Abstract
The magnetic resonance imaging (MRI) appearances of recurrent prostate cancer following radical prostatectomy have been documented in the radiology literature; however little has been written on the range of normal post-operative appearances. Common routes of surgical access for radical prostatectomy include retropubic and transperineal, although newer minimally invasive methods are gaining increasing acceptance. Specifically the range of appearances of the anastomotic site, the prostatic bed, the position of the bladder base, periurethral tissue, levator sling, rectum and residual seminal vesicles (if present) are demonstrated. A non-enhancing low signal nodule is frequently seen at the vesicourethral anastomosis or within the seminal vesicle remnant and usually represents fibrosis. Appearances following different surgical accesses do not differ tremendously, although the retropubic fat pad is reduced or absent following a retropubic approach. Anterior rectal-wall scarring may be present following a transperineal approach. Other post-surgical findings that may mimic disease include a lymphocoele and injected bladder-neck bulking agent. Many patients referred for MRI following radical prostatectomy will have a pathological study showing disease recurrence, although in non-pathological studies the radiological features can differ significantly. It is important for the radiologist to be aware of the spectrum of normal post-surgical appearances so not to confuse these with locally recurrent disease.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In this article, we discuss and illustrate the spectrum of imaging features of the normal post-prostatectomy pelvis, in order to ensure the radiologist does not confuse these with locally recurrent disease.
Surgical therapy
Radical prostatectomy is the most frequently used treatment for carcinoma confined to the prostate [1–3]. It involves removal of the entire prostate gland and the seminal vesicles, with the formation of an anastomosis between the membranous urethra and the bladder. One or both of the neurovascular bundles surrounding the gland are preserved if possible in order to salvage potency [3, 4].
The two traditional approaches to radical prostatectomy are transperineal and retropubic [5, 6]. An advantage of the retropubic prostatectomy is the ability to perform a concomitant pelvic lymphadenectomy, and as a result this has become the most common method of radical prostatectomy. Nowadays, due to earlier disease detection, more men are diagnosed with early-stage disease for which lymphadenectomy is not mandatory. Consequently, transperineal prostatectomy has had a resurgence, as it can be performed with less blood loss, operative time, and overall patient morbidity than retropubic prostatectomy [7]. Rectal injury occurs with greater frequency with transperineal prostatectomy than with the retropubic approach. However, if the rectum is adequately prepared, any injury promptly recognised and repaired, and post-operative care appropriate, the great majority of cases will not lead to attendant morbidity [8, 9]. Transperineal prostatectomy is comparable to retropubic prostatectomy for obtaining adequate surgical margins, and also in terms of quality-of-life outcome measures, particularly continence and potency [10, 11]. Laparoscopic radical prostatectomy initially had no advantage over the standard of open retropubic prostatectomy; however with advances in medical technology, laparoscopic radical prostatectomy is increasingly being utilised [12]. Based on follow-up of 1,000 consecutive cases, an evaluation by Guillonneau et al. confirmed that laparoscopic radical prostatectomy provides satisfactory results in regard to local tumour control and biochemical recurrence [13]. The latest technology includes further minimally invasive approaches such as robotic-guided surgery [14–16].
General MR imaging findings post-operatively
MR imaging technique
Our protocol involves the patient being positioned supine with a phased-array pelvic receiver coil placed at the patient’s pelvis. Patients are administered 10 mg of hyoscine butylbromide intramuscularly as a spasmolytic agent just prior to the scan but do not undergo bowel preparation or air insufflation. MR imaging sequences at 1.5 Tesla include axial T1-weighted (T1W) and fast spin-echo T2-weighted (T2W) sequences of the whole pelvis. Small field-of-view high resolution T2W sequences in both the coronal and axial planes are then undertaken. No further sequences are routinely performed if the anatomy appears unremarkable. However if there is any asymmetry or focal thickening of any post-operative structure then this is evaluated with dynamic contrast-enhanced T1-weighted images [17, 18].
