Abstract
The purpose was to evaluate the image quality of high-spatial resolution MRA of the renal arteries at 1.5 T after contrast-agent injection of 0.2 mmol/kg body weight (BW) in an interindividual comparison to 3.0 T after contrast-agent injection of 0.1 mmol/kg BW contrast agent (CA). After IRB approval and informed consent, 40 consecutive patients (25 men, 15 women; mean age 53.9 years) underwent MRA of the renal arteries either at a 1.5-T MR system with 0.2 mmol/kg BW gadobutrol or at a 3.0-T MR scanner with 0.1 mmol/kg BW gadobenate dimeglumine used as CA in a randomized order. A constant volume of 15 ml of these contrast agents was applied. The spatial resolution of the MRA sequences was 1.0 × 0.8 × 1.0 mm3 at 1.5 T and 0.9 × 0.8 × 0.9 mm3 at 3.0 T, which was achieved by using parallel imaging acceleration factors of 2 at 1.5 T and 3 at 3.0 T, respectively. Two radiologists blinded to the administered CA and the field strength assessed the image quality and the venous overlay for the aorta, the proximal and distal renal arteries independently on a four-point Likert-type scale. Phantom measurements were performed for a standardized comparison of SNR at 1.5 T and 3.0 T. There was no significant difference (p > 0.05) between the image quality at 3.0 T with 0.1 mmol/kg BW gadobenate dimeglumine compared to the exams at 1.5 T with 0.2 mmol/kg BW gadobutrol. The median scores were between 3 and 4 (good to excellent vessel visualization) for the aorta (3 at 1.5 T/4 at 3.0 T for reader 1 and 2). For the proximal renal arteries, median scores were 3 for the left and right renal artery at 1.5 T for both readers. At 3.0 T, median scores were 3 (left proximal renal artery) and 4 (right proximal renal artery) for reader 1 and 3 (left/right) for reader 2 at 3.0 T. For the distal renal arteries, median scores were between 2 and 3 at both field strengths (moderate and good) for both readers. The κ values for both field strengths were comparable and ranged between 0.571 (moderate) for the distal renal arteries and 0.905 (almost perfect) for the proximal renal arteries. In the phantom measurements, a 40% higher SNR was found for the measurements at 3 T with gadobenate dimeglumine. High-spatial resolution renal MRA at 3.0 T with 0.1 mmol/kg BW gadobenate dimeglumine yields at least equal image quality compared with renal MRA at 1.5 T with 0.2 mmol/kg BW gadobutrol.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Implementation of three-dimensional (3D) contrast-enhanced (CE) MR-angiography (MRA)-first described by Prince et al. in 1994-was a milestone in non-invasive vascular imaging [1]. MRA became an established non-invasive diagnostic tool for the evaluation of the renal vasculature [2]. With 3D CE MRA, a contiguous 3D data set with high-spatial resolution can be acquired during a single breathhold, also allowing for depiction of smaller vessel parts. The development of parallel imaging techniques (PI) enabled to further improve spatial and temporal resolution and hence to improve vessel visualization [3–5]. However, the use of parallel imaging is at the expense of signal-to-noise ratio (SNR), which is at least inversely proportional to the square root of the acceleration factor [6].
The 3.0-T MR scanners, which have recently become more widely available, potentially allow for a doubled SNR [7, 8]. In practice, the increased SNR can either be spent on increasing the spatial resolution or further decreasing the acquisition time by parallel imaging. As dielectric artifacts have no major impact on fast gradient spoiled sequences, CE MRA substantially benefits from the transition to 3.0 T [9, 10]. However, for an optimization of body imaging at 3.0 T, MR protocols have to be adjusted for occurring changes in tissue T1 and T2 relaxation parameters, which are field-strength dependent [11]. Because the longitudinal relaxation time (T1) of un-enhanced blood increases with higher field strength, and because the relaxivity of standard contrast agents remains relatively unchanged, sensitivity to injected gadolinium-based agents for CE MRA may be enhanced [12, 13].
