Abstract
This study was conducted to assess the accuracy of US-guided directional vacuum-assisted removal (US-DVAR) in evaluating nonmalignant papillary breast lesions. This retrospective study was approved by the institutional review board at our institution; patient consent was not required. We reviewed the clinical and pathology findings from a total of 39 papillary lesions diagnosed at vacuum-assisted removal in 37 patients (age range, 26–60 years; mean age, 44.5 years). Over the follow-up period, we evaluated whether any histologic upgrade occurred and whether or not residual lesions were detected on follow-up imaging. US-DVAR of 39 lesions yielded tissue that was classified as benign in 35 and atypical in 4. Of the 35 lesions that were diagnosed as histologically benign at US-DVAR, 2 were surgically excised. Both of them yielded benign results. Of the 33 benign lesions that were not surgically excised, 28 (85%) were not seen at radiographic follow-up. Of the four lesions diagnosed as atypical at US-DVAR that were surgically excised, all the four were benign. None proved to be malignant. The upgrade rate was 0.0% (95% confidence interval, 0–9%). Among our patients, diagnosis by US-DVAR of benign papillary lesions proved to be accurate, and benign papillary lesions at US-DVAR did not need to be surgically excised for accurate diagnosis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Although percutaneous breast biopsy is a highly reliable method for the diagnosis of breast lesions, papillary lesions of the breast form such a wide spectrum that it is difficult to differentiate between a benign papilloma and a papillary carcinoma at histologic examination [1, 2]. In previous studies, the management of percutaneously identified papillary lesions has been controversial [3–19]. Several investigators recommend surgical excision even when papillary lesions are benign at core-needle biopsy because 10–21% of those lesions were upgraded to atypical ductal hyperplasia (ADH) and ductal carcinoma in situ (DCIS) when re-assessed after excision [9, 15, 16]. However, most previous studies used stereotactic guidance or US-guided automated core-needle biopsy with a 14-gauge or smaller needle [3, 4, 6–10, 14, 15, 17–19]. Our investigators questioned whether such surgical excisions, yielding benign pathology in 79–90% of cases, were avoidable and whether applying ultrasound (US)-guided directional vacuum-assisted removal (US-DVAR) to these papillary lesions could make surgical excision unnecessary for accurate diagnosis.
The current study was undertaken to determine the accuracy of US-DVAR in the evaluation of nonmalignant papillary breast lesions.
Material and methods
Our institutional review board approved this research study and waived the requirement for informed consent because it was retrospective.
Patients
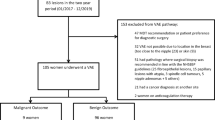
Between January 2003 and November 2005, 753 consecutive percutaneous, sonographically guided vacuum-assisted core biopsies of breast lesions were performed at our institution using the Mammotome system (Biopsys/Ethicon Endo-Surgery, Cincinnati, OH, USA). In 68 of 753 biopsies, the purpose was not removal but sampling of the lesions that were sonographically visible microcalcifications (n = 63) or heterogeneous areas (n = 5) rather than a mass. The remaining 685 procedures for 685 lesions were prospectively intended to remove the sonographically visible mass. Of these 685 lesions, 51 nonmalignant papillary lesions were diagnosed in 48 patients. Nonmalignant papillary lesions included papilloma, papillomatosis, sclerosing papilloma, atypical papilloma, and papilloma with atypical ductal hyperplasia [11]. Of the 51 papillary lesions, 8 were excluded because there was no subsequent surgical excision or long-term imaging follow-up for at least 2 years. We also excluded four cases in four patients who complained of nipple discharge as the purpose of this study was not to assess the therapeutic effect of DVAR, but the accurate diagnostic capability of DVAR.
The remaining 39 papillary lesions in 37 patients that manifested as masses on US made up the study population. The mean patient age was 44.5 years (range, 26–60 years). Of the 39 lesions, 30 had been diagnosed as papillary lesions at 14-gauge automated core-needle biopsy, and 6 had been considered papillary lesions due to imaging findings such as intraductal nodule with adjacent ductal dilatation. The remaining three had not been considered as possible papillary lesions as the findings were nonspecific. However the patients wanted their probable benign breast masses removed.
