Abstract
Characterisation and quantification of emphysema are necessary for planning of local treatment and monitoring. Sensitive, easy to measure, and stable parameters have to be established and their relation to the well-known pulmonary function testing (PFT) has to be investigated. A retrospective analysis of 221 nonenhanced thin-section MDCT with a corresponding PFT was carried out, with a subgroup analysis in 102 COPD stage III+IV, 44 COPD stage 0, and 33 investigations into interstitial lung disease (ILD). The in-house YACTA software was used for automatic quantification of lung and emphysema volume [l], emphysema index, mean lung density (MLD [HU]) and 15th percentile [HU]. CT-derived lung volume is significantly smaller in ILD (3.8) and larger in COPD (7.2) than in controls (5.9, p < 0.0001). Emphysema volume and index are significantly higher in COPD than in controls (3.2 vs. 0.5, p < 0.0001, 45% vs. 8%, p < 0.0001). MLD and 15th percentile are significantly smaller in COPD (−877/−985, p < 0.0001) and significantly higher in ILD (−777, p < 0.0006/−914, p < 0.0001) than in controls (−829/−935). A relevant amount of COPD patients apparently do not suffer from emphysema, while controls who do not fulfil PFT criteria for COPD also demonstrate CT features of emphysema. Automatic quantification of thin-section CT delivers convincing parameters and ranges that are able to differentiate among emphysema, control and ILD. An emphysema index of lower 20%, MLD higher than −850, and 15th percentile lower than −950 might be regarded as normal (thin-section, nonenhanced, B40, YACTA). These ranges might be helpful in the judgement of individual measures.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chronic obstructive pulmonary disease (COPD) is a slowly progressive disorder characterised by airway obstruction, which leads to emphysema [1]. The grading of COPD is based upon global parameters like lung function analysis, exercising capacity and frequency of exacerbation [2]. However, pulmonary function testing (PFT) and the six-minute-walk test (6MWT) are dependent on the motivation and collaboration of the patient. Also, healthy areas can partially compensate for diseased regions and the regional distribution of disease is not determined [3].
Nowadays, interventional as well as surgical treatment and systemic drug treatment have also gone in new directions in the local and systemic treatment of COPD. Therefore, a regional analysis is necessary for the planning and guidance of treatment [2]. For the definition of endpoints in clinical trials as well as for treatment monitoring, a quantification of the disease is necessary [4, 5]. Sensitive, easy to measure, as well as stable and reproducible parameters have to be established and their relation to the well-known parameters, e.g. PFT, have to be investigated. A single efficacy measure (such as FEV1) may not be the optimal outcome parameter for the assessment of the treatment effects of the various disease components of COPD [2]. Visual assessment on CT images is not sensitive, specific and reproducible enough for quantification of the disease [6–9]. Also, microscopic quantification is limited by being ex vivo and investigative; only a local snapshot of diffuse and heterogeneous disease is performed [2, 10].
Several studies have been performed to detect and quantify pulmonary emphysema and airway dimensions in COPD by using computed tomography (CT) [10–20]. These trials have been performed using single slice systems or are based on incremental HRCT [10]. The postprocessing and quantification of volumetric thin-section data acquired within a single breath-hold by the use of multidetector computed tomography (MDCT) poses challenges to computer technology, as manual interaction cannot be required on, for example, 300 slices. However, comparison of CT-derived values with the PFT standard and a range of certain diseases has not yet been performed. This study compares PFT and volumetric thin-section MDCT parameters derived consecutively in a dedicated chest department and analyses a subgroup of patients suffering from COPD to derive reference values for CT-based measurements. To emphasise the range and meaning of certain parameters, a comparison is made with a subgroup of patients suffering from an interstitial lung disease (ILD) and controls.
Methods and materials
MDCT
Patients that underwent nonenhanced MDCT (4-slice Siemens Volume Zoom, Forchheim, Germany) between July 2006 and December 2007 who met the technical inclusion criteria (automatic patient instruction for an inspiratory breath-hold, supine, 70 mAseff, 120 kV, collimation 4 × 1.25 mm, pitch 2, 1.25-mm slice thickness [typically DLPw = 127 mGy cm, E = 1.6 mSv according to International Commission on Radiological Protection 60], 1-mm increment, B40f algorithm) and had a PFT within 30 days of MDCT (median 1 day, range 0–30) were eligible for inclusion in the study. The scale of attenuation coefficients with this CT system ranges from −1,024 to +3,072 Hounsfield units (HU). The system was calibrated for water (with a standard phantom) periodically and after major maintenance, and for air daily.
