Abstract
The aim of our study was to determine the frequency of different hepatic arterial variants identified on abdominal CT angiography (CTA) with a 64-row CT system and a high resolution protocol. A total of 250 consecutive abdominal CTAs performed on a 64-row CT system were evaluated. Two radiologists in consensus analyzed arterial phase images; the anatomical findings were grouped according to Michels’ classification. An anomalous arterial pattern was observed in 34% of the cases. The most common anomaly was Michels type III (9.2%), followed by types II and V (5.2%), type VI (4.0%), types IV, VII, and IX (2.0%), and type VIII (0.6%). No cases of type X were detected. Unclassified variations were observed in 3.3% of the cases. The new generation of 64-row MDCT allows optimal visualization of splanchnic vascular anomalies with a minimally invasive examination. This visualization is extended to those vessels with a small caliber and slow flow resulting in difficult recognition by classic angiographic studies. The knowledge of anomalous arterial patterns could be very useful in the preoperative planning of surgical and interventional liver procedures.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Anatomical variants of hepatic vascular structures are part of the daily experience for hepatic surgeons and interventional radiologists. Modifications of the dominant scheme, in which the liver receives its total inflow from the hepatic branch of the celiac axis, occur in 12–49% of cases [1–3]. An aberrant hepatic artery refers to a branch that does not arise from its usual source. Under variant patterns, the lobes may receive blood supply from the superior mesenteric artery, left gastric artery, aorta, or other visceral branches. These vessels may be accessory, occurring in addition to the normal arterial supply, or replaced, representing the primary arterial supply to the lobe. Moreover, aberrant hepatic arteries can be of major surgical significance in operations of the upper intestinal tract, the liver, and the gallbladder and pancreas, especially for laparoscopic procedures [4–8]; they can also become a technical problem for infusion therapy and transarterial chemoembolization of neoplasms [9, 10]. In Michels’ classic autopsy series of 200 dissections [11], published in 1966, the basic anatomical variations in hepatic arterial supply were defined and this classification has served as the benchmark for all subsequent contributions in this area.
The advent of 64-row multidetector computed tomography (MDCT), thanks to isotropic spatial resolution, the extremely fast CT data acquisition, and the consequent optimization of enhancement, has made MDCT invaluable in the evaluation of vascular arterial anatomy, and in particular the splanchnic vessels [12]. The development of powerful and user-friendly software capable of managing the huge amount of data and of performing selective reconstruction of vessels enables CT angiography (CTA) to completely replace diagnostic angiography. This is particularly true of the evaluation of vascular anomalies, whose depiction on angiography is often a burden for the need for selective catheterization [13]. In the literature many studies have demonstrated CTA feasibility in the evaluation of abdominal vascular anatomy, without angiography comparison, for surgical or interventional planning [14–17].
Our study presents the frequency of normal and aberrant hepatic arteries encountered in abdominal computed tomography angiographies performed using 64-row MDCT with a high resolution protocol.
Materials and methods
Patient population
A total of 250 patients who underwent abdominal MDCT angiography examination at our institution between February 2006 and July 2008 were consecutively enrolled. These patients presented with a variety of pathological conditions, including known or suspected aortic and low limbs disease, secondary cancer of the liver, and primary cancer of pancreas and kidney. Subjects with allergy to iodinated intravenous (IV) contrast media, impaired renal function (creatinine concentration greater than 1.2 mg/dL), with previous hepatic or major abdominal surgery and all pathological conditions which may cause possible modifications of the vascular anatomy (i.e., parasitic flow in hepatocellular carcinoma) were excluded. Permission from the ethics committee was not requested as CT studies followed routine imaging protocols, and written informed consent was obtained from all patients.
Imaging techniques
MDCT was performed using a 64-row CT system (VCT; General Electric Healthcare, Milwaukee, USA). Acquisition parameters were as follows: detector collimation, 64 × 0.625 mm; reconstruction thickness, 0.4 mm; pitch, 1.3; tube voltage, 120 kVp; Smart mAs (Noise Index 18). A volumetric scan was obtained following the dynamic injection of nonionic iodinated contrast material (Xenetix 350; Guerbet, France) at a dose of 2 mL/kg of body weight, with a lower limit of 120 mL for patients weighing 60 kg or less and an upper limit of 150 mL for patients weighing over 75 kg. Contrast material was administered by means of an automatic double-head power injector (EZEM, Empower CTA, NY, USA) at a flow rate of 4 mL/s, through a plastic IV catheter placed in an antecubital vein. Data acquisition was triggered using the bolus tracking technique, with the region of interest placed in the abdominal aorta just below the diaphragmatic dome and the trigger threshold level set to the CT value of 100 HU with a 4-s delay.