Imaging evaluation
Bilateral mammography was performed with dedicated equipment (DMR; General Electric Medical Systems, Milwaukee, WI) until April 2005 and the Lorad/Hologic Selenia full field digital mammography system (Lorad/Hologic, Danbury, CT) from May 2005 to the present. Standard craniocaudal and mediolateral oblique views were routinely obtained, and additional mammographic views were obtained as needed. Ultrasonography (US) was performed using high-resolution ultrasonography units with 7.5- or 12-MHz linear array transducers (HDI 5000 or 3000, Philips-Advanced Technology Laboratories, Bothell, WA; Logic 9, GE Medical Systems, Milwaukee, WI). Prior to 14-gauge automated core-needle biopsy or directional vacuum-assisted removal, lesions were assigned to final assessment categories of the Breast Imaging Reporting and Data System (BI-RADS) [20], and these data were entered prospectively into a database using a computerized spreadsheet (Excel, Microsoft, Redmond, WA).
DVAR procedure
In our institution, an 8-gauge probe is used for lesions 1.5–3.0 cm in the greatest dimension and an 11-gauge probe for lesions less than 1.5 cm in the greatest dimension. However, when a papillary lesion was revealed at 14-gauge automated core-needle biopsy, or was considered to be a papillary lesion due to imaging findings, an 8-gauge probe was used for any lesion larger than 1.0 cm.
After administration of local anesthesia, the probe was inserted into the breast through a small skin incision and was guided into biopsy position under direct ultrasound visualization (HDI 5000, Philips-Advanced Technology Laboratories, Bothell, WA). Multiple core samples were taken until the mass was completely removed, as determined by real-time sonography of the biopsy site (Fig. 1). Forced scan pressure to disperse air artifacts and multi-directional sonographic images, such as views perpendicular to the biopsy needle, were applied in visualizing the residual mass. In our practice, we remove breast tissue surrounding the lesion at approximately four more sampling sites (12, 3, 6, and 9 o’clock directions) to ensure complete mass removal. Sonographic imaging data collected immediately after biopsy demonstrated the feasibility of complete lesion removal. The DVAR procedure was performed by three board-certified radiologists with 4–10 years of experience in breast imaging. The completeness of mass removal was recorded as “yes” or “no” for each patient, immediately after the procedure.
A 49-year-old woman with screening sonogram for dense breast on mammogram. The mammogram showed negative findings (not shown). An approximately 1.0-cm oval, indistinct isoechoic nodule with adjacent ductal dilatation (a, arrow) was noted in the upper portion of the right breast on a transverse sonogram. This lesion was classified as category 4a and considered to be a papillary lesion. Directional vacuum-assisted removal (DVAR) of the lesion was performed (b, arrows indicate the opened notch for capture of directional vacuum-assisted biopsy probe). The pathology was benign papilloma. A 2-year follow-up sonogram showed mild distortion (c, arrow) at the site of DVAR, considered to be a post-DVAR change
Follow-up
Surgical excision was performed in 6 of 39 papillary lesions. The remaining 33 lesions underwent imaging follow-up for an average of 29 months (range, 24-48 months). On US follow-up, mild distortion at the site of DVAR was considered to be a post-DVAR change, while space-occupying lesion was considered to be a residual. With respect to the follow-up exams, patients were advised to undergo 6- and 12-month follow-up sonography and 12-month follow-up mammography with annual mammographic and sonographic evaluations thereafter.
Outcome analysis
The clinical, pathologic and imaging findings from the 35 patients, including subsequent excisions and follow-up imaging studies, were reviewed. Data were entered into a computerized spreadsheet. With respect to follow-up data, the radiologist evaluated whether there was histologic upgrade at follow-up and whether follow-up imaging demonstrated residual lesions.
Upgrade rate
An “upgrade” in diagnosis was recorded when a patient had at least one benign lesion at DVAR, classified as ADH, DCIS, or invasive carcinoma at surgical excision or follow-up, or one atypical lesion at DVAR, classified as DCIS or invasive carcinoma at surgery or follow-up. The upgrade rate was determined by dividing the number of cases with upgrade in diagnosis by the total number of DVAR performed.