Subjects
From the 3,431 thin-section MDCT investigations performed during the inclusion period, 165 consecutive patients underwent 221 MDCT that allowed for study entry according to the technical and patient-related inclusion criteria. Patients were included only once per 3 months, resulting in 38 patients who were included twice, 6 were included three times and 2 four times. Indication for MDCT was COPD (48%), malignoma (28%), interstitial lung disease (15%) and other reasons (10%). The median age was 64 years (range 20–87), 71% of patients were smokers, 22% nonsmokers and in 7% the smoking history was unknown. Of the smokers the median acquired pack year (PY) history was 45 (range 1–200 PY). In 5% of smokers the PY history was unknown. As the analysis was carried out in retrospect, no ethical approval was required. The analysis was performed using pseudonymised data. Based on the PFT values and according to the admission diagnosis, subgroups of the 221 investigations were defined as follows:
- Control:
-
GOLD stage 0 COPD without ILD, (n = 44), which is the primary comparator
- ILD:
-
GOLD stage 0 COPD with ILD (n = 33)
- COPD:
-
stage III+IV COPD (n = 102)
α1-ATD and CT results were not noted for this analysis. Eventually respiratory artefacts (no severe ones seen visually) were ignored. Patients with PFT indicating stage I+II COPD were not analysed in a dedicated subgroup.
CT analysis
Using the in-house YACTA software, the stack of around 300 DICOM images per patient was analysed fully automatically in an unattended mode on a standard PC (1 GB RAM, Windows XP professional, 2 GHz Pentium® 4 processor) in around 9 min per patient, depending on the amount of emphysema [21]. Since soft tissue (greater than −750 HU), lung (less than −500 HU), and tracheobronchial tree were found fully automatically based on threshold values and an anatomical knowledge-based algorithm, no manual interaction was carried out. For emphysema detection, a threshold of −950 HU was used with a noise correction for voxels with a density of −910 to −949 HU that are surrounded by at least 4 voxels with a density of −950 HU or less. From the CT analysis, the volume of the segmented lung (LV), the volume of the segmented emphysema (EV), their ratio (pixel index = emphysema index, EI), mean lung density (MLD) and 15th percentile (15th) were calculated automatically. The 15th percentile was derived from a histogram recording the densities in Hounsfield units (HU) of all lung voxels and is defined as the threshold value for which 15% and 85% of all lung voxels have a lower and higher density value, respectively [22].
Pulmonary function test
All patients underwent PFTs that were performed with a postbronchodilatator on a body plethysmograph (MasterScreen Body; E. Jaeger; Hoechberg, Germany) according to the guidelines of the European Respiratory Society and the standards of the American Thoracic Society (ATS) [23–26]. The following inspired and expired lung volumes measured by spirometry and absolute lung volumes measured by body plethysmography were chosen for correlation with CT evaluation: forced vital capacity (FVC), forced expiratory volume in 1 s (FEV1), ratio of FEV1 to FVC (Tiffeneau index), total lung capacity (TLC), intrathoracic gas volume (ITGV), and residual volume (RV). To estimate the relative amount of hyperinflation from PFT, the ratio of RV/TLC was calculated. The European Coal and Steal Community (ECSC) predicted values were selected as our in-house standard [27].
Statistical analysis
All statistical analyses were performed using Excel 2003 (Microsoft, Redmond, WA 98052) and Smith Statistical Package 2.8 (Pomona College, Claremont, 91711 CA) using Pearson linear correlation analysis, box plots and t test after verification of normal distribution. A p value than 0.01 was considered to be significant.
Results
Automatic quantification was possible without interaction in all scheduled data. A high frequency of COPD patients affects the ranges of the overall analysis, resulting in a low FEV1 of 49%predicted and high ITGV, RV and TLC (112–169%predicted). This is also reflected in the range of CT-derived parameters that vary within a wide range, but demonstrate a relevant amount of emphysema with high a EI (median 26%) and low MLD/15th percentile (median −850/−962 HU).