It was felt that the standard sequences described above were diagnostically adequate in the majority of cases, and reflect the current working practice of most MRI units performing prostate studies in Europe. An endorectal coil was not used for these examinations, despite showing superior contrast resolution in the assessment of the pre-operative gland when compared to conventional imaging [19, 20]. In our tumour practice we do not use endorectal coil imaging when evaluating the pelvis following a radical prostatectomy. Although an endorectal coil may provide high resolution imaging of the prostate bed, our aim is to image the whole pelvis looking for local recurrence or adenopathy. In addition, a small or equivocal lesion at the prostate bed can be further evaluated with a transrectal ultrasound and biopsy.
We performed our evaluation by reviewing the scans of many patients who had had a prior radical prostatectomy and a disease-free pelvic MRI study at our institution over a 10-year period from 1997 to 2006. Although the MRI protocols varied over that time period, the standard technique outlined was achieved in the majority of patients, particularly latterly. In addition patients attended our unit with pelvic MRI scans already performed at other institutions, although the protocols used in these cases varied considerably.
Indications for a post-operative MRI study to exclude suspected recurrence include an elevated PSA level or an abnormal digital rectal examination.
Normal post-operative findings
In the coronal plane of imaging, the bladder base and levator sling descend caudally and anteriorly post-operatively into the space vacated by the resected prostate. The urogenital diaphragms tend to stay in a similar position (Fig. 1). Axially on imaging the mid pelvis, there is a notable increase in prominence of fat surrounding the descended bladder base and the cranial aspect of the levator sling, which have not yet descended into the upper part of the external anal sphincter. This fat has filled the space of the resected prostate (Fig. 2). To a variable degree, vas deferens and seminal vesicle remnants are present, visualised as a linear low/intermediate signal on both T1W and T2W imaging. There may be linear low signal (on both T1W and T2W imaging) in the anterior rectal wall in keeping with anterior rectal wall fibrosis. There may be a variable degree of low signal (on both T1W and T2W imaging) at the site of the anastomosis of the urethra and bladder in keeping with normal post-surgical fibrosis, which has even been quantified using transrectal ultrasound in previous studies [21, 22]. Wasserman et al. showed in 80% of patients that a hypoechoic soft-tissue lesion (average volume 1.7 cm3) was seen anterior to the anastamosis and indented the anterior bladder wall [21].
Coronal T2-weighted images in a 51-year-old man showing normal anatomy following a radical retropubic prostatectomy. a At the level of the anterior acetabulae, pre-operatively, the obturator internus muscles (OI) and urogenital diaphragms (UG) are present. Post-operatively the bladder has clearly descended caudally and also anteriorly. Levator muscles (L) are also now seen. b At the level of the mid acetabulae, the bladder neck (BN) and levator sling (L) can be seen to be low lying in the pelvis post-operatively. At the same level, pre-operatively, the mid prostate gland is shown (P)
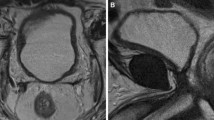
Axial T2-weighted images in a 51-year-old man showing normal anatomy following a radical retropubic prostatectomy. a At the level of the lower acetabulae, post-operatively, the seminal vesicle remnants (SVR) appear as intermediate/low signal [low signal on T1W (not shown)]. Note also the increased prominence of fat surrounding the bladder base, which has descended post-operatively. b At the level of the lower pubic symphysis, pre-operatively, the prostatic apex is visualised (Ap). Post-operatively, there is a linear low signal [also on the T1W image (not shown] between bladder and rectum in keeping with fibrosis (Fi). Note that the lower bladder is still in view and there is an increase in prominence of fat surrounding the bladder base and the levator muscles (L)
In the sagittal plane of imaging, post-operative bladder descent is striking (Fig. 3). A surgical incision within the lower anterior abdominal wall may confirm a retropubic approach, and linear fibrosis of Denonvilliers fascia is also indicative of post-surgical change.