Gadobutrol was the first MR contrast media that was specifically approved for CE MRA in 2003 with a 1-molar concentration. Compared to 0.5-M contrast agents, which had been used so far, 1-molar contrast agents yielded a higher signal intensity [14]. On the one hand, this is especially important for the application with parallel imaging techniques, because of the reduced SNR as discussed above [15]. On the other hand, the higher signal intensity of a 1.0-M contrast media formula may allow decreasing the contrast agent dose at 3.0 T. The relaxivity (plasma, 37°C) of gadobutrol at 1.5 T is 5.2 l*mmol-1*s-1 and 5.0 l*mmol-1*s-1 at 3.0 T [12]. Gadobenate dimeglumine is characterized by a slight protein binding and reveals a higher relaxivity (6.3 l*mmol-1*s-1 at 1.5 T, 5.5 l*mmol-1*s-1 at 3.0 T [12]) and thus yields a higher SNR than conventional contrast agents at the same concentration.
From theoretical thoughts as discussed above, it seems feasible to cut down contrast agent dose by using compounds with weak protein-binding properties at 3.0 T to achieve a similar enhancement as at 1.5 T [16]. Therefore, the purpose of this prospective study was to interindividually compare the image quality of renal MRA at 1.5 T with 0.2 mmol/kg BW gadobutrol to renal MRA at 3.0 T with 0.1 mmol/kg BW gadobenate dimeglumine.
Materials and methods
Subjects
After IRB approval and informed consent, 25 men and 15 women (mean age, 53.9 years) were enrolled in this study. Beside the well-known MRI contraindications, there were no special exclusion criteria for this study; all patients suitable for MRA exam were included. The patients were either referred to rule out renal artery stenosis (7/1.5 T, 5/3.0 T), to assess renal transplant dysfunction (4/1.5 T, 8/3.0 T), to be evaluated as a potential living kidney donor (1/1.5 T, 3/3.0 T), to detect re-stenosis after stenting of the renal arteries (5/1.5 T, 0/3.0 T) or to evaluate renal masses (4/1.5 T, 3/3.0 T).
MR imaging
All examinations were performed on 32-channel whole-body MR systems operating at 1.5 T (Magnetom Avanto, Siemens Medical Solutions, Erlangen, Germany) and 3.0 T (Magnetom Tim Trio; Siemens Medical Solutions), respectively using body matrix and spine matrix coils. The maximum FOV of each surface coil was 500 × 500 mm2; the number of elements was 2 × 3. There were no differences between the coils used at 1.5 T and 3.0 T. Based on a pre-defined randomization list, 40 patients were randomly assigned to either undergo an MRA exam at 1.5 T (n = 21) or at 3.0 T (n = 19). After 2D steady-state free precession sequences for localization of the renal arteries, a coronal three-dimensional fast low-angle shot (FLASH) sequence with Cartesian k-space sampling was acquired before and after intravenous bolus administration of a paramagnetic contrast agent. The detailed sequence parameters of the FLASH sequence for the MRA at 1.5 and 3.0 T are shown in Table 1. These sequences are part of the scanning protocols that are used in every day clinical routine at our institution and have been optimized for vessel imaging at the different magnetic field strengths before the study. The slab was tilted slightly from the coronal to the axial plane to include the abdominal aorta and the renal arteries.
For all examinations at 1.5 T, 15 ml of gadobutrol (Gadovist®, Bayer-Schering Pharma, Berlin, Germany) was administered independent of body weight, which equals a dose of 0.2 mmol/kg BW 1.0 M for a standard patient of 75 kg BW. For the examinations at 3.0 T, 15 ml of gadobenate dimeglumine (MultiHance®, Bracco, Milan, Italy) was used, which corresponds to an amount of 0.1 mmol/kg BW 0.5 M for a standard patient of 75 kg BW. All injections were performed through an 18-gauge needle catheter placed in an antecubital vein of the patients using an automated injector system (Spectris Solaris, Medrad, Indianola, PA, at 1.5 T and Spectris Solaris EP, Medrad, at 3.0 T) with a flow rate of 2 ml/s at 1.5 T and 2.5 ml/s at 3.0 T, followed by a saline chaser of 30 ml injected at the same corresponding flow rate. In order to assume data acquisition at k-space center (time-to-center 7.4 s at 3.0 T) with a shortened overall acquisition time of 19 s, a faster injection rate is needed at 3.0 T because lung passages stretch the bolus.
