Abstract
The purpose was to evaluate the ability of computer-aided detection (CAD) software to detect morphologically flat early colonic carcinoma using CT colonography (CTC). Twenty-four stage T1 colonic carcinomas endoscopically classified as flat (width over twice height) were accrued from patients undergoing staging CTC. Tumor location was annotated by three experienced radiologists in consensus aided by the endosocpic report. CAD software was then applied at three settings of sphericity (0, 0.75, and 1). Computer prompts were categorized as either true positive (overlapping tumour boundary) or false positive. True positives were subclassified as focal or non focal. The 24 cancers were endoscopically classified as type IIa (n=11) and type IIa+IIc (n=13). Mean size (range) was 27 mm (7–70 mm). CAD detected 20 (83.3%), 17 (70.8%), and 13 (54.1%) of the 24 cancers at filter settings of 0, 0.75, and 1, respectively with 3, 4, and 8 missed cancers of type IIa, respectively. The mean total number of false-positive CAD marks per patient at each filter setting was 36.5, 21.1, and 9.5, respectively, excluding polyps. At all settings, >96.1% of CAD true positives were classified as focal. CAD may be effective for the detection of morphologically flat cancer, although minimally raised laterally spreading tumors remain problematic.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
CT colonography (CTC) is increasingly utilized as a relatively non-invasive method of colonic investigation in both patients with symptoms suggesting possible colorectal neoplasia and asymptomatic individuals undergoing colorectal cancer screening [1]. Meta-analysis data suggest the procedure is robust for detection of “clinically significant” neoplasia, including larger adenomatous polyps and established cancer [2, 3]. However, one area of concern is the ability of CTC to reliably detect morphologically flat lesions [4]. The histopathological definition of a flat lesion is one where the height is less than twice the thickness of the surrounding mucosa [5], although a more practical definition used by both endoscopists and radiologists is a lesion whose width is over twice its height [5]. Flat lesions may be slightly elevated above the mucosa, flush with the mucosa, or even depressed below the surrounding mucosal surface, and are manifest on CTC as subtle areas of mural thickening [6–9]. Although there are some data suggesting CTC may depict these lesions [10], it is apparent that many are missed prospectively [6, 11, 12]. The clinical importance of flat lesions is debated, with some evidence they are no more significant than their polypoidal counterparts of similar size [13]. Undoubtedly a proportion of flat lesions are malignant [14], and failure to diagnose such lesions at CTC will often deny the patient potentially curative treatment.
Computer-aided detection (CAD) software systems are proving increasingly robust in detecting colonic neoplasia [15–17]. Because most CTC errors are perceptual [18–20], it seems likely CAD will play an increasingly important role in interpretation. The high clinical importance, but radiological subtlety of morphologically flat early colonic cancer make it an ideal target for CAD systems, but at the time of writing there is little if any literature on detection characteristics for such lesions. The purpose of our study was to evaluate the ability of computer-aided detection (CAD) software to detect morphologically flat early colonic carcinoma using CT colonography (CTC).
Materials and methods
Local ethical committee approval was obtained, and patients gave informed consent for the study.
Patient source
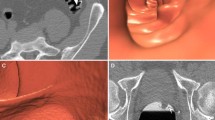
Patients were recruited from a single tertiary referral center. Patients with a known histological diagnosis of colorectal cancer (based on prior endoscopic biopsy) were referred to the center for treatment. As part of the pre-treatment workup, all patients were re-colonoscoped (to confirm the site of the tumour and to assess potential endoscopic respectability) by one of three experienced colonoscopists. Colonoscopy was immediately followed by same-day contrast-enhanced CT colonography (see below) for staging using the same bowel preparation (3 l of polyethylene glycol). Careful note of the endoscopic morphology of all diagnosed cancers was recorded by the colonoscopist using standard criteria (type 0 to 5) [5], together with the segmental location of the lesion. Maximal dimension was also documented (by assessment against adjacent open biopsy forceps). Those lesions classified by the endoscopist as superficial, i.e., depth of penetration no greater than the submucosa-type 0, were sub-classified as either polypoidal, i.e., protruding above the surrounding mucosa (type 0-I) or flat (“non-polypoidal”)-i.e., slightly elevated (height less then twice width; type 0-II). Type 0-II tumors were then further sub-classified as type 0-IIa (minimally elevated), type 0-IIb (completely flat), or type 0-IIc (depressed) (Fig. 1). Lesions showing a mix of these features were classified as such [5], depending on the predominant component (Fig. 1). Type II-a and type II-b lesions were classified as “laterally spreading” if their estimated maximal dimension was greater than 20 mm [21–23]. As per usual clinical practice at the referral center, not all synchronous polyps were removed by the colonoscopist, especially if less than 6 mm in size. For the purpose of the study, the colonoscopist recorded the presence (maximum diameter and segmental location) of any unremoved synchronous colonic polyps.