Sagittal T2-weighted images in a 51-year-old man showing normal anatomy following a radical prostatectomy. a Images near the midline show prostate and seminal vesicle (SV) pre-operatively. Post-operatively bladder descent is noted. Fibrosis of the lower anterior abdominal wall confirms a retropubic approach (F1). Linear fibrosis of Denonvilliers is also indicative of post-surgical change (F2). b Images parasagittally show prostate and seminal vesicle (SV) pre-operatively. Post-operatively, bladder descent and seminal vesicle remnant (SVR) are present
In normal post-surgical cases without recurrent disease there is usually no enhancement of the prostatic bed in the arterial phase after administration of IV gadolinium and sometimes some uniform but poor enhancement in the venous phase. This should be distinguished from disease, which usually shows avid heterogeneous enhancement in the arterial phase and often signal washout in the venous phase.
Metallic clip sutures may cause significant susceptibility artefacts within the post-operative pelvis, depending on their size, number and location, rendering imaging more difficult to interpret.
Imaging findings specific to technique
Retropubic prostatectomy surgical technique
A vertical lower-midline abdominal incision is performed from the umbilicus to the level of the symphysis, possibly followed by an extraperitoneal bilateral pelvic lymphadenectomy (if indicated). The retropubic fat is removed, and the endopelvic fascia is incised. The dorsal venous complex is then divided, and an incision is then passed to the urethra and bladder and advanced under the posterior aspect of the prostate [4]. The neurovascular bundle is swept off the prostate cranially and posteriorly. The membranous urethra is divided at the apex of the prostate. The prostate gland is mobilised cephalad and the anterior layer of Denonvilliers fascia divided (Fig. 4). The vas deferens and the seminal vesicles are then divided, the latter near to their tips, to prevent injury to the pelvic plexus, which lies close to the lateral aspect of the seminal vesicles. The bladder neck is then resutured.
T2-weighted images in a 66-year-old man with white lines representing the surgical planes of the radical retropubic prostatectomy approach. a A lower-midline abdominal incision to just cranial to the symphysis may be followed by an extraperitoneal bilateral pelvic lymphadenectomy if indicated. Retropubic fat is then removed, and the endopelvic fascia is incised, and the dorsal venous complex is divided. b An inverted V incision in the exposed prostatic fascial edge passes to the urethra and bladder and is advanced under the posterior aspect of the prostate. The neurovascular bundle is swept off the prostate cranially and posteriorly. c The membranous urethra is divided at the apex of the prostate. The prostate gland is mobilised cephalad and the anterior layer of Denonvilliers fascia divided
The imaging findings specific to post-retropubic prostatectomy cases include loss of the normal retropubic fat pad and of the dorsal venous complex (Fig. 5).
Specific T2-weighted MRI features in a 58-year-old man post-radical retropubic prostatectomy. Prominent retropubic fibrosis (RPF) with loss of the normal fat pad is often visualised following this approach. There is no evidence of fibrosis of the anterior anal sphincter (AS), which appears unremarkable, unlike following transperineal surgery
Transperineal prostatectomy surgical technique
An inverted-U incision is made in the perineum, with the ends medial to the ischial tuberosities and anterior to the mid anal line and with the apex in the mid perineum. The ischiorectal fossa is incised on either side of the central tendon, and the central tendon is divided. Dissection continues to the fibrous confluence posterior to bulbospongiosum. The levator ani muscles are visualised laterally and the rectourethralis centrally. The rectourethralis is divided (avoiding the rectum), and the rectum is swept posteriorly off the Denonvilliers aponeurosis. This in turn is incised transversely, and at the prostatic apex, the neurovascular bundles are separated from the urethra. The puboprostatic ligaments are divided several millimeters anterior to the anterior aspect of the prostate. The anterior prostate is then dissected caudal to the dorsal venous plexus, which is left intact and undisturbed, along with the retropubic fat pad (Fig. 6). Although the Denonvilliers fascia is divided in both transperineal and retropubic routes posteriorly to the urethra, it is divided further posteriorly and caudally using the transperineal approach, by the nature of the surgical access, and therefore this makes anterior sphincter/rectal wall fibrosis more likely [23, 24]. Fibrosis anterior to the sphincter is seen with both approaches but is more marked with the transperineal approach due to the access of the dissection plane.