For correct timing of the MRA, a test-bolus technique was applied, with an axial slice being positioned perpendicular to the aorta at the level of the renal arteries. The test-bolus technique has been used in our institution for 10 years. Due to the persistently good image with the test-bolus approach, we did not use a dynamic approach. From the time of peak enhancement in the test-bolus measurement, the time to center of the MRA sequence was subtracted to determine the MRA start delay [MRA start delay (s) = time of peak enhancement (s) - time to center (s)].
Image analysis
All postprocessing was performed on a dedicated post-processing workstation (Leonardo, Siemens Medical Solutions, Erlangen, Germany) by two radiologists independent from another (with 6 and 10 years’ experience in vascular MR imaging) who were blinded to field strength, the type of contrast agent used and any other patient data. These readers had access to the coronal source data and were also able to interactively make use of 3D post-processing capabilities, including multiplanar reformatting and maximum intensity projection.
The image quality of five vessel segments (aorta, left/right main renal arteries, left/right segmental renal arteries) was assessed on a four-point Likert-type scale, with 1 representing non-diagnostic (no signal enhancement within the vessel lumen), with 2 moderate (signal enhancement within the vessel lumen, but still inhomogeneous; incomplete delineation of the vessel border; evaluation possible with low diagnostic confidence), 3 good visualization (good signal enhancement within the vessel lumen, almost completely homogeneous; incomplete delineation of the vessel border; evaluation possible with satisfactory diagnostic confidence), and 4 representing excellent visualization (high and completely homogenous signal enhancement within the vessel lumen; optimal delineation of the vessel border; evaluation possible with high diagnostic confidence).
Venous overlay was defined as the simultaneous contrast enhancement of arterial and venous vessels, and it was assessed on a four-point scale, with 1 representing non-diagnostic; 2, major affecting diagnostic evaluation; 3, minor, not affecting diagnostic evaluation; 4, none.
Phantom measurements
As shown previously, SNR evaluation in data acquisition applyed using parallel imaging techniques is challenging [6]. This is because the non-uniform distribution of noise in images acquired with parallel imaging prohibits conventional region-of-interest (ROI)-based approaches for SNR evaluation. Therefore, phantom measurements were performed [17]. For this purpose a water-filled phantom constantly tempered to 37°C and filled with 18 vials of gadolinium contrast agent dissolved in a solution containing 70 mg/l bovine albumin was imaged 20 times with the MRA sequence at 1.5 T and 3.0 T. Sequence parameters for the measurements at 1.5 T and 3.0 T were exactly the same as for the patient measurements. SNR was calculated as follows [6]: the mean signal of the 20 measurements was used as the signal intensity (SI) of the phantom, and the pixel-wise calculated mean SD of the 20 measurements was used to describe the noise. SNR was then calculated: SNR = SI [mean SI of repeated measurements]/noise [mean SD repeated measurements]. The phantom contained nine vials of gadobenate dimeglumine and of gadobutrol with the following concentrations of gadolinium (mmol/l): 0.03125, 0.0625, 0.125, 0.25, 0.5, 1 and 2. These different concentrations were chosen to reflect the typical arterial signal that can be expected in patients after the bolus injection of the contrast agent [18]. The phantom was also used to determine the relaxivity of both contrast agents. For this purpose, a 2D saturation-recovery Turbo-FLASH sequence with linear encoding was repeatedly applied with inversion times TI ranging from the minimal value TI0 to TI = 800 ms in steps of 25 ms. A ROI was drawn manually inside each vial, and the T1 values were determined by fitting the signal offset S(ΔTI) = S(TI0+ΔTI) - S(TI0) to a mono-exponential recovery curve S0*[1-exp(-ΔTI/T1)]. The proportionality constant S0 is a function of the flip angle, the number of phase encoding lines, TE/T2, equilibrium magnetization and coil sensitivity, but is of no relevance for the purposes of this study. The fitted relaxation rates 1/T1 were plotted as a function of the concentration, and the relaxivity was calculated as the slope of the best linear fit to these data. Sequence parameters at 1.5 T were as follows: flip angle 12°, TR 278 ms, TI0 150 ms, acquisition matrix 112*192, sampling percentage 70, reconstructed matrix 320*384, TE 0.95 ms, bandwidth 900 Hz/pixel, four slices, slice thickness 8 mm and slice gap 8.8 mm. Sequence parameters at 3 T were: flip angle 8°, TR 177 ms, TI0 71 ms, acquisition matrix 176*192, sampling percentage 100, reconstructed matrix 352*384, TE 0.93 ms, bandwidth 965 Hz/pixel, five slices, slice thickness 8 mm and slice gap 12 mm. Each measurement was repeated ten times, and signals were averaged prior to post-processing in order to improve on SNR.