A moderate to good positive correlation is found between the static lung volumes ITGV, RV and TLC, and the CT-derived volumetric parameters lung volume, emphysema volume, and their ratio—the emphysema index (r = 0.71–0.93, Table 1, Fig. 1). However, the best correlation of each CT parameter is found with its respective counterpart in PFT (LV vs. TLC and ITGV, r = 0.93 and 0.85; EV vs. RV, r = 0.83; EI vs. RV/TLC, r = 0.60; Table 1). The MLD and 15th percentile correlate moderately with FEV1/FVC. (r = 0.75, Table 1).
Subgroup analysis
The range of MDCT quantification for the three analysed subgroups (control n = 44, ILD n = 33, COPD n = 102) are shown in Table 2 and Fig. 2.
a Box and whisker plot (minimum, 25% quartile, median, 75% quartile, maximum) of the CT-derived values for the three subgroups. Lung volume [ml] compared with the control images is significantly smaller in ILD (p = 0.000001) and significantly greater in COPD investigations (p = 0.000005). bEmphysema volume [ml] compared with the control images is not significantly different from ILD (p = 0.01) and significantly greater in COPD investigations (p = 9.5 × 10−17). cEmphysema index [%] compared with the control images is moderately significantly different from ILD (p = 0.001) and significantly higher in COPD investigations (p = 2.1 × 10−21). dMean lung density [HU] compared with the control images is significantly higher in ILD (p = 9.4 × 10−7) and significantly lower in COPD investigations (p = 4.7 × 10−9). e The 15thpercentile of the lung density histogram [HU] compared with the control images is significantly higher in ILD (p = 0.0006) and significantly lower in COPD investigations (p = 1.4 × 10−14)
The lung volume (LV) is significantly higher on COPD image data than control data (7.1 l vs. 5.9 l, p = 0.000005) (Table 2, Fig. 2a). On the other hand, lung volume on ILD image data is significantly smaller (3.8 l vs. 5.9 l, p = 0.000001). The ranges of the two groups overlap greatly. On images obtained from COPD patients, a moderate to good positive correlation is found between LV and the static lung volumes ITGV, RV and TLC (r = 0.80–0.88, Table 3). For ILD patients, correlation is even higher regarding ITGV and TLC (r = 0.93–0.96), while the correlation with RV is worse (r = 0.68). However, the correlation of LV with vital capacity is good (r = 0.91, Table 3) and with FEV1 it is moderate (r = 0.78, Table 3). For controls, correlation is moderate in FVC and TLC (r = 0.72–0.75, Table 3).
Emphysema volume (EV) of COPD patients is significantly higher than that in controls (3.2 l vs. 0.5 l, p = 9.5 × 10−17, Table 2) with only a minimal overlap. As expected, the values of EV in ILD and controls are low (0.2 l vs. 0.5 l, p = 0.01, Table 2) and appear fairly similar on the box plot (Fig. 2b). On images obtained from COPD patients, a moderate correlation is found between EV and the static lung volumes ITGV, RV and TLC (r = 0.71–0.76, Table 3). For controls, no useful correlations are found (|r| = 0.10–0.49, Table 3).
As the emphysema index (EI) is the ratio of LV and EV, its distribution is almost identical to that of EV (ILD 4%, COPD 45%, control 8%, Table 1). The best correlations for EI are those that are moderate with FEV1/FVC, ITGV, RV and TLC (|r| = 0.71–0.84, Table 1).
The mean lung density (MLD) is significantly higher in ILD than in the control data (−777 HU vs. −829 HU, p = 9.4 × 10−7). In contrast, MLD is significantly lower in COPD investigations (−877 HU vs. −829 HU, p = 4.7 × 10−9). The range overlap is only moderate (Table 2, Fig. 2d). On images obtained from COPD patients, a moderate positive correlation is found between MLD and FEV1/FVC (r = 0.69, Table 3), while for ILD patients a moderate negative correlation was found with FVC, FEV1, FEV1/FVC, ITGV and TLC (r = −70 to −0.83, Table 3). For controls, no useful correlations are found (|r| = 0.07–0.41, Table 3).