T2-weighted images in a 63-year-old man with white lines representing the surgical planes of the radical transperineal prostatectomy approach. a An inverted-U incision with the ends medial to the ischial tuberosities, anterior to the midanal line and with the apex in the mid perineum. The ischiorectal fossa is incised on either side of the central tendon, and the central tendon is divided. b Dissection continues to the fibrous confluence posterior to the membranous urethra. The surgeon will then visualise the levator ani muscles laterally and the rectourethralis centrally. c The rectourethralis is divided (avoiding the rectum). d The rectum is swept posteriorly off the Denonvilliers aponeurosis deep into the wound, proximal to the seminal vesicles. The Denonvilliers aponeurosis is incised transversely, and at the prostatic apex, the neurovascular bundles are separated from the urethra. e The puboprostatic ligaments are divided several millimetres anterior to the anterior aspect of the prostate
The imaging findings specific to post-transperineal prostatectomy cases include more low signal (on all sequences) fibrosis of the lower anterior anal sphincter and often anterior rectal wall fibrosis [8, 9]. There is no loss of the normal retropubic fat pad (Figs. 7, 8).
Specific T2-weighted MRI features in a 63-year-old man post-radical transperineal prostatectomy. On the left image, prominent fibrosis (Fi) is present in relation to the urethral anastomosis, and also just lateral to it. Note however there is no loss of the normal retropubic fat pad visualised following this approach, unlike following retropubic prostatectomy surgery. On the right image, there is further fibrosis of the lower anterior anal sphincter (Fi), which is pathognomic of this approach
Axial T2-weighted images in a 63-year-old man post-radical transperineal prostatectomy showing low signal fibrosis (white arrows) extending from the anal sphincter/anorectal anastomosis to the urethral anastomosis anteriorly. This should not be confused with disease, although it is only usually visualised following transperineal prostatectomy surgery. Black arrow shows vesicourethral anastomosis as a ring of uniform low signal
Laparoscopic and robotic surgical technique
Although initially technically challenging, advances in medical technology and laparoscopic aids such as ultrasonic cutting, coagulating surgical scalpels and improved optics have made the laparoscopic approach a popular method. A variety of techniques have been described, using both extra- and transperitoneal approaches. Laparoscopy has been shown so far to have an oncological efficacy equivalent to open procedures in terms of survival benefit, as well as having a low morbidity and good long-term functional results (continence and potency) [13]. However, the technique usually requires a long learning curve of 80-100 cases to transfer open surgical skills to the laparoscope in this setting, and hence its dissemination has been slow.
The technique of robotic radical prostatectomy is gaining increasing acceptance, and the latest models consist of a system composed of a four-armed robot connected to a remote surgeon at a master console. A three-dimensional display provides a unique magnified view (up to 16 times) of the surgical field, with foot pedals and hand-held devices used for control. The robot can also downscale movements by modulating the motion applied to it, and can, for instance, eliminate a surgeon’s hand tremor. Further potential advantages include a minimally invasive approach, and the need for less technical laparoscopic expertise permits a short learning curve of approximately 8-12 cases to transfer open surgery skills to this setting [25]. In experienced hands, the outcome of this procedure appears to be very favourable with minimal blood loss and operative morbidity, excellent functional results and oncological efficacy at 5 years equivalent to conventional laparoscopic and open procedures [26]. However, long-term data are not yet available for either of these minimally invasive approaches. The main drawbacks to the robotic approach are high operative costs, but also lack of tactile feedback (haptic perception), but these aside, this may be the future of the radical prostatectomy.
No MR findings are present to our knowledge that are specific to these techniques, other than the lack of open surgical excisions, and the presence of the laparoscopic portals in the anterior abdominal/pelvic wall. However, due to technical aspects of the procedures, the imaging features are considered very likely to be largely similar to those of a retropubic prostatectomy, although clearly with the absence of a large abdominal wall scar.