Colonic distension prior to same day CTC was achieved using automated carbon-dioxide insufflation (Protocol, EZEM, NY) and performed on a 64-detector–row CT scanner (Aquilion, Toshiba Medical Systems) according to the following protocol: 120 kV, 200–400 mA with automatic exposure control, 64 rows × 0.5 mm collimation, helical pitch 53.0 (effective dose 18 mSv). The supine scan acquisition was acquired 50 s following intravenous contrast administration [150 ml of iohexol 350 (Omnipaque, Daiichi-Sankyo Pharmaceutical)]. There were at least 3 weeks (range 3 to 6 weeks) between the original biopsy of the lesion and CTC.
Patient selection
Between October 2005 and May 2006, a total of 165 patients were referred to the center and underwent same-day colonoscopy and CTC. The endoscopic report of all patients was reviewed by the study coordinator who retrieved the records of all patients in whom the endoscopic classification of the tumour was grade 0-II (i.e., superficial or flat morphology). Those lesions in which the ultimate histopathological diagnosis was greater than T1 were excluded.
Dataset annotation
The CTC datasets of all patients with endoscopically flat T1 cancers were loaded onto a workstation equipped with CTC viewing software (Vitrea 3.9.01, Vital Images Minnetonka, MN) and reviewed in consensus by three radiologists experienced in CTC interpretation (over 200, 400, and 800 endoscopically validated cases, respectively), fully aware of the colonoscopic report. The radiologists located the cancer (and any unremoved polyps) and recorded the 2D axial image numbers for both supine and prone datasets (if applicable). Observers used segmental location (within one colonic segment of colonoscopy) and size (within 50%) to aid matching. Any uncertainty was resolved by face-to-face discussion between the observers.
A digital screen shot image of the tumour and any polyps was also taken to facilitate subsequent identification during CAD application (see below). Although the workstation used for initial dataset annotation had a CAD facility, this was not used by the radiologists so as not to bias the consensus-derived location of the cancer by prior knowledge of the position of any CAD prompts.
CAD application
After the ground truth dataset annotation was complete, one of the radiologists applied the CAD software (ColonCAD API 4.0, Medicsight plc) integrated into the Vitrea workstation to the datasets as described below.
Functionality and development of the commercially available CAD system used for the study have been described elsewhere [24, 25]. In brief, the software segments the colon from the CT dataset before applying a mathematical algorithm with the aim of detecting raised endoluminal objects, all of which are regarded as potential neoplasia. A sphericity filter is then applied that aims to facilitate discrimination between real polyps and false-positive prompts, due to haustral folds, for example, by analyzing every voxel on the candidate surface to determine whether or not it and its neighbors form part of the surface of a theoretical sphere. Detections whose sphericity is above a pre-determined threshold are prompted visually to the observer via small red dots superimposed over the region of interest on both 2D axial and 3D endoluminal views (Fig. 2) or by a yellow triangle when the polyp candidate is hidden behind a fold during 3D endoluminal analysis (Fig. 3). With the sphericity enhancement filter set at 1.0, only those voxels that potentially formed part of a perfect sphere were retained as prompts, and the others were dismissed as likely false positives. As the filter value reduces towards zero, voxels that may form part of an increasingly less perfect sphere are retained as prompts.
3D endoluminal CT colonographic image demonstrating the same lesion (arrow) as Fig. 2 barely visible behind a haustral fold (arrowhead). CAD alerts the reader to the hidden lesion via a yellow triangle
In the CAD workstation iteration used for the present study, the user could influence the sphericity threshold for prompted polyps via slider bars with a scale between 0 (most sensitive) and 1 (least sensitive). The recommended default sphericity setting for the software was 0.75 [26].
No data from the origin of datasets used for the present study had been used to develop the CAD algorithm previously.