Exact confidence intervals were calculated according to the formula given by Berry [21].
Results
Thirty-one benign papillomas (79.5%), three papillomatoses (7.7%), one sclerosing papilloma (2.6%), and four atypical papillomas (10.2%) were diagnosed at DVAR. Of four atypical papillomas, three had undergone 14-gauge automated core-needle biopsy, revealing benign papillomas.
Final assessments of 39 lesions based on combined mammographic and sonographic findings are shown in Table 1. Mammogram was performed in all included patients but one. Mammographic findings showed mass in 8 lesions, focal asymmetry in 4 lesions, and negative findings in the remaining 26 lesions. Sonographic findings were (1) a hypoechoic solid mass in 19 lesions (Fig. 2a), (2) a complex mass with solid and cystic component in 12 lesions, and (3) mass within a dilated duct in the remaining 8 lesions. There were no cases in which histological and imaging findings were considered discordant. At sonography, the mean lesion size was 9.0 mm (range, 3–20 mm).
A 49-year-old woman with a palpable mass in her right breast. The palpable mass was a 1.5-cm oval circumscribed mass. Another 1.0-cm oval indistinct hypoechoic nodule was noted in her left breast (a, arrow). Directional vacuum-assisted removal (DVAR) of the lesion was performed and the pathology was benign papilloma. One week later, a follow-up sonogram showed anoval hypoechoic hematoma (b, arrow) with surrounding edema. Six-month follow-up sonogram showed irregular hypoechoic distortion (c, arrows) at the site of DVAR, considered to be a post-DVAR change. At 2-year follow-up sonogram, the distortion had faded (d, arrows)
DVAR procedure
An 11-gauge directional vacuum biopsy device was used for 29 lesions, and an 8-gauge was used for the remaining 10 lesions. Twelve core samples were obtained on average per lesion (range, 4–25). For all patients, the completeness of mass removal was recorded as “yes.” No patient experienced serious adverse events, such as postprocedural bleeding or skin tear requiring a second procedure, during directional vacuum-assisted removal.
Follow-up
Of the 35 lesions that were diagnosed at DVAR as benign papilloma, sclerosing papilloma or papillomatoses, two lesions were surgically excised because the physician considered papillomatosis and florid papillomatosis to be high risk lesions. Surgery revealed no carcinoma but residual papilloma and no residual lesions.
Thirty-three (94.3%) of the 35 lesions that were diagnosed as benign papillary lesions were not surgically excised and underwent imaging follow-up (range, 24–48 months; mean, 29 months). At the 13-month follow-up sonography, one carcinoma was found in a different quadrant from where DVAR was performed. Of the 33 lesions that underwent imaging follow-up, 28 (85%) showed no sonographically visible residual lesions (Figs. 1c and 2c, d), while 5 (15%) showed residual lesions on the US follow-up (6–13 months). All of the residual lesions showed no interval change in size on serial follow-up sonography (24–32 months) since the residual lesions were detected on sonography.
All lesions (n = 4) with histological diagnosis of atypical papilloma at DVAR were surgically excised within 1 month of DVAR. No carcinomas were identified in the surgery of those four lesions. Surgical findings included benign papillomas in three cases and no residual lesion in one.
The frequency of upgrade rate and in DVAR of papillary breast lesions was 0% in benign papillary lesions (95% CI, 0–10%) and 0% in atypical papillomas (95% CI, 0–49%).
Discussion
Papillary lesions are a heterogeneous group of breast lesions, identified histologically by the presence of a fibrovascular stalk. Distinguishing malignant from benign papillary lesions can pose problems for the pathologist, particularly if only a small portion of the lesion is submitted for analysis. The most important histologic feature separating a benign papillary lesion from papillary carcinoma in situ is the presence of an atypical epithelial proliferation resembling low-grade ductal carcinoma, in which a normal myoepithelial cell layer is typically absent. Absence of this cell layer in a papillary lesion indicates a papillary carcinoma. Other intermediate lesions, such as atypical papillomas, are defined less precisely, and their correct classification depends on the percentage of the lesion revealing atypical epithelial proliferation, with or without an intact myoepithial cell layer [2].