Statistical analysis
All parameters were recorded in an electronic data sheet (Excel 2003, Microsoft Inc., Redmond, WA) and subjected to statistical analysis using SPSS version 14.0 for Windows software (SPSS 2006, SPSS Inc., Chicago IL). As the data of the reading are ordinal in character, the median values were determined. Differences between vessel conspicuity at 1.5 T and 3.0 T CE-MRA were compared with a Mann-Whitney-U test for ordinal data. A p-value of <0.05 was considered to indicate statistically significant differences. We assumed as null hypothesis H0 that there would be no significant difference in vessel conspicuity between 1.5-T MRA with 0.2 mmol/kg of contrast agent and 3.0-T MRA with 0.1 mmol/kg of contrast agent. Interobserver agreement was determined using kappa-statistics. Interobserver agreement was considered as slight, k ≤ 0.2; fair, k = 0.21–0.40; moderate, k = 0.41–0.60; substantial, k = 0.61–0.80 or almost perfect, k = 0.81–1.00.
Results
All MR examinations were performed successfully. None of the patients experienced an adverse event or any kind of problems due to the applied contrast agent or magnetic field strength. In all cases imaging of the renal vasculature was successful either with 0.2 mmol/kg BW gadobutrol at 1.5 T or 0.1 mmol/kg BW gadobenate dimeglumine at 3.0 T.
Phantom measurements
The phantom measurements showed on average a 40% higher SNR with gadobenate dimeglumine when compared to equal concentrations of gadobutrol at both field strengths. The higher enhancement can mainly be attributed to the higher relaxivity (R1) of gadobenate dimeglumine, which was measured as 7.4 and 5.2 l*mmol-1*s-1 at 1.5 T and 3.0 T, respectively, in comparison to 4.6 and 3.9 l*mmol-1*s-1 with gadobutrol at 1.5 T and 3.0 T. With both contrast agents a mean increase in SNR of 80% could be detected with the transition from 1.5 T to 3.0 T. An overview of the measured SNR values can be found in Fig. 1.
Analysis of image quality
All images were of adequate quality. The median scores of image quality for 0.2 mmol/kg BW gadobutrol and 0.1 mmol/kg BW gadobenate dimeglumine are listed in Table 2. Examples of very good image quality are seen in Figs. 2 and 3. A good to excellent vessel visualization (score 3 and 4) was the most common finding in almost all arterial vessels up to the proximal renal arteries at 1.5 T and 3.0 T. In comparison, the visualization of distal renal arteries revealed a lower median score of 2 to 3. There was no statistical significant difference between 1.5 T and 3.0 T (p > 0.05, Table 3) for all vessel segments. The venous overlay was not statistically significant between 1.5 T and 3.0 T either (p > 0.05). Venous overlay was most frequently rated as having minimal influence on diagnostic accuracy (mode score 3). An intraindividual comparison of image quality at 1.5 T and 3.0 T in a single patient pre- and post-reanastomosis of a transplant renal artery is shown in Fig. 4.