The observer applied the CAD at each of three sphericity settings (0, 0.75, and 1). At each setting the observer recorded whether the CAD has successfully detected the flat cancer present in each dataset [supine and prone (if visible on both)]. A successful detection was defined when at least one CAD mark directly overlapped any part of the tumor outline previously determined when the ground truth was established. CAD was deemed to have successfully classified the patient if there was at least one correct CAD detection of cancer on either the supine or prone datasets, or on both. The observer further sub-classified each correct CAD detection as “focal” or “non focal” (Fig. 4). A focal detection was ascribed when the CAD mark was located over a recognizable focal tumor elevation or protuberance beyond the contour of the main lesion. A non-focal detection was defined as a CAD mark correctly located over the cancer, but overlying no recognizable focal elevation or protuberance beyond the contour of the main lesion. All other CAD detections (including “true” polyp detections for the purpose of this study) were considered false positives. The observer classified all the CAD false positive detections at each filter setting as follows: 1-bulbous fold (prominent fold in otherwise well-distend segment), 2-segmental under distension, 3-fecal residue/residual fluid, 4-normal colonic anatomy, e.g., ileocaecal valve, redundant mucosa, internal hemorrhoid, normal fold, 5-extracolonic, 6-benign polyp, and 7-unexplained.
Statistical analysis
Data were collated and descriptive statistics (notably sensitivity) were calculated for the CAD at each of the three sphericity settings used. Categorical differences in detection according to tumor morphology sub-classification were analyzed using Fischer’s exact test.
Results
The final study cohort consisted of 24 patients (6 females, mean age 66 years, range 41 to 80 years). The final endoscopic classification of the 24 tumors was 0-IIa (n=11) and IIa+IIc (n=13). All 0-IIa tumors measured over 20 mm and were thus classified as lateral spreading.
Four tumors were located in the rectum, six in the sigmoid, four in the descending, six the transverse, three in the ascending colon, and one in the cecum. The mean size was 25 mm (range 7 to 60 mm). Of the 24 tumors, 3 were visible on the supine dataset only, 1 on the prone dataset only, and 20 on both supine and prone datasets.
CAD detection
Sensitivity for tumor detection increased as sphericity threshold decreased (from 54.1% at a sphericity of 1 to 83.3% at a sphericity of 0). Sensitivity for type IIa tumors was in general less than for type IIa+IIc, notably at a sphericity setting of 0 (p=0.03) (Fig. 5; Tables 1 and 2).
Each tumour detected by CAD had on average more than two correct CAD marks per scan position (other than at sphericity of 1 in the prone orientation) (Table 2; Fig. 6). The overwhelming majority of correct CAD detections were classified as focal (at least 96.2%), with a very small minority being non focal (Table 2; Fig. 7).
a 3D endolumonal images show a transverse colon type 0-IIa cancer with three correct CAD marks (red dots). b Colonoscopic image of the lesion in Fig. 6a
a 3D endoluminal CT colonographic image showing a CAD-detected type 0-IIa+IIc T1 ascending colonic carcinoma (arrows). The CAD detection (red dot) was classified as “focal” as located on a recognizable focal elevation. Yellow triangles represent further correct CAD marks on the hidden side of the lesion. b 3D endoluminal CT colonographic image shows a CAD-detected type IIa transverse colonic carcinoma (arrows). The two correct CAD marks were classified as non focal
False positives
False positives decreased with increasing sphericity. On average there were 19.3 (range 1–46), 11.6 (range 1 to 38), and 5.9 (range 0 to 21) false positives at sphericity settings of 0.75, and 1, respectively, for supine data. On average there were 21.3 (range 1 to 58), 12.9 (range 0 to 39), and 6.4 (range 0 to 15) false positives at sphericity settings of 0, 75, and 100, respectively, for prone data. The majority of false positives were related to normal colonic anatomy, although an increasing proportion (over 20% at sphericty setting of 0) were due to correct detections of small benign polyps. Excluding polyp detections, the mean number of CAD false positives was per patient was 36.5, 21.1, and 9.5 at sphericity settings of 0, 0.75, and 1, respectively (Table 3).
Discussion
It is widely accepted that the majority of colorectal cancers arise from benign polypoidal precursors [27]. However, there is an increasing recognition that non-polypoidal (i.e., “flat”) adenomas also may progress to invasive cancer. Although originally believed to be particularly prevalent in Japanese patients, it is now clear that flat adenomas are found throughout the world. For example, a recent study found flat or depressed colorectal neoplasia in 22.7% of North Americans [28], with similar data from Europe [29]. Although the overall incidence of invasive carcinoma in non-polypoidal lesions is in the order of 2% [14, 28], recent reports suggest up to 20% of early cancers (the prime target for any screening test) may have this morphology [14]. Furthermore, non-polypoidal flat cancers are smaller than polypoidal tumours of the same stage. Given this, strategies to increase detection of non-polypoidal neoplasia, such as use of dye spray, are increasingly adopted in endoscopic screening programs. The ability of CTC to detect such lesions will undoubtedly be subject to increasing scrutiny.