The management of papillary breast neoplasm has undergone some transformation over the years. In the early 20th century, mastectomy was recommended for intraductal papilloma [22]. Bloodgood recommended that papillomas be treated by surgical excision, a recommendation that was followed [23]. In 1999, Liberman et al. suggested that a percutaneous core biopsy with imaging concordance may be sufficient for the diagnosis and management of papillary lesions and speculated that the larger volume of tissue acquired with the vacuum-assisted device as opposed to the automated needle could be advantageous in women with papillary breast neoplasm [3]. Mercado et al. reported that papillary lesions of the breast diagnosed as benign at stereotactic directional vacuum-assisted biopsy may not require surgical excision when there is concordance between the radiological findings and the histopathological results [6]. However, both Liberman et al. and Mercado et al. have recently reported that papillary lesions diagnosed as benign at core-needle biopsy should be surgically excised because a substantial number of lesions were upgraded to ADH and DCIS at excision [15, 16].
Among the published studies, there continues to be a lack of agreement about the management of benign papillomas diagnosed with core-needle biopsy, with some lesions being followed up with imaging and others surgically excised [3, 4, 6–10, 15–17]. Moreover, these investigations used various needle gauges, biopsy types and imaging guidance, mainly stereotactic biopsy or US-guided, automated core-needle biopsy with 14-gauge or smaller needles (Table 2). A summary of false-negative biopsy results from these studies (Table 3) shows false-negative results from US 14-gauge automated or stereotactic 11-gauge vacuum-assisted biopsy in up to 14% of cases but none from US-guided DVAR (11- or 8-gauge). While Sydnor et al. showed that there was no significant relationship between needle type (automated core or vacuum-assisted) or the number of cores obtained and the presence of malignancy at excision [17], they did not evaluate whether guided imaging affected the false-negative rate or underestimation rate.
In this study, percutaneous US-DVAR proved reliable for diagnosis of benign papillary lesions, with 0% false-negative biopsy results from benign papillary lesions (95% CI; 0–9%) and 0% underestimation of atypical papillary lesions. One possible explanation for this low false-negative rate is that 8-gauge needles were used for removal rather than sampling in 9 (24%) of 37 cases. Page et al. showed that, in papillomas with foci of ADH, the area of ADH comprised less than 25% of the entire papilloma, while in 63% of cases the surrounding breast tissue showed ADH at excision. They concluded that samples of papillomas obtained with core-needle biopsy may not be representative of the entire lesion and that sampling of breast tissue surrounding the papilloma is warranted at repeat biopsy [24].
We tried to remove all sonographically visualized abnormality, and 28 lesions (85%) showed no sonographically visible residual lesions during follow-up. This result is higher than the value of 73% reported in Fine et al. [25] and 17–50% in previous reports on papillary lesions [4, 6]. Parker has stated that an 8-gauge needle device would be preferable in cases where the patient wishes to have lesions between 1.5 and 2.5 cm removed completely [26]. The amount of tissue submitted for biopsy, furthermore, is closely related to the accuracy of the diagnosis. Specimens obtained with an 11-gauge vacuum probe are significantly larger than those obtained with a 14-gauge needle and automatic gun [27].
A second explanation for the low false-negative rate is that ultrasound was used for imaging guidance during biopsy. US-guided percutaneous breast biopsy has several advantages over stereotactic biopsy [28], including real-time visualization of the inserted needle and the residual mass that is targeted. Although residual tissue can be obscured by air artifacts, they can be dispersed by forced pressure with the probe, and multidirectional sonographic images, such as views perpendicular to the biopsy needle, are useful in visualizing the residual mass [29].
