cho in the PTM was a significant marker for residual cancer. This result has the advantage that only a post-treatment 1H MRS is required to detect the residual cancer rather than requiring both the pre- and post-treatment 1H MRS to evaluate a change in ratios. For those patients with primary tumours and cervical metastatic nodes the method has the added advantage of not relying upon correctly predicting which tumour sites will leave behind a residual mass.
All four patients in this study with persistent Cho peaks in the PTM had residual cancer resulting in a positive predictive value of 100% for 1H MRS. All these cancers were confirmed clinically within 6 months of treatment and it is presumed that these PTMs probably consisted of gross residual cancer. There were five false negative results reducing the negative predictive value for 1H MRS to 81%. Three of these cases were at nodal sites that showed a volume reduction before enlarging again more than 6 months after treatment; in one case the relapse did not occur until 1.5 years after the end of treatment. It is postulated that 1H MRS was not capable of detecting small islands of viable tumour cells buried within the inflammatory PTM which later acted as the seeds for cancer regrowth. Confining the analysis of results to the detection of those residual cancers that were diagnosed within the first 6 months of treatment would have improved the sensitivity of the early post-treatment 1H MRS. However, even then 1H MRS missed residual cancer when the volume of residual tumour was small or the VOI could only sample the necrotic portion of the tumour. There were no false positive results in this study as none of the 21 patients with a noncancerous PTM had a Cho peak on their early post-treatment 1H MRS. While 1H MRS cannot exclude cancer, especially those cases that relapse later on, it may improve confidence in excluding the presence of gross tumour early after treatment, thus enabling close surveillance to be undertaken instead of surgery.
The main limitation of the study is that the head and neck is technically a difficult region in which to obtain high quality spectra because of susceptibility artefacts and motion. The spectra in this study could have been improved by using antisusceptibility devices and respiratory gating, but antisusceptibility devices are not well tolerated by patients with head and neck cancer, while swallowing, which is a major cause of motion, cannot be corrected using current MRI gating techniques. The other limitation is that the study was performed at 1.5 T rather than at higher magnetic field strengths of 3 T which are becoming increasingly available and increase the signal-to-noise ratio. However, this was a prospective study that was started several years ago and the technique could not be changed during the long period it took to recruit the patients and obtain the long-term clinical follow-up necessary to evaluate this group of patients where the majority had noncancerous PTM under conservative management. Despite the drawbacks, this study was performed using a magnet strength and technique that are widely available and so the results are directly applicable to current clinical practice. Furthermore, it remains to be seen whether future advances in MR spectroscopy will improve the quality of the spectra in the head and neck sufficiently to allow detection of foci of viable tumour cells buried within an inflammatory mass.
In conclusion, a residual mass was commonly found at the site of the primary or nodal HNSCC 6 weeks after completion of CRT. This prospective study set out to investigate the value of Cho as a marker to identify residual cancer within the PTM using a widely available 1H MRS technique and a clinical endpoint. The persistence of Cho after treatment was uncommon and therefore changes in Cho ratios between the pre- and post-treatment 1H MRS could not be used to identify residual cancer. However, the presence of Cho in the PTM had a high positive predictive value for residual cancer. The persistence of Cho should improve radiological confidence in identifying residual cancer, so that a more aggressive approach to confirming the diagnosis, including examination under general anaesthesia and repeated biopsies, can be undertaken. The absence of Cho did not exclude cancer and so 1H MRS cannot be used as a substitute for close clinical and radiological follow-up. On the other hand it could be argued that the disappearance of Cho early after treatment improves confidence in excluding a gross tumour mass, so that a more conservative approach of close surveillance can be undertaken which will identify the minority of cancers that relapse later on.