As the 15 th percentile of the lung density histogram is a similar parameter to MLD, its distribution is very similar to that of the MLD (ILD −914 HU, COPD −985 HU, control −935 HU, Table 2). However, the overlap between COPD and controls is less (p = 1.4 × 10−14), while that between ILD and controls is wider (p = 0.0006) (Fig. 2e). Also, correlation partners of the 15th percentile are similar to those of the MLD: on images obtained from COPD patients, a very moderate positive correlation is found with FEV1/FVC (r = 0.62, Table 3), while for ILD patients a moderate negative correlation was found with FVC, FEV1, FEV1/FVC, ITGV and TLC (r = −63 to −0.80, Table 3). For controls, no useful correlations are found (|r| = 0.14–0.48, Table 3).
Discussion
Trials using basically different techniques, as used here, have to be compared very carefully with one other, taking the respective collective and technology into account (Table 4) [10, 28].
Nakano used an incremental CT technique with a 2-mm slice thickness and a lower threshold of −960 HU [19]. A clear advantage of this approach is the low radiation dose, which is applied by discontinuous imaging. On the other hand, a pseudovolumetric approach requires manual interaction in the semiautomated quantification procedure, i.e. for manual extraction of the tracheobronchial tree, which is frequently ignored and therefore rated as emphysema. This results in an overestimation of emphysema by HRCT compared with thin-section CT of the whole lung [21, 29]. The inhomogeneous distribution of emphysema is addressed insufficiently [29]. Furthermore, the incremental approach is less useful in longitudinal trials, as the reproducibility of measurements is limited by the breathing level of each slice [6]. While the means detected by Nakano of EI = 32% and FEV1 = 48%predicted [19] were close to our parameters (EI = 45% and FEV1 = 45%predicted, Table 2), the resulting correlation of EI vs. FEV1 of r = −0.53 is higher (r = −0.35, Table 3). Both r values do not indicate a strong correlation between FEV1 as a functional parameter and EI as a morphological measure. The volumetric approach of MDCT also offers additional options, for example, by the intelligent improvement of anatomical segmentation of the volume data set. Unlike the 2D approach used in HRCT, MDCT allows for 3D postprocessing with reliable and accurate quantification. Software tools for fully automatic quantification of the lung parenchyma are available [21].
The Stolk group used Pulmo-CMS software in a multicentre trial for calculation of almost the same parameters as those in our trial [2]. In their approach, a thick section of 5 mm averages the high resolution of MDCT. This might be understood to be a way of reducing radiation exposure, as a setting of 40 mAs was used; however, a high tube voltage of 140 kV limits this effect [2]. The multicentricity with five different systems makes comparison between different centres demanding and a dedicated CT phantom was therefore implemented [2]. A density drift detected in this way led to the exclusion of all patients from one centre and some more from other centres, reported in the appendix to [2] (available online). Instead of a spirometric gating, a volume correction was applied to compensate for a different inspiration depth in the longitudinal course (appendix to [2], available online). Unfortunately, the CT measurements for emphysema characterising parameters such as EI, MLD and the 15th percentile were not reported [2]. A thick-section approach appears to be inadequate for the imaging of such an inhomogeneous disease as emphysema [30, 31].
In a trial comparing inspiratory and expiratory images, Zaporozhan et al. found correlations between PFT and inspiratory CT quantification that are comparable to the COPD subgroup analysed here: LV vs. TLC, r = 0.9 (cf. r = 0.88 here); EV vs. ITGV, r = 0.83 (cf. 0.71 here); EV vs. RV, r = 0.88 (cf. 0.74 here) [30]. A very recent trial that also compares paired inspiratory and expiratory CT with a standard radiation dose of 8 mSv provides few direct measures [32]. Therefore, the comparability with other trials is very limited (Table 4).