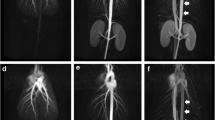
A 30-mm-thin MIP of a renal MRA of a 25-year-old female patient after dilatation of a distal stenosis of the right renal artery (a). Coronal source data of the 3D CE MRA of the same patient at 1.5 T and a contrast agent dose of 0.2 mmol/kg BW gadobutrol. Both readers rated the image quality with mode score 4, representing excellent visualization (b)
Source images of a 3D renal MRA at 3.0 T after administration of 0.1 mmol/kg BW in a 46-year-old male patient after renal transplantation. Both readers estimated image quality as excellent (score 4) (a). The 30-mm-thin MIPs clearly demonstrate the anastomosis between the donor renal artery and the external iliac artery on the left side. Both readers depicted the short, circular 50% stenosis of the graft artery (b)
The 30-mm-thin MIPs of a 3D renal MRA at 3.0 T of a 21-year-old female patient after renal transplantation shows a high grade stenosis of the graft artery (a). On the caudal pole of the renal graft there is a defective area of parenchymal contrast enhancement. Three coronal source images of a 3D MRA at 1.5 T of the same patient after re-operation of the described vessel stenosis (b). The defective area on the caudal pole of the renal graft is regressing
In terms of image quality, both readers found no difference of the vessel conspicuity with 0.2 mmol/kg gadobutrol at 1.5 T compared to 0.1 mmol/kg gadobenate dimeglumine at 3.0 T (Table 3). Vessel conspicuity was assessed according to the presented Likert scale. Aorta, proximal and distal renal arteries were depicted without significant differences (p > 0.05). Figure 5 shows an example of bad image quality with a low signal-to-noise ratio, noisy background and an inhomogenous signal within the vessel lumen.
Analysis of interobserver agreement
The overall agreement between the two readers regarding image quality was almost perfect at 1.5 T with gadobutrol used as contrast agent (κ=0.827) and also almost perfect at 3.0 T with gadobenate dimeglumine (κ=0.818). For the proximal renal arteries, interobserver agreement was considered as substantial to almost perfect for both administered contrast agents. For the distal renal arteries, interobserver agreement was assessed as slightly worse. An entire overview of the κ-values for the different vascular areas and for both readers is given in Table 4.
Discussion
The results of this study suggest that renal contrast-enhanced MRA with a dose of 0.1 mmol/kg BW gadobenate dimeglumine at 3.0 T is feasible and shows at least comparable image quality to renal MRA obtained at 1.5 T with 0.2 mmol/kg BW gadobutrol. As we used different sequence parameters of the MRA sequence at 1.5 T and 3.0 T and altered the amount and kind of contrast agent at the same time, the results cannot be allocated to a single parameter, but only to a combination of the contrast agent-specific MRA sequences and the specific contrast agents.
Three-dimensional CE-MRA is routinely performed with extracellular contrast media based on gadolinium as the paramagnetic ion. Gadopentetate dimeglumine (Gd-DTPA, Magnevist®; Bayer-Schering Pharma, Berlin, Germany) was the first contrast agent to be approved for clinical use in 1988 [19] and has been used as 0.5-M standard extracellular Gd-chelate so far. Gadopentetate dimeglumine already allows for good MRA image quality, but with increasing spatial resolution and the widespread use of parallel imaging further limiting the available SNR standard Gd-chelates are often not sufficient for high-spatial resolution MRA. Dedicated contrast agents, such as gadobutrol and gadobenate dimeglumine, are advantageous for these indications [20, 21]. Gadobutrol 1.0 is an extracellular, macrocyclic contrast agent. As gadobutrol has a lower osmolality and viscosity compared to Gd-DTPA, a high concentration 1.0 M formula can be offered [22], providing twice the concentration of other currently used 0.5-M Gd-based contrast media. A substantially enhanced image quality with higher SNR and CNR values was described at field strengths below 3.0 T for 1.0 M gadobutrol [23, 24]. Gadobutrol has a similar relaxivity as the other Gd-chelates, but the higher concentration reduces the effect of bolus dispersion that occurs during the pulmonary passage [20]. For gadobenate dimeglumine (MultiHance®, Bracco, Milan, Italy), another 0.5-M paramagnetic contrast agent, the 0.5-M Gd-chelate has a transient binding to albumin, which has been implicated as the cause for improved intra-arterial enhancement [18, 25]. Depending on the field strength, 0.5-M gadobenate dimeglumine reveals a higher (20–50%) relaxivity than other contrast agents due to this weak protein interaction [26]. This behavior has been confirmed in our own relaxivity measurements and is responsible for the known higher SNR and CNR [21, 27] of gadobenate dimeglumine as given in the literature compared to other 0.5-M contrast agents. As both contrast agents lead to a higher CNR, they seem to be useful for perfusion-weighted (PW) techniques and high-spatial resolution MRA to compensate for the SNR loss.
