Abstract
The purpose of this study was to compare the value of pelvic ultrasound with color Doppler and magnetic resonance imaging (MRI) in: (1) the diagnosis of placental adhesive disorders (PADs), (2) the definition of the degree of placenta invasiveness, (3) determining the topographic correlation between the diagnostic images and the surgical results. Fifty patients in the third trimester of pregnancy with a diagnosis of placenta previa and at least one previous caesarean section underwent color Doppler ultrasound (US) and MRI. The sonographic and MRI diagnoses were compared with the final pathologic or operative findings. Outcomes at delivery were as follows: normal placenta (n = 38) and PAD (n = 12). MR and US Doppler showed no statistically difference in identiyfing patients with PAD (P = 0.74), while MRI was statistically better than US Doppler in evaluating the depth of placenta infiltration (P < 0.001). MRI accurately characterized the topography of invasion in 12/12 (100%) of the cases, while US accurately characterized the topography of invasion in 9/12 (75%) of the cases. In conclusion, we confirmed that pelvic US is highly reliable to diagnose or exclude the presence of PAD and found MRI to be an excellent tool for the staging and topographic evaluation of PAD.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Placenta accreta, placenta increta and placenta percreta represent a spectrum of placental adhesive disorders (PADs). Placenta accreta is the least severe of the three entities with penetration of the decidua by the chorionic villi. Placenta increta is penetration of the myometrium by the chorionic villi. Placenta percreta is the most severe of the implantation anomalies with invasion of both the myometrium and uterine serosa often with extension into neighbouring organs [1].
In a large series, placenta accreta occurred in approximately 9% of women with placenta previa and 0.004% of women without placenta previa, being therefore almost exclusive of the former condition [1]. In women with placenta previa, placenta accreta risk varied from 2% in women less than 35 years old with no previous caesarean section to 39% in women at or over 35 years of age with two or more caesarean sections. In these women, caesarean section and advanced maternal age were independent risk factors [2]. The central contributory role of previous caesarean delivery is illustrated by the fact that nulliparous women with placenta previa have a risk of placenta accreta of 1–3% [2]. Really, the tenfold increased frequency of abnormal placentation with a reported incidence of 1 of 533 deliveries is primarily attributed to the rising caesarean delivery rate, which reached an all-time high of 29.1% in the USA in 2004 [2]. Patients with PAD are at increased risk of peripartum complications. There is an increased risk of damage to adjacent visceral structures and of haemorrhage that can be severe and life threatening, requiring multiple blood transfusion and hysterectomy, with an overall reported mortality as high as 7% [3]. This life-threatening obstetrical condition is generally diagnosed at the time of delivery, often resulting in emergency treatment with a greater risk of morbidity [4].
In this regard, preoperative diagnosis of placenta abnormalities and the correct identification of the topography of placental invasion are crucial in minimizing risks and planning a safer surgery.
Pelvic ultrasound is the most commonly used imaging modality for the diagnosis of placenta accreta [5]. The current place of MRI is mainly as a confirmatory exam in placenta imaging in cases of equivocal ultrasound, due to high soft-tissue contrast and multiplanar imaging capabilities [6, 7].
The purpose of this study was to compare, in a group of patients with a high risk of abnormal placental implantation due to placenta previa and at least one previous caesarean delivery , the value of pelvic ultrasound (US) with color Doppler and MRI in: (1) the diagnosis of PAD, (2) the definition of the degree of placenta invasiveness, (3) determining the topographic correlation between the diagnostic images and the surgical results.
Materials and methods
Between March 2006 and June 2007 56 patients in the third trimester of pregnancy (mean age 31 years; range 22–38 years) with diagnosis of placenta previa and at least one previous caesarean section were referred to us for detailed color Doppler and MRI. Fifty women for whom complete information was available regarding clinical and pathological diagnosis were included in this study.
The study protocol was approved by our institutional review board. Written informed consent was obtained from all patients before MRI.
All pelvic ultrasonography scans were performed by registered sonographers using Siemens Sonoline Elegra (Siemens, Issaqua, Wash.) US equipment.
US characterization for placenta accreta and its varieties was first established according to Finberg and Williams diagnostic criteria [8].
Briefly, gray-scale B-mode sonography was first used to screen the placental tissue in a systematic fashion. Careful attention was paid to homogeneity and echogenicity patterns of the placenta. In the absence of normal subplacental venous complex or in the presence of placental sonolucent lakes and/or irregularities of the bladder uterine serosa assessment of the placenta was completed using superimposed color Doppler flow. Doppler power settings were at the level approved for fetal use.
Magnetic resonance scans were performed on a Siemens Magnetom Avanto 1.5-T scanner (Siemens Medical Solution, Malvern, Pa.) equipped with high-performance gradients and phase array coils.
Patients were supine, with their feet entering the magnet bore first to minimize feelings of claustrophobia.
A small amount of fluid in the urinary bladder aided the evaluation of the uterine and bladder serosa; therefore, all US Doppler and MR exams were obtained with a partially full bladder.
Following a localizer scan, sagittal, axial and coronal half-Fourier acquisition with single-shot turbo spin echo (HASTE) (min/90.0 repetition time, ms/echo time, ms), (256 × 224 matrix, 4-mm thickness with no gap, echo train length of 94, receiver bandwidth of 62.50 kHz) were acquired during breath-hold.
Next, sagittal, coronal and axial true fast-imaging with steady-state precession (True FISP) (3.5/1.8 repetition time, ms/echo time, ms), 256 × 224 matrix, one signal acquired, 5-mm thickness with no gap, 50 flip angle, receiver bandwidth of 125 kHz were acquired.