In a recent trial, Lee et al. investigated a collective of patients suffering from emphysema (mean FEV1/FVC = 59%predicted; cf. median FEV1/FVC = 71%predicted in the present study), using a similar CT and postprocessing technique, which results in a lower mean MLD of −880 HU (cf. median MLD = −850 HU here) [33]. On the other hand, the emphysema index, FEV1, RV and TLC were almost comparable (EI = 21%, cf. 26% here; FEV1 = 45%predicted, cf. 49% here; RV = 150%predicted, cf. 169%predicted here; TLC = 110%predicted, cf. 112%predicted here) [33]. Comparison of these results with the COPD subgroup reaches a different estimation that the subgroup suffered from more severe emphysema (median FEV1/FVC = 34%predicted, MLD = −877 HU, EI = 45%, FEV1 = 33%predicted, RV = 266%predicted, TLC = 128%predicted) [33]. This comparison points out that emphysema involves various disease components resulting in a spectrum of different parameters, each describing a certain part of the disease. Lee et al. conclude that combined analysis of each component of COPD is important, not only to assess disease severity, but also to understand the pathogenesis of COPD [33]. Even with a similar MLD in both trials (−880 HU vs. −877 HU in the COPD subgroup, Table 2), a relevant different EI (26% vs. 45%, Table 2) is calculated, which cannot be the result of the slightly different techniques (e.g. algorithm B30 vs. B40 [33]). A concomitant hyperinflation, or air trapping, as assumed by the Lee group, might be a possible reason for this relevant difference in EI, while the MLD are comparable. The parameter that might overcome this limitation is the 15th percentile (−985 HU, Table 2), which is not provided by Lee et al. [33]. While Lee et al.’s correlation between emphysema volume and FEV1 was moderate (r = −0.55), our result was even poorer (r = −0.20, Table 3) [33] and almost identical regarding FEV1 vs. MLD (r = 0.44 vs. r = 0.43, Table 3). Taking into account that a correlation coefficient of |r| = 0.55 is not a strong correlation, the relation between FEV1 as a parameter based upon bronchial function and MLD or 15th percentile as morphological measures of parenchymal destruction should be loose. As mentioned by Lee et al., a CT snapshot of a COPD lung consists of a variety of phenotypes including at least bronchial, parenchymal, and combined stages [31–34]. Therefore, the extent of the emphysema is only partially predicted by the severity of the pulmonary functional changes [35, 36]. This is also reflected in our observation of a subgroup of patients with normal PFT demonstrating MDCT findings with clear pathological features and CT quantifications (Table 2, Fig. 2a–e), and also COPD patients with almost no emphysematous destruction (Table 2, Figs. 2a–e, 3) [35].
CT of a 36-year-old man with 20 PY and an FEV1 of 31% predicted, indicating severe COPD (PFT done within 1 week, FEV1/FVC = 64%predicted). In contrast, lung emphysema quantification resulted in an emphysema index of 0.7%predicted, a mean lung density of −768 HU and a 15th percentile of −880 HU, indicating almost normal lung parenchyma. Visual assessment of the CT image also shows no significant emphysema, highlighted as yellow overlay (window width 1,000 HU, level −800 HU)
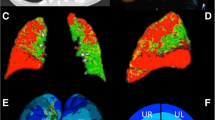
EV and EI correlate best with ITGV, RV and TLC (and therefore also RV/TLC as a parameter of trapped air). Frequently, a negative correlation of EI and EV with FEV1 is expected [19, 33]. However, EV and EI are measures of most peripheral parenchymal destruction, while FEV1 refers to the more central airways. These values should only correlate very loosely in a negative way, which is in fact the case in this analysis (r = −0.2 to −0.35 in COPD, Table 3). Currently there is no threshold for normal EV and EI values available, but this would be helpful in the interpretation of CT-derived quantification data. Furthermore, there is a subgroup of patients without clinical diagnosis of COPD (also within those suffering from ILD) in which an elevated EV and EI indicate a certain amount of emphysema (Figs. 2b, 4, 5, 6). These patients might suffer from undiagnosed and/or subclinical emphysema. The 15th percentile is less sensitive to the possible averaging of coexisting emphysema and fibrosis in the same patient as in MLD (Table 3). The measure of lung density parameters appears most useful in elucidating the influence of “high-density” lung diseases like fibrosis upon these values. In fact, some emphysema patients also suffer from a coexisting fibrous lung disease (Fig. 5). Vice versa, a case in which COPD was diagnosed by PFT whereas CT did not show any relevant emphysema was also seen (Fig. 3).