Imaging analysis
For the purposes of the study, only the CT data obtained during the arterial phase were downloaded onto an off-line workstation (ADW 4.3; General Electric Healthcare, Milwaukee, USA) for image postprocessing and analysis. Arterial phase images were analyzed by two experienced radiologists in consensus. If consensus was not reached, the opinion of a third radiologist was requested. Radiologists interactively analyzed the volumetric datasets on the workstation, being free to use axial slices as well as any possible reconstruction algorithms, including multiplanar reformation (MPR), maximum intensity projection (MIP), and volume rendering (VR). Images were considerate adequate for the purpose of the study if the relevant vasculature in the appropriate phase of contrast enhancement was depicted sufficiently to predict the vascular anatomy. The anatomical findings were classified as normal if the common hepatic artery originating from the celiac trunk and the right and left hepatic arteries arising from hepatic artery after gastroduodenal branching were observed. Anatomical variations were grouped according to Michels’ classification (Table 1). Arterial variants not included in Michels’ classification were recorded separately. The frequency of various anomalies was calculated.
Results
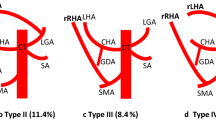
All 250 exams were considered technically adequate and none of the patient data were excluded by the subsequent analysis. We studied 143 males (57.2%) and 107 females (42.8%) (mean age, 63.4 ± 16.2 years). As illustrated in Table 2, normal anatomy was observed in 165 cases (66.0%), while 85 cases (34.0%) showed an anomalous hepatic arterial pattern. The anomalies consisted of Michels type II in 13 cases (5.2%) (Fig. 1), type III in 23 cases (9.2%) (Fig. 2), and type IV in 5 cases (2.0%). Type V and VI occurred in 13 (5.2%) and 10 (4.0%) cases, respectively. Michels type VII was found in five cases (2.0%) and type VIII in two cases (0.8%) (Fig. 3). Type IX was observed in five cases (2.0%). There were no cases in which the hepatic trunk was found to be replaced by the LGA, described by Michels as type X. We found additional, previously unclassified variations in nine cases (3.6%) with the hepatic arteries arising directly from the aorta and with the replaced or accessory arteries arising separately from the celiac trunk, the inferior pancreatic-duodenal artery (IPD) (Fig. 4), and the Buhler arch.
Discussion
At the time of writing, this is the only study to present such a large series in which hepatic vascular arterial anatomy has been depicted by 64-row CTA with a high resolution protocol. In Table 2, a comparison with the frequency of arterial variants reported by other DSA [18, 19] and CTA [20–22] series based on Michels’ classification is provided. Our study presents a frequency of replaced hepatic artery (types II and III) comparable with the findings of other angiographic studies; a possible explanation for the concordance of the results might be that a replaced hepatic artery usually has a normal caliber with a good flow inside and can thus also be revealed by a non-state-of-the-art catheterization of the celiac trunk or the SMA or with a normal CTA protocol. Only type IV has had a higher result than with DSA and this could be due to population variability.
On the other hand, Table 2 demonstrates a general difference in the number of accessory hepatic arteries detected (Michels types V, VI, and VII), with lower rates of these anatomical patterns in the recently published angiographic series than in this study. This difference might be due to the small size of the accessory branches, resulting in the general underestimation of the accessory arteries on angiography. As there is usually a wide deviation in the rate of aberrant LHA from the LGA in radiological publications, it is assumed that the reason lies in the more difficult angiographic identification of the LGA itself; it is easily overlooked if the tip of the catheter is positioned too far into the celiac trunk on selective angiographies and the procedure lacks adequate aortography [23, 24]. Compared with other CTA studies, performed on 4/16-row scanners, we found a similar frequency of these anomalies and this demonstrated that global visualization of abdominal vessels obtained with CTA, with no state-of-the-art technology, permits accessory vessels not easily depicted by DSA to be recognized.
