Abstract
The aim was to compare the performances of contrast-enhanced (CE) ultrasonography (US) and spiral computed tomography (CT) in the detection and characterization of portal vein thrombosis complicating hepatocellular carcinoma (HCC). We studied 50 patients with HCC who had biopsy-proven portal vein thrombi that had been detected with US and color Doppler US. Thirteen of the thrombi involved the main portal trunk and 37 the segmental branches. CEUS and CT were performed within a week of thrombus biopsies. For each imaging technique, diagnoses of thrombosis (present/absent) and thrombus nature (malignancy/benignancy) were made by experienced readers under blinded conditions and compared with pathological findings to determine accuracy rates for thrombus detection and characterization. Forty-four of the 50 thrombi were pathologically diagnosed as malignant and the remaining six were benign. CEUS detected 50/50 (100%) thrombi and correctly characterized 49/50 (98%). CT detected 34/50 (68%) thrombi and correctly characterized 23 of these 34 (68%). CEUS outperformed CT in terms of both thrombus detection (P < 0.0001) and characterization (P = 0.0001). CEUS appears to be significantly superior to CT for detection and characterization of portal vein thrombosis complicating HCC, and it should be considered in the staging of these tumors.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Portal-vein thrombosis can complicate clotting-cascade disorders, inflammatory, myeloproliferative, and neoplastic diseases, portal-vein hypertension, injection therapies (percutaneous, endoscopic, etc.) and splenectomy [1–8]. In clinical practice, it is most commonly encountered in patients with hepatocellular carcinoma (HCC), especially those with end-stage disease [4]. The detection and characterization of portal-vein thrombi is of paramount importance in HCC patients because malignant thrombi represent an absolute contraindication for liver transplantation, resective surgery, and percutaneous ablation techniques, and they are also a relative contraindication for trans-arterial chemoembolization [8, 9].
Thus far, intra-arterial digital subtraction angiography has been regarded as the “gold standard” for detection of portal-vein thrombosis [10, 11], although other non-invasive radiological techniques have also been proposed for this purpose [12–21]. Tri-phasic spiral computed tomography (CT) is used worldwide for the non-invasive assessment of the portal system [12, 13], and it is considered a fundamental tool for the detection of portal-vein thrombosis. It identifies thrombi involving the main portal trunk with 86% sensitivity and a specificity of 100% [13]. However, it is reportedly less sensitive in thrombus characterization [12], and its diagnostic accuracy has never been evaluated in comparative, prospective studies. Ultrasonography (US) and color Doppler US (cDUS), which are generally the first imaging techniques used in patients with chronic liver disease, have produced results similar to those of tri-phasic CT for detection and characterization of portal vein thrombi [6, 14, 15]. In a recent study, however, CEUS appeared to be superior to US and cDUS for both the detection and characterization of thrombi involving the hepatic and portal venous systems in patients with hepatic malignancies, and in a limited subset of patients, the results obtained with CEUS also proved to be better than those of spiral CT [20].
In the present study, we compared the performance of CEUS and spiral CT in the detection and characterization of portal vein thrombi. CEUS and spiral CT findings were collected prospectively from 50 patients with HCC accompanying cirrhosis and biopsy-proven portal vein thrombosis.
Materials and methods
Patients
This prospective study, conducted in accordance with the ethical principles of the Helsinki declaration, was approved by the Institutional Ethics Committee. Each participant provided written, informed consent to all study procedures.
The study population consisted in 374 cirrhotic patients with diagnosis of HCC who were consecutively referred to the US outpatient clinic of our department between January 2004 and June 2006.