CT of a 70-year-old man with 80 PY and a FEV1 of 60%predicted, indicating moderate COPD (PFT done within 1 week, FEV1/FVC = 42%predicted). In contrast, lung emphysema quantification resulted in an emphysema index of 46%, a mean lung density of −883 HU and a 15th percentile of −984 HU, indicating severe emphysematous destruction of the lung parenchyma. Visual assessment of the CT image verifies this interpretation of the quantification results as severe emphysema, highlighted as yellow overlay (window width 1,000 HU, level −800 HU)
CT of a 57-year-old man with 30 PY and a FEV1 of 49%predicted, indicating severe COPD (PFT done on the same day, FEV1/FVC = 40%predicted). Lung emphysema quantification resulted in an emphysema index of 31%, an almost normal mean lung density of −839 HU and a low 15th percentile of −978 HU, indicating severe emphysematous destruction of the lung parenchyma in combination with a diffuse high-density disease. Visual assessment of the CT image verifies this interpretation of fibrous interstitial lung disease (usual interstitial pneumonia, UIP, verified histopathologically) combined with severe emphysema, highlighted as yellow overlay (window width 1,000 HU, level −800 HU)
CT of a 73-year-old man with 50 PY and a FEV1 of 42%predicted, indicating severe COPD (PFT done on the same day, FEV1/FVC = 33%predicted). Lung emphysema quantification resulted in an emphysema index of 44%, a mean lung density of −878 HU and a 15th percentile of −978 HU, indicating severe emphysematous destruction. Visual assessment of the CT image verifies this interpretation of severe emphysema, highlighted as yellow overlay (window width 1,000 HU, level −800 HU)
CT-based emphysema quantification is an attractive target for characterisation of COPD into its phenotypes and comorbidities, and for treatment planning and monitoring [2, 31]. For longitudinal analysis, the underlying imaging technique has to be kept constant [2, 10]. In this respect, radiation exposure is a critical issue [37]. The actual therapies depend on repeated imaging for intervention guidance, escalation, or removal, as some are reversible. Also, systemic therapies need monitoring for dose finding and identification of responders. Therefore, any dose that is not applied in an installed CT protocol is multiplied by the repetitions for monitoring. This is also the reason for not routinely applying paired inspiratory and expiratory CT acquisition. A recent simulation trial suggested a reduction from 150 to at least 50 mAs using a certain system, without any loss of information [37]. Other parameters (e.g. kernel) need to be kept constant, as relevant changes have been observed [28].
Limitations
Because the enrolment for this trial was done in retrospect, a selection bias is probable, as at least “indication for CT” and “referred to chest hospital” were additional criteria. This composition is also the prospective target for respective emphysema-modifying trials. A result is also the mixture of the resulting collectives, which is also caused by the fact that, for example, ILD and emphysema will coexist within a subgroup of patients (Fig. 5). This is, however, also the case in clinical routine. Purified (smaller) groups would not reflect this circumstance.
Also, no real gold standard, i.e. disease workup, was used, as this was a noninvasive and in vivo trial. It reflects a typical clinical collective with various stages of lung diseases. Furthermore, histopathology is limited to regional emphysema assessment while CT enables global analysis. Both factors are required for indication, guidance and monitoring of COPD-modifying therapies.
While the total collective has an acceptable size, the subgroups are substantially smaller because of strict inclusion criteria. The subgroup analysis is inhomogeneous and consists of a range of patients suffering from COPD and controls. In addition, the ILD group is inhomogeneous, consisting of patients suffering from UIP and NSIP to various extents. The use of those software-based quantification tools is not planned in ILD. However, these images were helpful as models to identify and explain the demands of patients suffering simultaneously from “low- and high-density” lung diseases, which may superimpose one another.
Neither prospective spirometric gating nor retrospective volume correction has been done [2], because neither the respective hardware nor the software function was available.
Conclusion
-
The fully automatic quantitative assessment of emphysema is possible using thin-section volumetric MDCT. The resultant values characterise emphysema better than FEV1. CT-based quantification, furthermore, delivers local information. Both are essential in the individual planning of surgical, interventional or systemic treatment for COPD.