In previous studies, 16–62% of cases consisted of microcalcifications unassociated with mass [3, 4, 6, 8, 15–17], while our cases were all masses without microcalcifications. Stereotactic guidance is usually preferred for microcalcifications revealed by mammography. But noncalcified lesions can be easily obscured during stereotactic biopsy by overlapping densities caused by the local anesthetic infiltration or bleeding, making it difficult to be certain of complete sampling and removal of the lesion [30]. Valdes et al. reported that malignancy is missed significantly less frequently with stereotactic biopsy [31], however, we are not sure whether their results derived from the guidance method or the type/gauge of needle used. They used an 11-gauge directional vacuum-assisted biopsy device with stereotactic guidance and a 14-gauge automated core-needle biopsy device with sonographic guidance, so the comparison is not straightforward.
In recent reports suggesting subsequent excision for benign papillary lesions diagnosed by core-needle biopsy, Liberman et al. did not include data using the US-guided vacuum-assisted device [15]. Mercado et al. did include such data (n = 3) [16], but none of the papillary lesions considered benign at US-DVAR turned out to be malignant at subsequent excision. According to recommendations by Liberman et al.[15] and Mercado et al. [16], if surgical excision should be needed for the papillary lesions due to possible upgraded diagnosis, surgical excision will require needle localization for nonpalpable lesions, as 95% of our cases were (37 of 39 cases). If the wire inadvertently dislodges, migrates or is transected, the surgeon can become disoriented and excise the wrong tissue [32]. Surgical excision could be the gold standard because we believe that it can give us an accurate histological diagnosis, but it is time-consuming, uncomfortable, and potentially nerve racking; moreover, it can occasionally be an unreliable method of diagnosis [33].
One limitation of our study is the small number of cases; papillary lesions of the breast are relatively uncommon. However, in our review of the literature, our study was one of the largest series on papillary lesions with single-type image (US or stereotaxis)-guided vacuum-assisted biopsy. Our findings are consistent with results from US guidance (3–35 cases) [12, 16, 34] or stereotactic guidance (2–34 cases) [3, 6, 8, 12, 15, 16, 31, 34]. Sixteen percent (8 of 51 lesions) of available cases in our study that revealed nonmalignant papillary lesions were excluded due to insufficient follow-up data. In seven of those eight lesions, DVAR was performed in the last year of the study period, that is, with less than 2 years follow-up. This exclusion rate seems to be acceptable considering the data of previous literature [3, 6, 8, 10, 15, 17, 31]. In these studies, 19–35% of papillary lesions were excluded due to insufficient follow-up data. In two studies that included cases with a follow-up period less than 2 years [12, 34], the mean follow-up was 14.7 and 19 months. This means that the half of study population without surgical excision was followed up for less than 2 years. Further studies with a large patient population for US-DVAR are needed to confirm our findings.
The second limitation is that the 15% of 33 lesions that underwent imaging follow-up showed residual lesions on US follow-up. As several investigators reported, complete removal of all imaging evidence of a breast lesion by using the available percutaneous biopsy methods does not ensure complete excision of carcinoma, even if there is no evidence of the residual lesion on radiographs [35–38]. The aim of this study was not to determine the ability of complete histologic excision with DVAR but rather to study the accuracy of histologic diagnosis by large tissue sampling. A subsequent follow-up imaging study is mandatory for patients with papillary lesions who underwent DVAR, especially for patients with multiple papillomas. Several studies support that multiple papillomas are associated with an increased risk of breast cancer [13, 24, 39]. Even when a surgical excision is performed, follow-up imaging study is necessary.
Another limitation is that 33 (95%) of 35 benign papillary lesions underwent imaging follow-up rather than surgical excision. Moreover, 15% of those 33 lesions showed a residual lesion. These lesions may continue to grow, and a longer follow-up should be necessary. However, our study showed that benign papilloma at US-DVAR fit the “probably benign” definition of a less than 2% chance of carcinoma at 2-year follow-up, allowing a recommendation of imaging follow-up rather than immediately subsequent surgical excision. In our study, US-DVAR revealed atypical papilloma in four cases, all of which were benign or showed no residual lesions at subsequent excision. However, the number of these cases was too small. Subsequent excision is still recommended for atypical papilloma or papilloma with atypical ductal hyperplasia because the available percutaneous biopsy methods do not ensure complete removal of the atypical lesion, even when imaging reveals no evidence of residual lesions [35]. Our study just showed that the underestimation rate for atypical papilloma at DVAR may be lower than that of atypical papillomas in previous studies, if only atypical papilloma shows mass on US and is removed by US-guided vacuum-asssisted device.