In 98/374 (26%) cases, the US and cDUS examinations (see “Imaging techniques” for methods) also revealed portal vein thrombosis. Forty-eight of these patients had tumor characteristics, liver function parameters and/or concomitant pathology that rendered them ineligible for curative/palliative treatment (surgery, percutaneous ablative treatment, trans-arterial chemoembolization). The remaining 50/98 (51%) underwent percutaneous US-guided biopsies of the thrombus [22] and were enrolled in the study (Table 1). Biopsies were performed by aspiration with a 21-gauge Chiba needle (Ecoject, Hospital Service, Rome, Italy) in 38/50 (76%) patients and with a cutting technique using a 21-gauge needle (Biomol, Hospital Service) in the remaining 12 (24%) patients. These latter patients had thrombi involving segmental portal branches, and the angle between the main axis of the vein and the optimal needle-insertion pathway to the thrombus exceeded 45 degrees. In these cases, back and forth movements of the needle during aspiration biopsy can dislodge the tip of the needle from the thrombus, resulting in sampling errors. Within a week of the thrombus biopsy, each enrolled patient was examined with CEUS and spiral CT, as described below.
Imaging techniques
All US examinations considered in this study (the initial US and cDUS that revealed the portal vein thrombosis as well as CEUS) were performed with an Aloka Prosound SSD 5500 ePHD (extended Pure Harmonic Detection) scanner (Aloka, Tokyo, Japan) and multifrequency, convex-array transducers (3.0 – 6.0 MHz).
The US and cDUS examinations were carried out as previously described [20]. A preliminary gray-scale US examination of the upper abdomen was performed with imaging in the sagittal, transverse, oblique, and intercostal planes. Intrahepatic branches of the portal, splenic, mesenteric, and hepatic veins and the inferior vena cava were then examined with cDUS. The cDUS images (in which red and blue indicated flow toward and away from the transducer, respectively) were displayed with simultaneous B-mode gray-scale or Doppler spectral images. Flow settings were selected based on the individual flow velocity, and during each examination the color gain was increased to the highest value compatible with artifact-free images. If a thrombus was detected, the Doppler-encoded area was reduced to maximize the color sensitivity and frame rate, and the thrombus was carefully examined for internal color signals. Any signal detected was subjected to Doppler spectral analysis using a sample volume of 1.5–3.0 mm without angle correction [6, 20, 23].
For the CEUS examination, the scanner was used in a bubble-specific imaging mode, i.e., with a mechanical index (MI) of 0.04 or lower, second harmonic filtering, and phase detection. SonoVue (Bracco, Milan, Italy) was used as a contrast agent, in accordance with the manufacturer’s instructions. The lyophilized powder (25 mg) was reconstituted in 5.0 ml of 0.9% sodium chloride solution to produce a solution containing sulfur hexafluoride microbubbles at a concentration of 8 μl/ml. Shortly after preparation, this solution was administered as a 2.4-ml bolus via a 19-gauge intravenous cannula in an antecubital vein, and the line was flushed with 5.0 ml of 0.9% sodium chloride solution. A chronometer displayed on the screen was used to determine the temporal characteristics of flow enhancement. If a thrombus was detected, up to two additional boluses of SonoVue were injected, and the thrombus was examined more closely for pulsating enhancing signals within its boundaries. When present, these signals were subjected to Doppler spectral analysis, as described above. Each examination was digitally recorded (Premium Digital Videocassettes and DVCAM Model DSR-20 DMP recorder, both from Sony Corporation, Tokyo, Japan).
Spiral CT was performed with a multi-slice CT system (Sensation 16, Siemens, Erlangen, Germany) using a conventional protocol developed for detection and staging of hepatic tumors [24]. In all cases, non-enhanced and contrast-enhanced CT series (1.5-mm slice thickness, 1.5-mm collimation, 120 kvp, 120 mAs with 2- and 5-mm axial reconstructions) were performed. Enhanced images were obtained after power injection of 120-ml contrast medium (Iomeron 400; Bracco, Milan, Italy) at a rate of 4 ml/s followed by a bolus of 50 ml of 0.9% sodium chloride solution. Bolus-tracking software was used to determine the precise temporal characteristics of the arterial and portal phases of enhancement. The entire liver was imaged in 4–5 s.
Criteria for imaging diagnoses
The findings obtained with each imaging technique were reviewed under blinded conditions by two trained, experienced reviewers (R.V. and G.G. for US, cDUS, and CEUS; C.F. and V.A. for CT). For each patient, a diagnosis of thrombosis or non-thrombosis was recorded for each imaging technique; in the former case, the thrombus was also classified as benign or malignant. All diagnoses represented consensus decisions reached by the two reviewers and were based on the criteria shown in Table 2. Imaging findings were reported using terminology published elsewhere [13, 20, 23] (Figs. 1, 2 and 3).