-
Values for LV, EV, EI, MLD and the 15th percentile, which are to be expected in the given technical environment (thin-section, nonenhanced, B40 algorithm, quantification by YACTA), are provided. Based on the 75% quartile, an emphysema index of up to 20%, a mean lung density larger than −850 HU and a 15th percentile larger than −950 HU might regarded as normal (Table 2, Fig. 2).
-
In patients with normal MLD, emphysema might be superimposed by some kind of high-density lung disease, and the 15th percentile is less sensitive to this fact.
Abbreviations
- 6MWT:
-
six-minute-walk test
- COPD:
-
chronic obstructive pulmonary disease
- EI:
-
emphysema index
- EV:
-
emphysema volume
- FEV1 :
-
forced expiratory volume in 1 s
- FVC:
-
forced vital capacity
- ILD:
-
interstitial lung disease
- ITGV:
-
intrathoracic gas volume
- LV:
-
lung volume
- MLD:
-
mean lung density
- NSIP:
-
nonspecific interstitial pneumonia
- PFT:
-
pulmonary function test
- RV:
-
residual volume
- TLC:
-
total lung capacity
- UIP:
-
usual interstitial pneumonia
References
American Thoracic Society (1995) Standards for the diagnosis and care of patients with chronic obstructive lung disease. Am J Respir Crit Care Med 52:S77–S83
Stolk J, Putter H, Bakker EM et al (2007) Progression parameters for emphysema: a clinical investigation. Respir Med 101:1924–1930
Kemerink GJ, Kruize HH, Lamers RJ et al (1997) CT lung densitometry: dependence of CT number histograms on sample volume and consequences for scan protocol comparability. J Comput Assist Tomogr 21:948–954
Bankier AA, Madani A, Gevenois PA (2002) CT quantification of pulmonary emphysema: assessment of lung structure and function. Crit Rev Comput Tomogr 43:399–417
Coxson HO, Rogers RM, Whittall KP et al (1999) A quantification of the lung surface area in emphysema using computed tomography. Am J Respir Crit Care Med 159:851–856
Gierada DS, Yusen RD, Pilgram TK et al (2001) Repeatability of quantitative CT indexes of emphysema in patients evaluated for lung volume reduction surgery. Radiology 220:448–454
Stolk J, Dirksen A, van der Lugt AA et al (2001) Repeatability of lung density measurements with low-dose computed tomography in subjects with alpha-1-antitrypsin deficiency-associated emphysema. Invest Radiol 36:648–651
Stolk J, Ng WH, Bakker ME et al (2003) Correlation between annual change in health status and computer tomography derived lung density in subjects with alpha1-antitrypsin deficiency. Thorax 58:1027–1030
Bakker ME, Stolk J, Putter H et al (2005) Variability in densitometric assessment of pulmonary emphysema with computed tomography. Invest Radiol 40:777–783
Madani A, De Maertelaer V, Zanen J (2007) Pulmonary emphysema: radiation dose and section thickness at multidetector CT quantification—comparison with macroscopic and microscopic morphometry. Radiology 243:250–257
Bergin C, Müller NL, Nichols DM et al (1986) The diagnosis of emphysema: a computed tomographic-pathologic correlation. Am Rev Respir Dis 133:541–546
Müller NL, Staples CA, Miller RR et al (1988) “Density mask”: an objective method to quantitate emphysema using computed tomography. Chest 94:782–787
Gevenois PA, de Maertelaer V, De Vuyst P et al (1995) Comparison of computed density and macroscopic morphometry in pulmonary emphysema. Am J Respir Crit Care Med 152:653–657
Gevenois PA, De Vuyst P, de Maertelaer V et al (1996) Comparison of computed density and microscopic morphometry in pulmonary emphysema. Am J Respir Crit Care Med 154:187–192
Gevenois PA, De Vuyst P, Sy P et al (1996) Pulmonary emphysema: quantitative CT during expiration. Radiology 199:825–829
Kinsella M, Müller NL, Abboud RT et al (1990) Quantitation of emphysema by computed tomography using a “density mask” program and correlation with pulmonary function tests. Chest 97:315–321
Nishimura K, Murata K, Yamagishi M et al (1998) Comparison of different computed tomography scanning methods for quantifying emphysema. J Thorac Imaging 13:193–198