These studies also share the finding of a relatively stable percentage of Michels type VIII and IX patterns, probably owing to the low frequency of these anomalies. Michels type X anomaly, in which the hepatic trunk is replaced by the LGA, is confirmed to be the rarest anomaly, given that it has only been reported in one study after Michels’ original classification [25].
However, in our study the prevalence of anomalies not classified by Michels’ system does not differ compared with those reported in other publications.
The preoperative knowledge of these anomalies assumes high surgical significance, in particular in liver surgery and laparoscopic procedures where surgeons have a limited vision of the surgical field. For this reason, the possibility of obtaining a complete arterial pattern, with no substantial additional cost to the patient, during standard imaging liver studies, seems to be invaluable for improvement of surgical technique and reduction of morbidity costs.
Finally, the knowledge of liver vascular anatomy is also useful for the placement of an infusion pump for liver metastasis treatment, where it is fundamental to recognize the presence of anomalous vessels, which preclude the treatment of liver parenchyma supplied by aberrant arteries [26, 27]. Moreover, recognition of aberrant vessels could also be useful in transcatheter arterial chemoembolization and radioembolization with yttrium-90, where all extrahepatic vessels that originate from hepatic arteries should be occluded to prevent severe complications such as pancreatitis and gastroduodenal ulcerations [28].
Conclusions
The new generation of 64-row MDCT allows optimal visualization of splanchnic vascular anomalies with a minimally invasive examination, as demonstrated in other preliminary CTA series obtained with 4/16-row CT systems. This visualization is also extended to those vessels with a small caliber and slow flow, resulting in difficult recognition by classic angiographic studies. The application of submillimetric protocols could also improve recognition of small accessory branches compared with 4/16-row CTA, but further studies are needed to confirm these findings. Preoperative knowledge of the range of arterial anomalies and their specific frequencies is of greater importance than ever in the planning and performance of specific surgical and interventional radiological procedures in the upper abdomen.
References
Kornblith PL, Boley SJ, Whitehouse BS (1992) Anatomy of the splanchnic circulation. Surg Clin North Am 72:1–30
Soin AS, Friend PJ, Rasmussen A, Saxena R, Tokat Y, Alexander GJM, Jamieson NV, Calne RY (1996) Donor arterial variations in liver transplantation: management and outcome of 527 consecutive grafts. Br J Surg 83:637–641
Kadir S (1991) Atlas of normal and variant angiographic anatomy. Saunders, Philadelphia
Abouljoud MS, Kim DY, Yoshida A, Arenas J, Jerius J, Malinzak L, Raoufi M, Brown KA, Moonka DK (2005) Impact of aberrant arterial anatomy and location of anastomosis on technical outcomes after liver transplantation. J Gastrointest Surg 9:672–678
VanDamme JP (1993) Behavioral anatomy of the abdominal arteries. Surg Clin North Am 73:699–725
Weiglein AH (1996) Variations and topography of the arteries in the lesser omentum in humans. Clin Anat 9:143–150
Klingler PJ, Seelig MH, Floch NR, Branton SA, Freund MC, Katada N, Hinder RA (2004) Aberrant left hepatic artery in laparoscopic antireflux procedures. Surg Endosc 18:807–811
Hemming AW, Finley RJ, Evans KG, Nelems B, Fradet G (1992) Esophagogastrectomy and the variant left hepatic artery. Ann Thorac Surg 54:166–168
Kapoor V, Brancatelli G, Federle MP, Katyal S, Marsh JW, Geller DA (2003) Multidetector CT arteriography with volumetric three-dimensional rendering to evaluate patients with metastatic colorectal disease for placement of a floxuridine infusion pump. AJR 181:455–463
Allen PJ, Stojadinovic A, Ben-Porat L, Gonen M, Kooby D, Blumgart L, Paty P, Fong Y (2002) The management of variant arterial anatomy during hepatic arterial infusion pump placement. Ann Surg Oncol 9:875–880
Michels NA (1966) Newer anatomy of the liver and its variant blood supply and collateral circulation. Am J Surg 112:337–347