Other imaging findings
Seminal vesicle appearances
The seminal vesicles are highly variable in their MR appearance post-operatively, with none, all or, most often, just residual lateral tips present [27]. The seminal vesicles are seldom completely removed and the lateral ends can be identified as a small remnant having the characteristic appearance of a seminal vesicle. This appearance includes high signal on T2W imaging, a bilobulated contour, but possibly showing some post-operative scarring as evidenced by distortion and low signal on T2W imaging. Prominent linear low signals on all sequences can be present and are in keeping with extensive normal post-operative fibrosis which may be present unilaterally or bilaterally (Fig. 9).
The seminal vesicles are highly variable in appearance post-operatively, with none, all or just residual lateral tips present. Axial T2-weighted images show a linear low signal in keeping with normal post-op fibrosis (arrow) and b some residual high signal as well as low signal in keeping with residual gland mixed with fibrosis (arrow). c An intermediate signal mass within the residual left seminal vesicle (arrow), which was subsequently confirmed as recurrent disease
Occasionally however, a small non-linear area of intermediate signal can be representative of recurrent disease [27]. The vasa deferentia usually have a normal anatomical site and course post-operatively apart from their medial ends, which are divided. They are intimately related to the seminal vesicles and are also usually linear and low in signal on all sequences.
Anastomotic appearances
At the vesicourethral anastomosis, there can be a variable degree of signal, which can reflect ongoing exuberant fibrosis. In the vast majority of cases this is usually very low signal on all sequences, and this is not a difficult radiological distinction [21, 22]. The degree of soft tissue can be quite marked, often reflecting the amount of haemorrhage at the time of surgery. Occasionally this can be a low to intermediate signal on T2W and mimic the intermediate signal of a tumour recurrence. In this difficult situation, a dynamically enhanced contrast study can be performed, following administration of intravenous gadolinium. Recurrent disease may enhance avidly in the arterial phase and possibly wash out in the venous phase. Subtle venous phase enhancement or no enhancement would be expected with fibrosis (Fig. 10). However, no study to our knowledge has specifically assessed the accuracy of dynamic contrast-enhanced MRI for the detection of post-prostatectomy recurrence. Transrectal ultrasound and biopsy can also be used in this regard [21, 22].
Axial T2-weighted images at the vesicourethral anastomosis in a 61-year-old man following radical prostatectomy, where there is a variable degree of low signal, which can reflect exuberant fibrosis (arrows). This is usually very low signal on all sequences. Occasionally this can be low to intermediate signal on T2W and mimic the intermediate signal of a tumour recurrence. In this situation, disease may be excluded with a post-contrast study
Silastic injection
In patients suffering post-operative incontinence, silastic or similar agents can be injected around the urethral sphincter mechanism in order to act as a bulking agent [28]. This appearance should not be confused with recurrent or residual disease (Fig. 11). It typically appears as three or four well-defined ovoid areas of homogeneous intermediate signal with an outer low signal rim. It does not enhance following intravenous gadolinium administration.
Axial T2-weighted image at the vesicourethral anastomosis in a 69-year-old man following radical prostatectomy. In patients suffering post-operative incontinence, silastic can be infused around the internal urethral sphincter to act as a bulking agent. This appearance is shown (arrows) and should not be confused with recurrent disease
Residual prostate gland
Residual gland can mimic recurrent disease, with both presenting intermediate signal on T2W imaging and low signal on T1W imaging. Residual gland is typically shaped like normal gland, is homogeneous, well defined and, if present, is invariably at the anastomosis. In essence it usually appears very similar to a large defect following a transurethral resection of the prostate, although there have been no studies to our knowledge comparing the accuracy of this distinction between residual gland and recurrence. In case such a distinction cannot be made, then following intravenous gadolinium, enhancement patterns may be different, with recurrent disease often enhancing avidly and heterogeneously during the arterial phase of enhancement, and possibly washing out during the venous phase. Residual gland may enhance (often uniformly) during the venous phase. These features accompanied by a lack of a complete PSA drop post-operatively in patients with residual glandular tissue can also assist in this judgement (Fig. 12).