Nakano Y, Sakai H, Muro S et al (1999) Comparison of low attenuation areas on CT between inner and outer segments of the lung in COPD patients: incidence and contribution to lung function. Thorax 54:384–389
Nakano Y, Muro S, Sakai H et al (2000) Computed tomographic measurements of airway dimensions and emphysema in smokers: correlation with lung function. Am J Respir Crit Care Med 162:1102–1108
Mishima M, Itoh H, Sakai H et al (1999) Optimized scanning conditions of HRCT in the follow-up of pulmonary emphysema. J Comput Assist Tomogr 23:380–384
Heussel CP, Achenbach A, Buschsieweke C et al (2006) Quantification of pulmonary emphysema in multislice-CT using different software tools. Fortschr Röntgenstr 178:987–998
Newell JD, Hogg JC, Snider GL (2004) Report of a workshop: quantitative computed tomography scanning in longitudinal studies of emphysema. Eur Respir J 23:769–775
Miller MR, Hankinson J, Brusasco V et al (2005) ATS/ERS Task Force. Standardisation of spirometry. Eur Respir J 26:319–338
Wanger J, Clausen JL, Coates A et al (2005) Standardisation of the measurement of lung volumes. Eur Respir J 26:511–522
American Association for Respiratory Care (AARC) (2001) Clinical practice guideline: body plethysmography: 2001 revision & update. Respir Care 46:506–513
American Thoracic Society (1994) Standardization of spirometry, 1994 update. Am J Respir Crit Care Med 152:1107–1136
Quanjer PhH, Tammeling GJ, Cotes JE et al (1993) Lung volumes and ventilatory flows. Report working Party “Standardization of Lung Function Tests” European Community of Steal and Coal and European Respiratory Society. Eur Respir J 6 Suppl 16:5–40
Ley-Zaporozhan J, Ley S, Weinheimer O et al (2008) Quantitative analysis of emphysema in 3D using MDCT: influence of different reconstruction algorithms. Eur J Radiol 65:228–234
Achenbach T, Buschsieweke C, Gerhards A et al (2005) Does HRCT-emphysema index represent the entire lung? Fortschr Röntgenstr 177:655–659
Zaporozhan J, Ley S, Eberhardt R, Weinheimer O et al (2005) Paired inspiratory/expiratory volumetric thin-slice CT scan for emphysema analysis: comparison of different quantitative evaluations and pulmonary function test. Chest 128:3212–3220
Orlandi I, Moroni C, Camiciottoli G et al (2004) Spirometric-gated computed tomography quantitative evaluation of lung emphysema in chronic obstructive pulmonary disease: a comparison of 3 techniques. J Comput Assist Tomogr 28:437–442
Akira M, Toyokawa K, Inoue Y et al (2009) Quantitative CT in chronic obstructive pulmonary disease: inspiratory and expiratory assessment. AJR Am J Roentgenol 192:267–272
Lee YK, Oh YM, Lee JH et al (2008) KOLD Study Group. Quantitative assessment of emphysema, air trapping, and airway thickening on computed tomography. Lung 186:157–165
Fujimoto K, Kitaguchi Y, Kubo K et al (2006) Clinical analysis of chronic obstructive pulmonary disease phenotypes classified using high-resolution computed tomography. Respirology 11:731–740
Hersh CP, Jacobson FL, Gill R et al (2007) Computed tomography phenotypes in severe, early-onset chronic obstructive pulmonary disease. COPD 4:331–337
Gelb AF, Hogg JC, Muller NL et al (1996) Contribution of emphysema and small airways in COPD. Chest 109:353–359
Zaporozhan J, Ley S, Weinheimer O et al (2006) Multi-detector CT of the chest: influence of dose onto quantitative evaluation of severe emphysema: a simulation study. J Comput Assist Tomogr 30:460–468
Author information
Authors and Affiliations
Corresponding author
Additional information
This manuscript includes major parts of the doctoral thesis of Romy Hantusch and Simon Hartlieb.
Rights and permissions
About this article
Cite this article
Heussel, C.P., Herth, F.J.F., Kappes, J. et al. Fully automatic quantitative assessment of emphysema in computed tomography: comparison with pulmonary function testing and normal values. Eur Radiol 19, 2391–2402 (2009). https://doi.org/10.1007/s00330-009-1437-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-009-1437-z