The CTC appearance of non-polypoidal colorectal neoplasia (shallow plaque-like areas of mural thickening) is well described [6, 9]. Anecdotally, although flat lesions are generally believed “invisible” at CTC, the small amount of available literature concerning their depiction is more encouraging. Using a dataset containing 22 non-polypoidal lesions and 3 readers, Fidler et al. reported a prospective sensitivity of between just 15% and 65% [6]. However, double reading resulted in 100% flat adenoma detection, and 19 of the 22 lesions were visible at least in retrospect. A recent report by the ESGAR CTC study group investigators found that most of the large polyps missed by expert observers were non polypoidal, emphasizing that their detection is difficult [12]. However, almost all lesions were visible in retrospect and most were detected by at least one reader. Moreover, in the present study all 24 tumors were identifiable in retrospect by 3 unblinded experienced radiologists. Although in his series, Pickhardt et al. prospectively achieved high sensitivity for “flat colorectal lesions” [10], most data to date suggests non-polypoidal neoplasia, while visible on CTC, is often missed prospectively because of perceptual error [6, 9, 11].
Computer-aided detection software has proved successful in scenarios where radiologist must pick up subtle abnormalities that appear infrequently, notably in screening mammography. Non-polypoidal neoplasia is therefore potentially an ideal target for colon CAD software. Our study used a single vendor CAD system, developed ostensibly for detection of raised polyps [25]. Importantly, the present study employed an external validation paradigm [30]-no datasets from the hospital donating the cancers had been used previously to develop the CAD software. We specifically selected early cancers rather than non-malignant flat lesions since the former have unquestionable clinical significance, and it is important they are detected by CTC.
Overall CAD sensitivity was clinically acceptable with 71% detection at the manufacturer’s currently recommended default operating point for the CAD, rising to 83% at a sphericity setting of 0. As would be expected, although sensitivity increased as sphercity was reduced, this occurred at the expense of increased false-positive detections (i.e., decreased specificity). Most false-positive detections were due to normal colonic anatomy, and previous work has suggested such prompts are easily dismissed by most experienced radiologists [24]. Although fewer CAD false positives are desirable, it is still unclear at what threshold the benefit of CAD diminishes significantly. It could be argued, for example, that a CAD generating 25 prompts, one of which correctly identifies a subtle flat cancer originally missed by a radiologist, is more useful than an algorithm with fewer false positives, but that fails to detect the cancer. Indeed, recent data suggest that current CAD systems may be relatively poor at detecting cancer as opposed to polyps [16]. In the case of flat cancers, improved detection algorithms may inevitably engender decreased specificity.
Interestingly, the vast majority of CAD detections were related to a “focal” protuberance on the cancer. Also, detection of the lateral spreading type 0-IIa lesions (minimally raised) was overall inferior to type 0-IIa+IIc lesions, possibly because the CAD detects a focal “protuberance” at the junction of the main lesion and the central depression. Colon CAD systems rely on differences in geometric shape between raised colonic polyps and surrounding colonic wall and haustral folds. As recently reviewed by Yoshida and Dachman [31], various computational models have been proposed to take advantage of the shape difference, including sphere fitting [32], volumetric shape index [33], surface curvature with rule-based filter [34], surface normal overlap [35], and more recently Haussian matrix [36]. False-positive detections, for example, due to haustral folds, then are often reduced by various techniques, including gradient concentration [17] or application of a edge displacement field [37]. Recent publications have confirmed the increasingly robust detection characteristics of colon CAD systems for detecting polypoidal neoplasia [15], but it remains unclear whether such systems will reliably detect non-polypoidal malignancy. While some workers have successfully proposed algorithms based on fuzzy merging and wall thickness analysis to detect colonic masses (i.e., large cancers) [38], again the ability of such systems to detect subtle raised lesion remains unknown. It is reassuring, however, that even endoscopically confirmed non-polypoidal lesions often possess focal raised areas that may be targeted successfully by CAD. Indeed, on average the CAD in the current study placed at least two marks on each cancer detected. It is clear, however, that some “smooth” type 0-IIa lateral spreading tumors will prove difficult to detect reliably and will likely need new algorithms, possibly based on mural thickening.