In conclusion, our results suggest that papillary lesions of the breast that are diagnosed as benign at US-DVAR may not require surgical excision as long as the surgical excision was for accurate diagnosis. More data, based on a larger series, are required to test this conclusion.
References
Fenoglio C, Lattes R (1974) Sclerosing papillary proliferations in the female breast. A benign lesion often mistaken for carcinoma. Cancer 33:691–700
Tavassoli FA (1992) Pathology of the breast. Elsevier, New York
Liberman L, Bracero N, Vuolo MA et al (1999) Percutaneous large-core biopsy of papillary breast lesions. AJR Am J Roentgenol 172:331–337
Philpotts LE, Shaheen NA, Jain KS, Carter D, Lee CH (2000) Uncommon high-risk lesions of the breast diagnosed at stereotactic core-needle biopsy: clinical importance. Radiology 216:831–837
Ioffe O, Berg W, Silverberg S (2000) Analysis of papillary lesions diganosed on core needle biopsy of the breast: management implications [abstract]. Mod Pathol 13:23A
Mercado CL, Hamele-Bena D, Singer C et al (2001) Papillary lesions of the breast: evaluation with stereotactic directional vacuum-assisted biopsy. Radiology 221:650–655
Irfan K, Brem RF (2002) Surgical and mammographic follow-up of papillary lesions and atypical lobular hyperplasia diagnosed with stereotactic vacuum-assisted biopsy. Breast J 8:230–233
Rosen EL, Bentley RC, Baker JA, Soo MS (2002) Imaging-guided core needle biopsy of papillary lesions of the breast. AJR Am J Roentgenol 179:1185–1192
Puglisi F, Zuiani C, Bazzocchi M et al (2003) Role of mammography, ultrasound and large core biopsy in the diagnostic evaluation of papillary breast lesions. Oncology 65:311–315
Renshaw AA, Derhagopian RP, Tizol-Blanco DM, Gould EW (2004) Papillomas and atypical papillomas in breast core needle biopsy specimens: risk of carcinoma in subsequent excision. Am J Clin Pathol 122:217–221
Agoff SN, Lawton TJ (2004) Papillary lesions of the breast with and without atypical ductal hyperplasia: can we accurately predict benign behavior from core needle biopsy? Am J Clin Pathol 122:440–443
Ivan D, Selinko V, Sahin AA, Sneige N, Middleton LP (2004) Accuracy of core needle biopsy diagnosis in assessing papillary breast lesions: histologic predictors of malignancy. Mod Pathol 17:165–171
Gendler LS, Feldman SM, Balassanian R et al (2004) Association of breast cancer with papillary lesions identified at percutaneous image-guided breast biopsy. Am J Surg 188:365–370
Carder PJ, Garvican J, Haigh I, Liston JC (2005) Needle core biopsy can reliably distinguish between benign and malignant papillary lesions of the breast. Histopathology 46:320–327
Liberman L, Tornos C, Huzjan R, Bartella L, Morris EA, Dershaw DD (2006) Is surgical excision warranted after benign, concordant diagnosis of papilloma at percutaneous breast biopsy? AJR Am J Roentgenol 186:1328–1334
Mercado CL, Hamele-Bena D, Oken SM, Singer CI, Cangiarella J (2006) Papillary lesions of the breast at percutaneous core-needle biopsy. Radiology 238:801–808
Sydnor MK, Wilson JD, Hijaz TA, Massey HD, Shaw de Paredes ES (2007) Underestimation of the presence of breast carcinoma in papillary lesions initially diagnosed at core-needle biopsy. Radiology 242:58–62
Ashkenazi I, Ferrer K, Sekosan M et al (2007) Papillary lesions of the breast discovered on percutaneous large core and vacuum-assisted biopsies: reliability of clinical and pathological parameters in identifying benign lesions. Am J Surg 194:183–188
Ko ES, Cho N, Cha JH, Park JS, Kim SM, Moon WK (2007) Sonographically-guided 14-gauge core needle biopsy for papillary lesions of the breast. Korean J Radiol 8:206–211