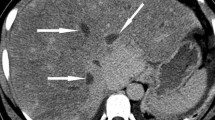
Oblique US and cDUS images of an HCC nodule successfully treated by percutaneous radio-frequency ablation. a US reveals an iso-echoic area at the tumor site that is smaller than the treated tumor (small arrows). Nearby, a blood vessel can be seen with a hypoechoic image (thrombus) within its lumen (arrowhead). b Oblique CEUS scan with longitudinal view of the portal vein during the arterial phase. The treated HCC nodule appears as a non-enhancing area. Within the lumen of the peripheral branch of the portal vein near the treated tumor, there is a diffusely enhanced image (arrow) representing a thrombus with internal neovascularity (blooming pattern). c During the portal phase of the CEUS scan shown in b, enhancement persists within the thrombus tissue (arrows). d) Spiral CT of the involved area showed a non-enhancing area at the site of the treated HCC nodule (arrow) but failed to detect the small thrombus within the segmental branch of the portal vein
Oblique US and cDUS with longitudinal views of the portal vein. a US reveals isoechoic material within the lumen of the portal vein (arrow). b Early arterial phase of CEUS: the material within the lumen of the portal vein appears strongly enhanced (arrow), while the lumen itself is non-enhanced. c Portal phase of CEUS: enhancement persists at the thrombus site (arrow), while enhancement begins in the lumen itself. d Early arterial and portal phases of CT: during the arterial phase (left), the thrombus was barely visible and displayed minimal internal enhancement, whereas in the portal phase (right), within the lumen of the portal vein there is an area of hypo-attenuation (arrow) that contains a focus of enhancement (malignant thrombus)
Statistical analysis
Thrombus detection rates for CEUS and CT were calculated as the percentages of all cases with diagnoses of thrombosis based on the criteria shown in Table 2.
To determine the accuracy of US, cDUS, CEUS, and CT in thrombus characterization, the imaging diagnoses made using the criteria in Table 2 were checked against the pathologic diagnosis. In this analysis the sensitivity [true positives/(true positives + false negatives)] and specificity [true negatives/(false positives + true negatives)] of the four methods were also computed with their 95% confidence intervals (CIs). For this analysis, cases were classified as false negatives when the imaging method yielded a benign diagnosis for a thrombus found to be malignant based on biopsy findings. Cases in which the imaging technique had failed entirely to detect the thrombus were excluded in the analysis of the performance of that method.
Chi-square and Fisher’s exact tests were used to evaluate differences in the performance of the various techniques, as appropriate [25]. A P value less than 0.05 was considered indicative of statistical significance. All tests were two-sided. Stata software (StataCorp, 2002, Stata Statistical Software 7.0, College Station, Tex., USA) was used to analyze data.
Results
Based on the initial US/cDUS findings, 13 of the 50 (26%) portal vein thrombi examined in this study were located in the main portal trunk, while the remainder 37/50 (74%) involved segmental branches of the portal vein; 33/50 (66%) completely occluded the involved vein, and 17/50 (34%) were only partially occlusive.
None of the thrombus biopsies were associated with complications. Based on pathological findings, 44/50 (88%) thrombi were classified as malignant, and the remaining six (12%) were regarded as benign. All 50 diagnoses were consistent with the subsequent evolution of the disease. During clinical follow-up lasting 12–25 months (mean, 18 ± 5 months), four of the six thrombi classified as benign disappeared. The remaining two decreased in size, and their appearance changed from hypoechoic to hyperechoic. Two patients with benign thrombi (including one whose thrombus had disappeared) have died from causes unrelated to the thrombosis (i.e., stroke, myocardial infarction). The remaining four are alive and well. The 44 patients whose thrombi were classified as malignant were followed for 3–25 months (mean, 13 ± 3 months), during which time thrombus extension was documented in all cases. Thirty-four (77.2%) of these patients have died, all from causes related to the progression of the thrombus and/or neoplastic disease.