T2-weighted images in a 59-year-old man following radical prostatectomy. Residual gland is not usually difficult to distinguish from recurrent disease. Residual prostate has the shape, site and signal characteristics of native gland, presenting an intermediate signal on T2W imaging (arrows) and low signal on T1W imaging (not shown). It may appear very similar to a large transurethral resection defect. If there is still difficulty in this distinction, then enhancement patterns may be different, and a lack of complete PSA drop post-operatively in patients with residual glandular tissue can also assist in this judgement
Lymphocoele
Following a pelvic lymphadenectomy, a lymphocoele may commonly be observed. It is typically high signal on T2W sequences and low signal on T1W imaging and does not usually enhance [29]. It is usually self limiting and is not to be confused with more sinister pathologies (Fig. 13). It is most commonly sited in the pelvic side wall.
Coronal T2-weighted images in a 59-year-old man following radical prostatectomy. Images show a lymphocele which may commonly be observed in the pelvic side wall (black arrows). It is usually self limiting and is not to be confused with more sinister pathologies. Incidental note is also made of residual gland post-surgery (white arrows)
Conclusion
A non-enhancing ring of low signal is frequently seen at the vesicourethral anastomosis in keeping with fibrosis. Seminal vesicle remnants are quite variable in appearance but are usually present to some degree and usually also contain fibrosis. Appearances following the different surgical accesses do not differ tremendously, although the retropubic fat pad and dorsal venous complex is much reduced or absent following a retropubic approach. Anterior rectal wall scarring may be present following a transperineal approach. Other benign appearances post-operatively, such as the visualisation of residual gland or a bulking agent, should be recognised so that the radiologist can distinguish these findings from active recurrent disease.
References
Petit JH, Chen MH, Loffredo M, Sussman B, Renshaw AA, D’Amico AV (2006) Prostate-specific antigen recurrence and mortality after conventional dose radiation therapy in select men with low-risk prostate cancer. Cancer 107:2180–2185
Hricak H, Schoder H, Pucar D et al (2003) Advances in imaging in the postoperative patient with a rising prostate-specific antigen level. Semin Oncol 30:616–634
Geary ES, Dendinger TE, Freiha FS, Stamey TA (1995) Nerve sparing radical prostatectomy: a different view. J Urol 154:145–149
Ghavamian R, Zincke H (1999) An updated simplified approach to nerve-sparing radical retropubic prostatectomy. BJU Int 84:160–163
Young HH (1905) The early diagnosis and radical cure of carcinoma of the prostate. Bull Johns Hopkins Hosp 16:315–321
Millin T (1947) Carcinoma of the prostate: radical retropubic prostatectomy. In: Millen TE (ed) Retropubic urinary surgery. Williams and Wilkins, Baltimore, pp 15–17
Harris MJ, Thompson IM (1996) The anatomic radical perineal prostatectomy: a contemporary and anatomic approach. Urology 48:762–768
Lassen PM, Kearse WS (1995) Rectal injuries during radical perineal prostatectomy. Urology 45:266–269
Dahm P, Silverstein AD, Weizer AZ et al (2003) A longitudinal assessment of bowel related symptoms and fecal incontinence following radical perineal prostatectomy. J Urol 169(6):2220–2224
Korman HJ, Leu PB, Huang RR, Goldstein NS (2002) A centralized comparison of radical perineal and retropubic prostatectomy specimens: is there a difference according to the surgical approach? J Urol 168:991–994
Yang BK, Young MD, Calingaert B et al (2004) Prospective and longitudinal patient self-assessment of health-related quality of life following radical perineal prostatectomy. J Urol 172:264–268
Schuessler WW, Schulam PG, Clayman RV, Kavoussi LR (1997) Laparoscopic radical prostatectomy: initial short-term experience. Urology 50:854–857