Laghi A, Iannaccone R, Catalano C, Passariello R (2001) Multislice spiral computed tomography angiography of mesenteric arteries. Lancet 358:638–639
Song SY, Chung JW, Lim HG, Park JH (2006) Nonhepatic arteries originating from the hepatic arteries: angiographic analysis in 250 patients. J Vasc Interv Radiol 17:461–469
Guiney MJ, Kruskal JB, Sosna J, Hanto DW, Goldberg SN, Raptopoulos V (2003) Multi-detector row CT of relevant vascular anatomy of the surgical plane in split-liver transplantation. Radiology 229:401–407
Brennan DD, Zamboni G, Sosna J, Callery MP, Vollmer CMV, Raptopoulos V, Kruskal JB (2007) Virtual Whipple: preoperative surgical planning with volume-rendered MDCT images to identify arterial variants relevant to the Whipple procedure. AJR 188:W451–W455
Kamel IR, Kruskal JB, Pomfret EA, Keogan MT, Warmbrand G, Raptopoulos V (2001) Impact of multidetector CT on donor selection and surgical planning before living adult right lobe liver transplantation. AJR 176:193–200
Kawamoto S, Montgomery RA, Lawler LP, Horton KM, Fishman EK (2003) Multidetector CT angiography for preoperative evaluation of living laparoscopic kidney donors. AJR 180:1633–1638
Covey AM, Brody LA, Maluccio MA, Getrajdman GI, Brown KT (2002) Variant hepatic arterial anatomy revisited: digital subtraction angiography performed in 600 patients. Radiology 224:542–547
Koops A, Wojciechowski B, Broering DC, Adam G, Krupski-Berdien G (2004) Anatomic variations of the hepatic arteries in 604 selective celiac and superior mesenteric angiographies. Surg Radiol Anat 26:239–244
Winston CB, Lee NA, Jarnagin WR, Teitcher J, DeMatteo RP, Fong Y, Blumgart LH (2007) CT angiography for delineation of celiac and superior mesenteric artery variants in patients undergoing hepatobiliary and pancreatic surgery. AJR 188:W13–W19
Coskun M, Kayahan EM, Özbek O, Çakır B, Dalgıç A, Haberal M (2005) Imaging of hepatic arterial anatomy for depicting vascular variations in living related liver transplant donor candidates with multidetector computed tomography: comparison with conventional angiography. Transplant Proc 37:1070–1073
Stemmler BJ, Paulson EK, Thornton FJ, Winters SR, Nelson RC, Clary BM (2004) Dual-phase 3D MDCT angiography for evaluation of the liver before hepatic resection. AJR 183:1551–1557
Ishigami K, Yoshimitsu K, Irie H, Tajima T, Asayama Y, Hirakawa M, Honda H (2006) Accessory left gastric artery from left hepatic artery shown on MDCT and conventional angiography: correlation with CT hepatic arteriography. AJR 187:1002–1009
Kemeny MM, Hogan JM, Goldberg DA et al (1986) Continuous hepatic artery infusion with an implantable pump: problems with hepatic arterial anomalies. Surgery 99:501–504
Uva P, Arvelakis A, Rodriguez-Laiz G, Lerner S, Emre S, Gondolesi G (2007) Common hepatic artery arising from the left gastric artery: a rare anatomic variation identified on a cadaveric liver donor. Surg Radiol Anat 29:93–95
Sahani DV, Krishnamurthy SK, Kalva S, Cusack J, Hahn PF, Santilli J, Saini S, Mueller PR (2004) Multidetector-row computed tomography angiography for planning intra-arterial chemotherapy pump placement in patients with colorectal metastases to the liver. J Comput Assist Tomogr 28:478–484
Kapoor V, Brancatelli G, Federle MP, Katyal S, Marsh JW, Geller DA (2003) Multidetector CT arteriography with volumetric three-dimensional rendering to evaluate patients with metastatic colorectal disease for placement of a floxuridine infusion pump. AJR 181:455–463
Murthy R, Nunez R, Szklaruk J, Erwin W, Madoff DC, Gupta S, Ahrar K, Wallace MJ, Cohen A, Coldwell DM, Kennedy AS, Hicks ME (2005) Yttrium-90 microsphere therapy for hepatic malignancy: devices, indications, technical considerations, and potential complications. Radiographics 25:S41–S55
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
De Cecco, C.N., Ferrari, R., Rengo, M. et al. Anatomic variations of the hepatic arteries in 250 patients studied with 64-row CT angiography. Eur Radiol 19, 2765–2770 (2009). https://doi.org/10.1007/s00330-009-1458-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-009-1458-7