Table 3 summarizes the thrombus detection rates for the CEUS and CT and the correct characterization rates for the four imaging modalities tested. Results are shown for the total set of thrombi and for subsets based on characteristics revealed by the initial US/cDUS examinations (degree of occlusiveness and involvement vs. noninvolvement of the main portal trunk).
CEUS identified 50/50 (100%) of the thrombi, while only 34/50 (68%) were visualized on enhanced spiral CT. Of the 16 thrombi that were not seen on spiral CT, 13 were located in segmental branches of the portal vein, and 15 were malignant. Neither CEUS nor CT revealed any additional portal-vein thrombi that had not been detected in the original US/cDUS examination.
In thrombus characterization, the US diagnosis was concordant with pathologic findings in 38/50 (76%) cases; corresponding rates for cDUS were slightly lower [32/50 (64%)]. Thus, for the characterization of portal vein thrombosis, the overall sensitivity of US was 86.4% (95% CI: 72.6–94.8), and that of cDUS was 54.3% (95% CI: 52.4–81.4).
CEUS correctly characterized all but one of the 50 thrombi, displaying a sensitivity of 98.0% (95% CI: 88.0–99.9) with a specificity of 100% (95% CI:54.1–100). Seven of the 50 (14%) thrombi appeared non-enhancing (including all six of those that were benign). The single malignant thrombus without enhancement was located in a small-caliber segmental branch of the portal vein deep within the seventh liver segment. The remaining 43 malignant thrombi all had enhancing patterns [20] (Figs. 2c,d and 3b,c).
As noted above, spiral CT failed to detect 16 of the 50 portal vein thrombi. Limiting our analysis to the 34 that were visualized on CT, we found that 11 (32.4%) were incorrectly characterized. Therefore, spiral CT showed a sensitivity of 67.6% (95% CI: 49.5–82.6) and a specificity of 60% (95% CI: 14.7–94.7) in thrombus characterization.
CEUS proved to be far superior to CT in terms of thrombus detection (P < 0.0001) and characterization rates (P = 0.0001). As for the characterization of thrombi, it was also more sensitive than cDUS (P = 0.03) and US (P = 0.2), although its superiority in the latter case was not statistically significant. Due to the limited number of benign thrombi in the series, it was not possible to evaluate the differences between CEUS, US, cDUS, and CT in terms of their specificities in thrombus characterization.
Discussion
Our study showed that CEUS is a very reliable technique for evaluating the patency of the portal venous system. Its sensitivity proved to be significantly higher than that of tri-phasic spiral CT for both the detection and characterization of portal vein thrombosis, and in the latter setting, it was also significantly more sensitive than cDUS, confirming previous reports [18–21].
Several factors contribute to the superior results we observed with CEUS. First, low-MI CEUS imaging following intravenous bolus injection of a microbubble agent allows real-time examination with a diagnostic interval of about 3–4 min, which is more than sufficient for complete examination of the portal system. At present, no other imaging technique offers an observation period of this length. The microbubble agent also generates excellent visual contrast between the thrombus tissue and the lumen of the veins [20]. Thus, in the arterial phase, malignant thrombi appeared as enhancing tissue within a non-enhanced lumen (Fig. 3c), while in the portal phase, regardless of their nature, the thrombi appear as a poorly- or non-enhancing images within a strongly enhanced lumen. For thrombus characterization, the main advantage of CEUS over other radiological techniques is its ability to detect very small arterial vessels within tissue [18, 20, 26]. Arterial neovascularization within a neoplastic thrombus results in arterial enhancement, which, on real-time imaging, can be easily distinguished from venous enhancement by its intermittent pulsation. Confirmation of the signal’s arterial nature with Doppler spectral analysis has been shown to indicate malignancy with very high specificity [18–21]. False positives caused by the incorrect placement of the Doppler sample volume at a point in which the hepatic artery crosses over the portal vein has been reported for CEUS [20] and for cDUS [6]. However, although the risk of this type of error must be kept in mind, it is actually quite low.