References
Corvo R (2007) Evidence based radiation oncology in head and neck squamous cell carcinoma. Radiother Oncol 85:156–170
Bezabeh T, Odlum O, Nason R et al (2005) Prediction of treatment response in head and neck cancer by magnetic resonance spectroscopy. AJNR Am J Neuroradiol 26:2108–2113
Shah GV, Gandhi D, Mukerji SK (2004) Magnetic resonance spectroscopy of head and neck neoplasms. Top Magn Reson Imaging 15:87–94
Mukherji SK, Schiro S, Castillo M, Kwock L, Muller KE, Blackstock W (1997) Proton MR spectroscopy of squamous cell carcinoma of the extracranial head and neck: in vitro and in vivo studies. AJNR Am J Neuroradiol 18:1057–1072
Star-Lack J, Spielman D, Adalsteinsson E, Kurhanewicz J, Terris DJ, Vigneron DB (1998) In vivo lactate editing with simultaneous detection of choline, creatine, NAA, and lipid singlets at 1.5T using PRESS excitation with applications to the study of brain and head and neck tumors. J Magn Reson 133:243–254
King AD, Yeung DK, Ahuja AT et al (2005) Human cervical lymphadenopathy: evaluation with in vivo 1H MRS at 1.5T. Clin Radiol 60:592–598
Bisdas S, Baghi M, Huebner F et al (2007) In vivo proton MR spectroscopy of primary tumours, nodal and recurrent disease of the extracranial head and neck. Eur Radiol 17:251–257
Vanhamme L, van den Boogaart A, van Huffel S (1997) Improved method for accurate and efficient quantification of MRS data with use of prior knowledge. J Magn Reson 129:35–43
Van den Boogaart A, van Ormondt D, Pijnappel WWF, de Beer R, Ala-Korpela M (1994) Removal of the water resonance from H-1 magnetic resonance spectra. In: McWhirter JG (ed) Mathematics in signal processing III. Clarendon, Oxford, England, pp 175–195
Ojiri H, Mendenhall WM, Stringer SP, Johnson PL, Mancuso AA (2002) Post-RT CT results as a predictive model for the necessity of planned post-RT neck dissection in patients with cervical metastatic disease from squamous cell carcinoma. Int J Radiat Oncol Biol Phys 52:420–428
Yao M, Graham MM, Hoffman HT et al (2004) The role of post-radiation therapy FDG PET in prediction of necessity for post-radiation therapy neck dissection in locally advanced head-and-neck squamous cell carcinoma. Int J Radiat Oncol Biol Phys 59:1001–1010
McCollum AD, Burrell SC, Haddad RI et al (2004) Positron emission tomography with 18F-fluorodeoxyglucose to predict pathologic response after induction chemotherapy and definitive chemoradiotherapy in head and neck cancer. Head Neck 26:890–896
Rogers JW, Greven KM, McGuirt WF et al (2004) Can post-RT neck dissection be omitted for patients with head-and-neck cancer who have a negative PET scan after definitive radiation therapy? Int J Radiat Oncol Biol Phys 58:694–697
Le QT, Koong A, Lieskovsky YY et al (2008) In vivo 1H magnetic resonance spectroscopy of lactate in patients with stage IV head and neck squamous cell carcinoma. Int J Radiat Oncol Biol Phys 71:1151–1157
Schwarz AJ, Maisey NR, Collins DJ, Cunningham D, Huddart R, Leach MO (2002) Early in vivo detection of metabolic response: a pilot study of 1H MR spectroscopy in extracranial lymphoma and germ cell tumours. Br J Radiol 75:959–966
Jagannathan NR, Kumar M, Seenu V et al (2001) Evaluation of total choline from in-vivo volume localized proton MR spectroscopy and its response to neoadjuvant chemotherapy in locally advanced breast cancer. Br J Cancer 84:1016–1022
Meisamy S, Bolan PJ, Baker EH et al (2004) Neoadjuvant chemotherapy of locally advanced breast cancer: predicting response with in vivo 1H MR spectroscopy—a pilot study at 4T1. Radiology 233:424–431
Murphy PS, Viviers L, Abson C et al (2004) Monitoring temozolomide treatment of low-grade glioma with proton magnetic resonance spectroscopy. Br J Cancer 90:781–786
Kimura T, Sako K, Tanaka K et al (2004) Evaluation of the response of metastatic brain tumors to stereotactic radiosurgery by proton magnetic resonance spectroscopy, 201TICI single-photon emission computerized tomography, and gadolinium-enhanced magnetic resonance imaging. J Neurosurg 100:835–841
Lichy MP, Bachert P, Henze M, Lichy CM, Debus J, Schlemmer HP (2004) Monitoring individual response to brain-tumour chemotherapy: proton MR spectroscopy in a patient with recurrent glioma after stereotactic radiotherapy. Neuroradiology 46:126–129
Graves EE, Nelson SJ, Vigneron DB (2001) Serial proton MR spectroscopic imaging of recurrent malignant gliomas after gamma knife radiosurgery. AJNR Am J Neuroradiol 22:613–624
Chen CY, Li CW, Kuo YT et al (2006) Early response of hepatocellular carcinoma to transcatheter arterial chemoembolization: choline levels and MR diffusion constants—initial experience. Radiology 239:448–456
Pucar D, Shukla-Dave A, Hricak H et al (2005) Prostate cancer: correlation of MR imaging and MR spectroscopy with pathologic findings after radiation therapy—initial experience. Radiology 236:545–553
Huang W, Roche P, Shindo M, Madoff D, Geronimo C, Button T (2000) Evaluation of head and neck tumor response to therapy using In vivo 1H MR spectroscopy: correlation with pathology. Proc Intl Soc Mag Reson Med 8:552
Acknowledgement
The work described in this study was fully supported by a grant from the Research Grants Council of the Hong Kong Special Administrative Region, China (Project number 4300/04).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
King, A.D., Yeung, D.K.W., Yu, Kh. et al. Monitoring of treatment response after chemoradiotherapy for head and neck cancer using in vivo 1H MR spectroscopy. Eur Radiol 20, 165–172 (2010). https://doi.org/10.1007/s00330-009-1531-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-009-1531-2