Reducing the amount of administered contrast is not only of economic concern, but is also strongly required in patients with chronic renal failure. With the recently and immediately after the end of our study postulated association of nephrogenic systemic fibrosis-a severe and potentially lethal disease in patients with impaired renal function-a minimization of the administered dose of gadolinium seems appealing [28, 29]. The postulated association between nephrogenic systemic fibrosis and the application of higher doses of Gd-chelates questions the safety of Gd-chelates in patients with impaired renal function [29–31]. Therefore, the possibility of using lower doses of gadobenate dimeglumine without loss in image quality is very appealing. NSF almost exclusively occurred in patients with an end-stage chronic renal failure and a glomerular filtration rate of less than 15 ml/min after administration of larger amounts of contrast agent up to 40 ml. In these renally impaired patients, the half-life time of standard Gd-chelates can be as high as 50 h compared to up to 3 h in healthy patients. Therefore, at this time it is recommended to use as little Gd-chelates as possible in patients with drastically reduced glomerular filtration rates [32].To maintain a high diagnostic quality-even with a reduced amount of contrast agent-imaging can be either performed at higher field strength with higher inherent SNR or a contrast agent with higher relaxivity can be applied. A further potential benefit of high-field MRA is the prolonged longitudinal tissue relaxation time T1 in background tissue and unenhanced blood. Overall, the sensitivity to injected gadolinium-based contrast agents for contrast-enhanced MRA is enhanced at 3.0 T[33]. In consequence, there is an increased contrast between contrast-enhanced vascular structures and the surrounding background tissue in T1-weigthed images at 3.0 T [10, 34].
In our study a state-of-the-art 3D CE MRA was acquired with submillimeter isotropic voxels of 0.9 × 0.8 × 0.9 mm3 at 3.0 T and compared to state-of-the-art 1.5-T MRA with a voxel size of 1 × 0.8 × 1 mm3. Using a parallel imaging acceleration factor of 3 at 3.0 T, an acquisition time of 19 s was reached, while the acquisition time was 26 s at 1.5 T with a parallel imaging factor of 2. The shorter acquisition times at 3.0 T are particularly valuable for critically ill patients and for a better, motion-free depiction of the distal part of the renal arteries. This might be especially helpful for the accurate detection and grading of distal renal artery lesions, such as fibromuscular dysplasia. Decreasing the voxel size, applying higher factors of parallel imaging and reducing the slice thickness substantially diminish the SNR. Even though this should be detrimental to vessel delineation, there was no statistically significant difference at both field strengths regarding vessel conspicuity. This implies that at 3.0 T, a single dose of higher relaxivity contrast agent with a weak albumin interaction is sufficient for enhancement of the target vessels. Using the same reading guidelines, it is of interest that the overall interobserver agreement regarding image quality is better at 3.0 T than at 1.5 T. The faster injection of the contrast agent bolus that is required for the fast MRA acquisition at 3.0 T did not lead to more venous contamination; this might have been expected because shortening of the acquisition time leads to a small time window within exact arterial contrast enhancement before venous overlay is possible.
Some limitations of our study need to be mentioned. As we altered the sequence parameters of the MRA sequence and the amount and kind of contrast agent at the same time, the results cannot be allocated to a single parameter. We did not differ among the various forms of renal diseases of the examined patients either. Thus, comparability of the vasculature and therefore image quality might be restricted to a certain extent. Moreover, the concentration of Gd-containing contrast agents in the renal arteries depends on factors such as body mass, blood volume as well as circulation time [35]. They are affected by patient-based hemodynamic parameters such as heart rate, blood pressure and cardiac ejection fraction, which we did not take into account.