The superiority of CEUS over CT in the detection of portal vein thrombosis is a reflection of physical differences between the two methods in terms of contrast sensitivity, temporal resolution, and tracer diffusibility. First, CEUS has a high intrinsic sensitivity, which allows single bubbles the size of a red blood cell to be imaged and tracked at a depth of more than 10 cm from the transducer. This picomolar sensitivity is the result of the stable, resonant oscillatory behavior of the microbubbles. The echo from a single resonant bubble is more than a thousand billion times stronger than the echo from a red blood cell [27]. Furthermore, the nonlinear acoustic signature of the echo allows those generated by tissue to be suppressed with nonlinear detection methods such as pulse inversion [28]. As a result, the intrinsic contrast between contrast-enhanced blood and tissue in a CEUS image is very high. Enhanced CT, conversely, employs a soluble contrast agent whose effect is to increase attenuation, a property identical to that imaged in tissue. Therefore, if subtraction is not used, detectable vascular enhancement relies on an incremental increase in local attenuation within an image resolution voxel that is typically the volume of a very large number of red blood cells. With iodine-based contrast agents and the CT technology currently available in clinical settings, detectable increases in attenuation with a volume of contrast equal to that of a single red blood cell are inconceivable. Therefore, US is more sensitive to its tracer contrast material than tri-phasic CT. Second, the microbubbles used for US contrast agents remain exclusively within the vascular tree [29], so that in all enhancement phases, the microbubble signal reflects the relative volume of blood within the field of view. Iodinated contrast agents for CT, in comparison, diffuse through the vascular endothelium into the tissue interstitium, giving rise to the so-called interstitial phase of enhancement [30, 31]. Tumor neovascularity is known to be characterized by a hyperpermeable endothelium [32] that allows CT contrast agents to diffuse more rapidly into the tumor interstitium, so that “wash-out” in the portal venous phase can be masked by interstitial contrast. As a result, the contrast gradient between liver parenchyma and malignant thrombus tissue is lower on CT than on CEUS [33]. Third, CEUS imaging is performed in real time, typically at frame rates of 10–20 Hz, with a low MI, which produces stable, non-disruptive oscillation within the microbubble pool. Contrast-related imaging commences a few second after injection and is monitored continuously throughout the arterial, portal, and late phases [33]. Therefore, peak enhancement in the arterial phase is always imaged and can be easily identified within the target thrombus. Even when bolus tracking is used, contrast enhancement on CT is dependent on an assumption of uniformity in arterial transit kinetics that may not hold true for every patient. Transit times can be affected by local vascular abnormalities, such as aneurysms or arterio-venous and arterio-portal shunts, which are not uncommon in cirrhotic patients with HCC, as well as by hypertrophy of the thrombus-feeding artery/ies. Therefore, in the early arterial phase, image acquisition may not coincide precisely with the moment of maximum perfusion of thrombus tissue with the contrast agent. In this case, the visual contrast between thrombus tissue and surrounding parenchyma may not be high enough to ensure the detection of the thrombus.
Our data confirm previous reports on the limited sensitivity of CT in thrombus characterization [12], but the thrombus detection rates we observed with CT are lower than those reported by other investigators [12–14]. These discrepancies are probably due in large part to the fact that the majority of the thrombi examined in previous studies were located in the main portal trunk, whereas over two-thirds of the thrombi we analyzed involved segmental branches of the portal vein, where the sign of portal vein enlargement is not particularly useful. Furthermore, small thrombi that are continuous with the tumor mass can be easily missed by CT, which is unable to distinguish between tumor and thrombus tissues [12–14, 23].
It is also true, however, that all of the thrombi considered in this study were initially identified with US/cDUS, and this factor represents an undeniable bias in favor of CEUS detection over that of CT. This is the major limitation of our study. A second limitation is the low rate of benign thrombi in the series we studied. Under these circumstances, it is difficult to evaluate the specificities of the different techniques in thrombus characterization. On the other hand, as previously reported, in most cases portal vein thrombi in HCC patients are indeed malignant [20].