Guillonneau B, el-Fettouh H, Baumert H et al (2003) Laparoscopic radical prostatectomy: oncological evaluation after 1,000 cases at the Montsouris Institute. J Urol 169:1261–1266
Menon M, Shrivastava A, Tewari A et al (2002) Laparoscopic and robot assisted radical prostatectomy: establishment of a structured program and preliminary analysis of outcomes. J Urol 168:945–949
Menon M, Tewari A, Baize B, Guillonneau B, Vallancien G (2002) Prospective comparison of radical retropubic prostatectomy and robot-assisted anatomic prostatectomy: the Vattikuti Urology Institute experience. Urology 60:864–868
Joseph JV, Rosenbaum R, Madeb R, Erturk E, Patel HR (2006) Robotic extraperitoneal radical prostatectomy: an alternative approach. J Urol 175:945–950; discussion 951
Villers A, Puech P, Mouton D, Leroy X, Ballereau C, Lemaitre L (2006) Dynamic contrast enhanced, pelvic phased array magnetic resonance imaging of localized prostate cancer for predicting tumor volume: correlation with radical prostatectomy findings. J Urol 176:2432–2437
Buckley DL, Roberts C, Parker GJ, Logue JP, Hutchinson CE (2004) Prostate cancer: evaluation of vascular characteristics with dynamic contrast-enhanced T1-weighted MR imaging-initial experience. Radiology 233:709–715
Fütterer JJ, Engelbrecht MR, Jager GJ, Hartman RP, King BF, Hulsbergen-Van de Kaa CA, Witjes JA, Barentsz JO (2007) Prostate cancer: comparison of local staging accuracy of pelvic phased-array coil alone versus integrated endorectal-pelvic phased-array coils. Local staging accuracy of prostate cancer using endorectal coil MR imaging. Eur Radiol 17:1055–1065
Sella T, Schwartz LH, Swindle PW, Onyebuchi CN, Scardino PT, Scher HI, Hricak H (2004) Suspected local recurrence after radical prostatectomy: endorectal coil MR imaging. Radiology 231(2):379–385
Wasserman NF, Kapoor DA, Hildebrandt WC, Zhang G, Born KM, Eppel SM, Reddy PK (1992) Transrectal US in evaluation of patients after radical prostatectomy. Part I. Normal postoperative anatomy. Radiology 185:361–366
Wasserman NF, Reddy PK (1993) Use of transrectal ultrasound in follow-up of postradical prostatectomy. Urology 41(1 Suppl):52–56
Janoff DM, Parra RO (2005) Contemporary appraisal of radical perineal prostatectomy. J Urol 173:1863–1870
Lance RS, Freidrichs PA, Kane C, Powell CR, Pulos E, Moul JW, McLeod DG, Cornum RL, Brantley Thrasher J (2001) A comparison of radical retropubic with perineal prostatectomy for localized prostate cancer within the Uniformed Services Urology Research Group. BJU Int 87:61–65
Ahlering TE, Skarecky D, Lee D, Clayman RV (2003) Successful transfer of open surgical skills to a laparoscopic environment using a robotic interface: initial experience with laparoscopic radical prostatectomy. J Urol 170(5):1738–1741
Le CQ, Gettman MT (2006) Laparoscopic and robotic radical prostatectomy. Expert Rev Anticancer Ther 6(7):1003–1011
Sella T, Schwartz LH, Hricak H (2006) Retained seminal vesicles after radical prostatectomy: frequency, MRI characteristics, and clinical relevance. AJR Am J Roentgenol 186:539–546
Colombo T, Augustin H, Breinl E, Schips L, Hubmer G (1997) The use of polydimethylsiloxane in the treatment of incontinence after radical prostatectomy. Br J Urol 80:923–926
Hricak H, Williams RD, Spring DB, Moon KL Jr, Hedgcock MW, Watson RA, Crooks LE (1983) Anatomy and pathology of the male pelvis by magnetic resonance imaging. AJR Am J Roentgenol 141(6):1101–1110
Author information
Authors and Affiliations
Corresponding author
Additional information
No grant was applied for or received in the production of this work.
Rights and permissions
About this article
Cite this article
Allen, S.D., Thompson, A. & Sohaib, S.A. The normal post-surgical anatomy of the male pelvis following radical prostatectomy as assessed by magnetic resonance imaging. Eur Radiol 18, 1281–1291 (2008). https://doi.org/10.1007/s00330-008-0867-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-008-0867-3