In summary, our study shows that at 3.0 T a submillimeter high-spatial resolution MRA with 0.1 mmol/kg BW gadobenate dimeglumine yields at least comparable image quality to state-of-the art 1.5-T MRA with 0.2 mmol/kg BW gadobutrol.
References
Prince MR (1994) Gadolinium-enhanced MR aortography. Radiology 191:155–164
Tan KT, van Beek EJ, Brown PW et al (2002) Magnetic resonance angiography for the diagnosis of renal artery stenosis: a meta-analysis. Clin Radiol 57:617–624
Pruessmann KP, Weiger M, Scheidegger MB, Boesiger P (1999) SENSE: sensitivity encoding for fast MRI. Magn Reson Med 42:952–962
Griswold MA, Jakob PM, Heidemann RM et al (2002) Generalized autocalibrating partially parallel acquisitions (GRAPPA). Magn Reson Med 47:1202–1210
Nael K, Michaely HJ, Villablanca P et al (2006) Time-resolved contrast enhanced magnetic resonance angiography of the head and neck at 3.0 Tesla: Initial results. Invest Radiol 41:116–124
Reeder SB, Wintersperger BJ, Dietrich O et al (2005) Practical approaches to the evaluation of signal-to-noise ratio performance with parallel imaging: application with cardiac imaging and a 32-channel cardiac coil. Magn Reson Med 54:748–754
Campeau NG, Huston J, 3rd, Bernstein MA, Lin C, Gibbs GF (2001) Magnetic resonance angiography at 3.0 Tesla: initial clinical experience. Top Magn Reson Imaging 12:183–204
Runge VM, Biswas J, Wintersperger BJ et al (2006) CMR 2005: 6.03: The efficacy of MultiHance (gadobenate dimeglumine or Gd-BOPTA) at 1.5 and 3 T in a rat brain glioma model. Contrast Media Mol Imaging 1:68–69
Fenchel M, Nael K, Deshpande VS et al (2006) Renal magnetic resonance angiography at 3.0 Tesla using a 32-element phased-array coil system and parallel imaging in 2 directions. Invest Radiol 41:697–703
Michaely HJ, Nael K, Schoenberg SO et al (2005) The feasibility of spatial high-resolution magnetic resonance angiography (MRA) of the renal arteries at 3.0 T. Rofo 177:800–804
de Bazelaire CM, Duhamel GD, Rofsky NM, Alsop DC (2004) MR imaging relaxation times of abdominal and pelvic tissues measured in vivo at 3.0 T: preliminary results. Radiology 230:652–659
Rohrer M, Bauer H, Mintorovitch J, Requardt M, Weinmann HJ (2005) Comparison of magnetic properties of MRI contrast media solutions at different magnetic field strengths. Invest Radiol 40:715–724
Nael K, Michaely HJ, Lee M et al (2006) Dynamic pulmonary perfusion and flow quantification with MR imaging, 3.0T vs. 1.5T: initial results. J Magn Reson Imaging 24:333–339
Tombach B, Benner T, Reimer P et al (2003) Do highly concentrated gadolinium chelates improve MR brain perfusion imaging? Intraindividually controlled randomized crossover concentration comparison study of 0.5 versus 1.0 mol/L gadobutrol. Radiology 226:880–888
Schoenberg SO, Rieger J, Weber CH et al (2005) High-spatial-resolution MR angiography of renal arteries with integrated parallel acquisitions: comparison with digital subtraction angiography and US. Radiology 235:687–698
Trattnig S, Pinker K, Ba-Ssalamah A, Nöbauer-Huhmann IM (2006) The optimal use of contrast agents at high field MRI. Eur Radiol 16:1280–1287
Zech CJ, Herrmann KA, Huber A et al (2004) High-resolution MR-imaging of the liver with T2-weighted sequences using integrated parallel imaging: comparison of prospective motion correction and respiratory triggering. J Magn Reson Imaging 20:443–450