It is important to recall that the success of the CEUS examination, like that of other ultrasonographic studies, is strongly dependent on the skill, experience, and motivation of the sonographers; on the characteristics and compliance of the patient; and on the quality of the US equipment [6, 20, 23]. Used correctly, however, CEUS seems to offer several potential advantages over CT. It not only improves detection of small thrombi, it can also reliably identify those that are malignant. There is no doubt that intra-thrombus neovascularity can often be demonstrated with conventional cDUS and characterized by Doppler spectral analysis, and if this is the case, there is no indication for CEUS or for CT. However, false negatives were not uncommon with cDUS and spectral analysis in the present study, and in many cases like this CEUS can eliminate the need for biopsy. Furthermore, it is a valid non-invasive diagnostic technique for patients with absolute or relative contraindications to contrast-enhanced CT and MRI.
In conclusion, CEUS appeared to be a reliable tool for assessing portal vein patency in patients with HCC accompanying cirrhosis. It should be used for HCC staging in all patients with contraindications to CT/MRI. It should also be done in HCC patients who are potential candidates for liver transplantation or for surgical and percutaneous ablative therapies, even if CT findings are negative for portal vein thrombosis. Further studies are necessary to confirm our data and to assess the best diagnostic algorithm to evaluate portal vein system in patients with HCC accompanying cirrhosis.
References
McNamara C, Juneja S, Wolf M et al (2002) Portal or hepatic vein thrombosis as the first presentation of a myeloproliferative disorder in patients with normal peripheral blood counts. Clin Lab Haematol 24:239–242
Jansen HLA, Garcia-Pagan JC, Elias E et al (2003) Budd-Chiari syndrome: a review by an expert panel. J Hepatol 38:364–371
The liver cancer study group of Japan (1990) Primary liver cancer in Japan. Clinicopathologic features and results of surgical treatment. Ann Surg 211:277–287
Calvet X, Bruix J, Bru C et al (1990) Natural history of hepatocellular carcinoma in Spain. Five year’s experience in 249 cases. J Hepatol 10:311–317
Atri M, de Stempel J, Bret PM et al (1990) Incidence of portal vein thrombosis complicating liver metastasis as detected by duplex ultrasound. J Ultrasound Med 9:285–289
Lencioni R, Caramella D, Sanguinetti F et al (1995) Portal vein thrombosis after percutaneous ethanol injection for hepatocellular carcinoma: value of color Doppler sonography in distinguishing chemical and tumor thrombi. AJR Am J Roentgenol 164:1125–1130
Hassn AM, Al Fallouji MA, Ouf TI et al (2000) Portal vein thrombosis following splenectomy. Br J Surg 87:362–373
Nonami T, Yokoyama I, Iwatsuki S et al (1992) The incidence of portal vein thrombosis at liver transplantation. Hepatology 16:1195–1198
Llovet JM, Bustamante J, Castells A et al (2003) Natural history of unteated nonsurgical hepatocellular carcinoma: rationale for the design and evaluation of therapeutic trias. Hepatology 29:62–67
Bach AM, Hann LE, Brown KT et al (1996) Portal vein evaluation with US: comparison to angiography combined with CT arterial portography. Radiology 202:149–154
Kreft B, Strunk H, Flacke S et al (2000) Detection of thrombosis in the portal venous system: comparison of contrast-enhanced MR angiography with intraarterial digital subtraction angiography. Radiology 216:86–92
Taylor CR (1992) Computed tomography in the evaluation of the portal venous system. J Clin Gastroenterol 14:167–172
Tublin ME, Dodd GD 3rd, Baron RL (1997) Benign and malignant portal vein thrombosis: differentiation by CT characteristics. AJR Am J Roentgenol 168:719–723
Tessler FN, Gehring BJ, Gomes AS et al (1991) Diagnosis of portal vein thrombosis: value of color Doppler imaging. AJR Am J Roentgenol 157:293–296