Our study does have limitations. Although we included all non-polypoidal T1 tumors in our inclusion criteria, only two endoscopic sub-classification types were available (0-IIa and 0-IIa+IIc). However, it is not surprising we did not have any type II-b lesions in our cohort since such morphology is exceedingly rare [14]. The endoscopists in the present study defined a non-polypoidal lesion as one where the height is less than half the width. Others have defined such lesions as having a height less than 2.5 mm (the width of closed biopsy forceps). However, the definition we used is adopted by the working group created specifically to consider the definitions of flat neoplasia in Western endoscopic practice [5, 39]. We used a single CAD algorithm and, as discussed above, it is uncertain whether similar results would be obtained using different vendors. Finally, we did not incorporate a radiologist observer into our analysis, choosing to determine the “stand-alone” performance of the software, as have many others. The stand-alone detection characteristics of CAD for flat cancers have not been determined previously, and, based on our data, studies incorporating human readers are now appropriate.
In conclusion, the CAD system tested is relatively effective for detection of morphological non-polypoidal cancer, although some minimally raised lateral spreading tumors remain problematic.
References
Kim DH, Pickhardt PJ, Taylor AJ, Leung WK, Winter TC, Hinshaw JL, Gopal DV, Reichelderfer M, Hsu RH, Pfau PR (2007) CT colonography versus colonoscopy for the detection of advanced neoplasia. N Engl J Med 4(357):1403–1412
Halligan S, Altman DG, Taylor SA et al (2005) CT colonography in the detection of colorectal polyps and cancer: systematic review, meta-analysis, and proposed minimum data set for study level reporting. Radiology 237:893–904
Sosna J, Morrin MM, Kruskal JB, Lavin PT, Rosen MP, Raptopoulos V (2003) CT colonography of colorectal polyps: a metaanalysis. AJR Am J Roentgenol 181:1593–1598
Macari M, Bini EJ (2005) CT colonography: where have we been and where are we going. Radiology 237:819–833
The Paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach, and colon. Gastrointest Endosc 2003;58:S3–S43
Fidler JL, Johnson CD, MacCarty RL, Welch TJ, Hara AK, Harmsen WS (2002) Detection of flat lesions in the colon with CT colonography. Abdom Imaging 27:292–300
Taylor SA, Halligan S, Bartram CI (2003) CT colonography: methods, pathology and pitfalls. Clin Radiol 58:179–190
Macari M, Bini EJ, Jacobs SL, Lange N, Lui YW (2003) Filling defects at CT colonography: pseudo- and diminutive lesions (the good), polyps (the bad), flat lesions, masses, and carcinomas (the ugly). Radiographics 23:1073–1091
Park SH, Lee SS, Choi EK et al (2007) Flat colorectal neoplasms: definition, importance, and visualization on CT colonography. AJR Am J Roentgenol 188:953–959
Pickhardt PJ, Nugent PA, Choi JR, Schindler WR (2004) Flat colorectal lesions in asymptomatic adults: implications for screening with CT virtual colonoscopy. AJR Am J Roentgenol 183:1343–1347
Jensch S, van Gelder RE, Florie J et al (2007) Performance of radiographers in the evaluation of CT colonographic images. AJR Am J Roentgenol 188:W249–W255
Effect of directed training on reader performance for CT colonography: multicenter study. Radiology 242:152–161
O’Brien MJ, Winawer SJ, Zauber AG et al (2004) Flat adenomas in the National Polyp Study: is there increased risk for high-grade dysplasia initially or during surveillance. Clin Gastroenterol Hepatol 2:905–911
Soetikno R, Friedland S, Kaltenbach T, Chayama K, Tanaka S (2006) Nonpolypoid (flat and depressed) colorectal neoplasms. Gastroenterology 130:566–576
Summers RM, Yao J, Pickhardt PJ et al (2005) Computed tomographic virtual colonoscopy computer-aided polyp detection in a screening population. Gastroenterology 129:1832–1844
Mang T, Peloschek P, Plank C et al (2007) Effect of computer-aided detection as a second reader in multidetector-row CT colonography. Eur Radiol 17:2598–2607
Nappi J, Yoshida H (2002) Automated detection of polyps with CT colonography: evaluation of volumetric features for reduction of false-positive findings. Acad Radiol 9:386–397