Knopp MV, Schoenberg SO, Rehm C et al (2002) Assessment of gadobenate dimeglumine for magnetic resonance angiography: phase I studies. Invest Radiol 37:706–715
Kirchin MA, Runge VM (2003) Contrast agents for magnetic resonance imaging: safety update. Top Magn Reson Imaging 14:426–435
Michaely HJ, Dietrich O, Nael K et al (2006) MRA of abdominal vessels: technical advances. Eur Radiol 16:1637–1650
Herborn CU, Lauenstein TC, Ruehm SG et al (2003) Intraindividual comparison of gadopentetate dimeglumine, gadobenate dimeglumine, and gadobutrol for pelvic 3D magnetic resonance angiography. Invest Radiol 38:27–33
Tombach B, Heindel W (2002) Value of 1.0- M gadolinium chelates: review of preclinical and clinical data on gadobutrol. Eur Radiol 12:1550–1556
Goyen M, Lauenstein TC, Herborn CU et al (2001) 0.5 M Gd chelate (Magnevist) versus 1.0 M Gd chelate (Gadovist): dose-independent effect on image quality of pelvic three-dimensional MR-angiography. J Magn Reson Imaging 14:602–607
Knopp MV, von Tengg-Kobligk H, Floemer F, Schoenberg SO (1999) Contrast agents for MRA: future directions. J Magn Reson Imaging 10:314–316
Cavagna FM, Maggioni F, Castelli PM et al (1997) Gadolinium chelates with weak binding to serum proteins. A new class of high-efficiency, general purpose contrast agents for magnetic resonance imaging. Invest Radiol 32:780–796
Kirchin MA, Pirovano GP, Spinazzi A (1998) Gadobenate dimeglumine (Gd-BOPTA). An overview. Invest Radiol 33:798–809
Goyen M, Debatin JF (2003) Gadobenate dimeglumine (MultiHance) for magnetic resonance angiography: review of the literature. Eur Radiol 13(3):N19–N27
Thomsen HS (2006) Nephrogenic systemic fibrosis: a serious late adverse reaction to gadodiamide. Eur Radiol 16:2619–2621
Broome DR, Girguis MS, Baron PW et al (2007) Gadodiamide-associated nephrogenic systemic fibrosis: why radiologists should be concerned. AJR Am J Roentgenol 188:586–592
Grobner T (2006) Gadolinium-a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis? Nephrol Dial Transplant 21:1104–1108
Boyd AS, Zic JA, Abraham JL (2007) Gadolinium deposition in nephrogenic fibrosing dermopathy. J Am Acad Dermatol 56:27–30
Thomsen HS (2007) European Society of Urogenital Radiology guidelines on contrast media application. Curr Opin Urol 17:70–76
Rinck PA, Muller RN (1999) Field strength and dose dependence of contrast enhancement by gadolinium-based MR contrast agents. Eur Radiol 9:998–1004
Allkemper T, Heindel W, Kooijman H, Ebert W, Tombach B (2006) Effect of field strengths on magnetic resonance angiography: comparison of an ultrasmall superparamagnetic iron oxide blood-pool contrast agent and gadopentetate dimeglumine in rabbits at 1.5 and 3.0 tesla. Invest Radiol 41:97–104
Hany TF, McKinnon GC, Leung DA et al. (1997) Optimization of contrast timing for breath-hold three-dimensional MR angiography. J Magn Reson Imaging 7:551–556
Author information
Authors and Affiliations
Corresponding author
Additional information
Ulrike I. Attenberger and Henrik J. Michaely contributed equally.
Rights and permissions
About this article
Cite this article
Attenberger, U.I., Michaely, H.J., Wintersperger, B.J. et al. Three-dimensional contrast-enhanced magnetic-resonance angiography of the renal arteries: Interindividual comparison of 0.2 mmol/kg gadobutrol at 1.5 T and 0.1 mmol/kg gadobenate dimeglumine at 3.0 T. Eur Radiol 18, 1260–1268 (2008). https://doi.org/10.1007/s00330-008-0873-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-008-0873-5