American College of Radiology (2003) Breast Imaging Reporting and Data System (BI-RADS). American College of Radiology, Reston, VA
Berry CC (1990) A tutorial on confidence intervals for proportions in diagnostic radiology. AJR Am J Roentgenol 154:477–480
Dickinson C (1922) The breast physiologically and pathologically considered with relation to bleeding from the nipple. Am J Obstet Gynecol 3:31–34
Bloodgood J (1922) Benign lesions of the female breast for which operation is not indicated. JAMA 78:859–863
Page DL, Salhany KE, Jensen RA, Dupont WD (1996) Subsequent breast carcinoma risk after biopsy with atypia in a breast papilloma. Cancer 78:258–266
Fine RE, Whitworth PW, Kim JA, Harness JK, Boyd BA, Burak WE Jr (2003) Low-risk palpable breast masses removed using a vacuum-assisted hand-held device. Am J Surg 186:362–367
Parker S (2003) Ultrasound-guided needle procedures in the breast. In: Stavros AT (ed) Breast ultrasound. Lippincott Williams & Wilkins, Philadelphia, pp 742–777
Berg WA, Krebs TL, Campassi C, Magder LS, Sun CC (1997) Evaluation of 14- and 11-gauge directional, vacuum-assisted biopsy probes and 14-gauge biopsy guns in a breast parenchymal model. Radiology 205:203–208
Liberman L, Feng TL, Dershaw DD, Morris EA, Abramson AF (1998) US-guided core breast biopsy: use and cost-effectiveness. Radiology 208:717–723
Kim MJ, Kim EK, Lee JY et al (2007) Breast lesions with imaging-histologic discordance during US-guided 14G automated core biopsy: can the directional vacuum-assisted removal replace the surgical excision? Eur Radiol 17:2376–2383
Dershaw DD (2005) Stereotactic biopsy: equipment, devices, and technique. In: Feig SA (ed) Categorical course in diagnostic radiology. 91st Scientific Assembly and Annual Meeting of the Radiological Society of North America, pp 49–54
Valdes EK, Tartter PI, Genelus-Dominique E, Guilbaud DA, Rosenbaum-Smith S, Estabrook A (2006) Significance of papillary lesions at percutaneous breast biopsy. Ann Surg Oncol 13:480–482
Davis PS, Wechsler RJ, Feig SA, March DE (1988) Migration of breast biopsy localization wire. AJR Am J Roentgenol 150:787–788
Norton LW, Pearlman NW (1988) Needle localization breast biopsy: accuracy versus cost. Am J Surg 156:13B–15B
Plantade R, Gerard F, Hammou JC (2006) [Management of non malignant papillary lesions diagnosed on percutaneous biopsy]. J Radiol 87:299–305
Liberman L, Zakowski MF, Avery S et al (1999) Complete percutaneous excision of infiltrating carcinoma at stereotactic breast biopsy: how can tumor size be assessed? AJR Am J Roentgenol 173:1315–1322
March DE, Coughlin BF, Barham RB et al (2003) Breast masses: removal of all US evidence during biopsy by using a handheld vacuum-assisted device–initial experience. Radiology 227:549–555
Parker SH, Klaus AJ, McWey PJ et al (2001) Sonographically guided directional vacuum-assisted breast biopsy using a handheld device. AJR Am J Roentgenol 177:405–408
Perez-Fuentes JA, Longobardi IR, Acosta VF, Marin CE, Liberman L (2001) Sonographically guided directional vacuum-assisted breast biopsy: preliminary experience in Venezuela. AJR Am J Roentgenol 177:1459–1463
Carter D (1977) Intraductal papillary tumors of the breast: a study of 78 cases. Cancer 39:1689–1692
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, M.J., Kim, EK., Kwak, J.Y. et al. Nonmalignant papillary lesions of the breast at US-guided directional vacuum-assisted removal: a preliminary report. Eur Radiol 18, 1774–1783 (2008). https://doi.org/10.1007/s00330-008-0960-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-008-0960-7