Dachman AH, Lefere P, Gryspeerdt S, Morin M (2007) CT colonography: visualization methods, interpretation, and pitfalls. Radiol Clin North Am 45:347–359
Fidler JL, Fletcher JG, Johnson CD et al (2004) Understanding interpretive errors in radiologists learning computed tomography colonography. Acad Radiol 11:750–756
Slater A, Taylor SA, Tam E et al (2006) Reader error during CT colonography: causes and implications for training. Eur Radiol 16:2275–2283
Hart AR, Kudo S, Mackay EH, Mayberry JF, Atkin WS (1998) Flat adenomas exist in asymptomatic people: important implications for colorectal cancer screening programmes. Gut 43:229–231
Saito Y, Fujii T, Kondo H et al (2001) Endoscopic treatment for laterally spreading tumors in the colon. Endoscopy 33:682–686
Uraoka T, Saito Y, Matsuda T et al (2006) Endoscopic indications for endoscopic mucosal resection of laterally spreading tumours in the colorectum. Gut 55:1592–1597
Taylor SA, Halligan S, Burling D et al (2006) Computer-assisted reader software versus expert reviewers for polyp detection on CT colonography. AJR Am J Roentgenol 186:696–702
Halligan S, Altman DG, Mallett S et al (2006) Computed tomographic colonography: assessment of radiologist performance with and without computer-aided detection. Gastroenterology 131:1690–1699
Dehmeshki J, Halligan S, Taylor SA et al (2007) Computer assisted detection software for CT colonography: effect of sphericity filter on performance characteristics for patients with and without fecal tagging. Eur Radiol 17:662–668
Muto T, Bussey HJ, Morson BC (1975) The evolution of cancer of the colon and rectum. Cancer 36:2251–2270
Saitoh Y, Waxman I, West AB et al (2001) Prevalence and distinctive biologic features of flat colorectal adenomas in a North American population. Gastroenterology 120:1657–1665
Rembacken BJ, Fujii T, Cairns A et al (2000) Flat and depressed colonic neoplasms: a prospective study of 1000 colonoscopies in the UK. Lancet 355:1211–1214
Halligan S, Taylor SA, Dehmeshki J et al (2006) Computer-assisted detection for CT colonography: external validation. Clin Radiol 61:758–763
Yoshida H, Dachman AH (2005) CAD techniques, challenges, and controversies in computed tomographic colonography. Abdom Imaging 30:26–41
Kiss G, Van Cleynenbreugel J, Thomeer M, Suetens P, Marchal G (2002) Computer-aided diagnosis in virtual colonography via combination of surface normal and sphere fitting methods. Eur Radiol 12:77–81
Yoshida H, Nappi J, MacEneaney P, Rubin DT, Dachman AH (2002) Computer-aided diagnosis scheme for detection of polyps at CT colonography. Radiographics 22:963–979
Summers RM, Beaulieu CF, Pusanik LM et al (2000) Automated polyp detector for CT colonography: feasibility study. Radiology 216:284–290
Paik DS, Beaulieu CF, Rubin GD et al (2004) Surface normal overlap: a computer-aided detection algorithm with application to colonic polyps and lung nodules in helical CT. IEEE Trans Med Imaging 23:661–675
Kim SH, Lee JM, Lee JG et al (2007) Computer-aided detection of colonic polyps at CT colonography using a Hessian matrix-based algorithm: preliminary study. AJR Am J Roentgenol 189:41–51
Acar B, Beaulieu CF, Gokturk SB et al (2002) Edge displacement field-based classification for improved detection of polyps in CT colonography. IEEE Trans Med Imaging 21:1461–1467
Nappi JJ, Frimmel H, Dachman AH, Yoshida H (2004) Computerized detection of colorectal masses in CT colonography based on fuzzy merging and wall-thickening analysis. Med Phys 31:860–872
Update on the paris classification of superficial neoplastic lesions in the digestive tract. Endoscopy 37:570–578
Acknowledgements
This work was undertaken at UCLH/UCL and a proportion of funding was received from the Department of Health’s NIHR Biomedical Research Center’s funding scheme.
Medicsight plc, London, supplied the CAD software for this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Taylor, S.A., Iinuma, G., Saito, Y. et al. CT colonography: computer-aided detection of morphologically flat T1 colonic carcinoma. Eur Radiol 18, 1666–1673 (2008). https://doi.org/10.1007/s00330-008-0936-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-008-0936-7