Tanaka K, Numata K, Okazaki H et al (1993) Diagnosis of portal vein thrombosis in patients with hepatocellular carcinoma: efficacy of color Doppler sonography compared with angiography. AJR Am J Roentgenol 160:1279–1293
Finn JP, Kane RA, Edelman RR et al (1993) Imaging of the portal venous system in patients with cirrhosis: MR angiography versus duplex Doppler sonography. AJR Am J Roentgenol 161:989–994
Naik KS, Ward J, Irving HC et al (1997) Comparison of dynamic contrast-enhanced MRI and Doppler ultrasound in the pre-operative assessment of the portal venous system. Br J Radiol 70:43–49
Marshall MM, Beese RC, Muiesan P et al (2002) Assessment of portal venous system patency in the liver transplant candidate: a prospective study comparing ultrasound, microbubble-enhanced colour Doppler ultrasound, with arteriography and surgery. Clin Radiol 57:377–383
Ricci P, Cantisani V, Biancari F et al (2000) Contrast-enhanced color Doppler US in malignant portal vein thrombosis. Acta Radiol 41:470–473
Rossi S, Rosa L, Ravetta V et al (2006) Contrast-enhanced versus conventional and color Doppler sonography for the detection of thrombosis of the portal and hepatic venous systems. AJR Am J Roentgenol 186:1–11
Tarantino L, Francica G, Sordelli I et al (2006) Diagnosis of benign and malignant portal vein thrombosis in cirrhotic patients with hepatocellular carcinoma: color Doppler US, contrast-enhanced US, and fine-needle biopsy. Abdom Imaging 31:537–544
Vilana R, Bru C, Bruix J et al (1993) Fine-needle aspiration biopsy of portal vein thrombosis: value in detecting malignant thrombosis. AJR Am J Roentgenol 160:1285–1287
Dodd GD 3rd, Memel DS, Baron RL et al (1995) Portal vein thrombosis in patients with cirrhosis: does sonographic detection of intrathrombus flow allow differentiation of benign and malignant thrombus? AJR Am J Roentgenol 165:573–577
Rossi S, Garbagnati F, Lencioni R et al (2000) Unresectable hepatocellular carcinoma: percutaneous radiofrequency thermal ablation after occlusion of tumor blood supply. Radiology 217:119–126
Campbell MJ, Machin D (1999) Medical statistics: a commonsense approach, 3rd edn. Wiley, Chichester, 6:85–89; Appendix I:155–158
Hohmann J, Albrecht T, Hoffmann CW et al (2003) Ultrasonographic detection of focal liver lesions; increased sensitivity and specificity with microbubble contrast agent. Eur J Radiol 46:147–159
Ophir J, Parker KJ (1989) Contrast agents in diagnostic ultrasound. Ultrasound Med Biol 15:319–333
Burns PN, Wilson SR, Hope Simpson D (2000) Pulse inversion imaging of liver blood flow: an improved method for characterization of focal masses with microbubble contrast. Invest Radiol 35:58–71
Burns PN (2002) Contrast ultrasound technology. In: Solbiati L, Martegani A, Leen E, Correas J-M, Burns PN, Becker D (eds) Contrast-enhanced ultrasound of liver diseases. Springer, Milan, pp 1–19
Donahue KM, Burstein D, Manning WJ et al (1994) Studies of Gd-DTPA relaxivity and proton exchange rates in tissue. Magn Reson Med 32:66–76
Gardeur D, Lautrou J, Millard JC et al (1980) Pharmacokinetics of contrast media: experimental results in dog and man with CT implication. J Comput Assist Tomogr 4:178–185
Jain RK (1997) Vascular and interstitial physiology of tumours: role in cancer detection and treatment. In: Bicknell R, Lewis CE, Ferrara N (eds) Tumor angiogenesis. Oxford University Press, Oxford, pp 45–59
Wilson SR, Kim TK, Jang H-J et al (2007) Enhancement patterns of focal liver masses: discordance between contrast-enhanced sonography and contrast-enhanced CT and MRI. AJR Am J Roentgenol 189:W7–W12
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rossi, S., Ghittoni, G., Ravetta, V. et al. Contrast-enhanced ultrasonography and spiral computed tomography in the detection and characterization of portal vein thrombosis complicating hepatocellular carcinoma. Eur Radiol 18, 1749–1756 (2008). https://doi.org/10.1007/s00330-008-0931-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-008-0931-z