Finally, a sagittal T1 three-dimensional (3D) volume interpolated breath-hold examination (VIBE) (4.2/2/15 repetition time, ms/echo time, ms/inversion time, ms), 256 × 224 matrix, one signal acquired, 2.5-mm thickness with no intersection gap, 12 flip angle, receiver bandwidth of 62.50 kHz was acquired.
Breath-holding by the mother was utilized to minimize respiratory motion artifact. The entire examination time was 20–25 min.
The levels of placental invasion determined by US Doppler and MRI were labelled: 0, for absence of invasion; A, for accreta ); I for, increta; P, for percreta).
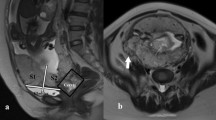
According to the previous topographic characterization of Palacios et al. [9], sagittal images obtained through MRI allow the division of anterior placenta invasion into two sectors, delimited by a plane perpendicular to the center of the so-called upper bladder axis. The uterine sectors bordering the upper and lower posterior bladder wall were called S1 and S2, respectively (Fig. 1).
A 32-year-old pregnant at 35 weeks gestation: normal placenta on MRI. Sagittal (a, b) and axial (c) T2 HASTE sequences show that placenta (*) is previa and is homogeneous in signal intensity and does not distort the normal contour of the uterus. The myometrial mantle is particularly well visualized (arrows) and there is a slight differences in signal intensity between the placenta and the myometrium. The division plane line perpendicular to the middle level of the upper bladder axis can separate two sectors on sagittal images (a, b). S1 is the uterine sector bordering with the upper posterior bladder wall and S2 is the uterine sector adjacent to the lower posterior wall
For the case of different grades of placenta penetration and combined S1 and S2 invasions, the score was assigned according to the highest percentage of invasion.
Images were interpreted prospectively by two reviewers, who were blinded to results of the US and the pathologic examinations. Interobserver agreement was assessed using k-statistics.
After this first analysis, a second interpretation was performed by the same two reviewers, who reached a consensus in evaluating both the degree of invasion and the topographic evaluation of invasion.
The consensus evaluation and the final degree of placental penetration and its specific topography were compared in the operating room according to clinical and anatomical criteria. In this regard, placenta was considered accreta if firmly attached to the endometrium and showing a non-self-controlled bleeding when detached, increta when requiring surgical curettage to remove invasive tissue deeply implanted in the myometrium and percreta when extending through the myometrium into the neighbouring organs. A true negative was defined as an uncomplicated placental removal without excessive bleeding after caesarean delivery.
All true positive diagnoses, as well as false-positive and negative diagnoses, were confirmed by pathologic examination (hysterectomy, curettings, or fragments of myometrium adherent to the placenta).
The diagnosis of placenta accreta required that the anchoring villi directly abut the myometrium [10]: invasive trophoblastic cells were evidenced with the Giemsa staining.
For both US and MRI, we calculated sensitivity and specificity by using binary diagnostic test tables. Measures of test accuracy [e.g., sensitivity, specificity, negative predictive value (NPV) and positive predictive value (PPV)] were computed and results expressed as point estimates and their 95% confidence intervals (95% CIs). Analyses were carried out using SAS System, version 8.20 (SAS, Cary, N.C.).
Results
Thirty-eight women were found to have a normal attached placenta, while 12 women ultimately had clinical and pathological confirmation of PAD. The mean gestational age at diagnosis with MRI/US was 30 weeks, with a range of 20–37 weeks. The US Doppler and MR examinations were performed in the same day in all patients.
Clinical findings and pathology reports divided the PAD cases as follows: placenta accreta (n = 7), placenta increta (n = 2), placenta percreta (n = 3). Total hysterectomy (seven cases) was performed in all cases of placenta increta and percreta and in two cases of placenta accreta with prevalent adhesiveness in the S2 sector facing the lower bladder posterior wall.
The comparison of the results between US Doppler and MR with the identification of placenta accreta is shown in Table 1, which presents test accuracy measures with confidence intervals.
The performance of US Doppler and MRI in evaluating the degree of invasion accreta, increta, and percreta (Figs. 2, 3, 4), is shown in Table 2.
A 34-year-old pregnant at 32 weeks gestation: placenta accreta. Transabdominal sagittal US Doppler scan with a 3.5-MHz probe (a) shows small sinuses into myometrium according to placenta accreta (arrow) with normal visualization of the bladder (b). Sagittal (b) and axial (c) HASTE show the irregular placenta-myometrium interface with placental tissue (arrow) without surrounding myometrium, indicative of placenta accreta at the level of S1. Note the normal myometrial mantle (small arrows)
A 30-year-old pregnant at 33 weeks gestation: placenta increta. Transabdominal sagittal US Doppler scan with a 3.5-MHz probe (a) shows multiple large sinus into myometrium according to placenta increta. Sagittal (b) and axial (c) T2 HASTE images show multiple foci of hypersignal located into the myometrium (arrows); these findings are indicative of invasion of the placental tissue deeply through the myometrium, (placenta increta)
A 28-year-old pregnant at 34 weeks gestation: placenta percreta. Transabdominal sagittal US Doppler scan with a 3.5-MHz probe (a) shows multiple irregular areas of the placenta (arrows) bulging into the myometrium, with no evidence of vessels in the bladder wall. Coronal T2 HASTE sequence (b) showing absent myometrium at the level of bladder (arrow), indicative of placenta percreta. Axial slice T2 HASTE image (c) showing massive invasion of the right parametrium (arrows)
Pelvic ultrasonography ruled out correctly the diagnosis of PAD in all 38/38 (100%) cases without PAD and correctly identified the diagnosis of PAD in 11/12 (91%) (Table 2); the false negative being one unrevealed case of posterior placenta accreta in a patient with multiple uterine myomas (Fig. 5).
A 38-year-old pregnant with multiple uterin myomas at 35 weeks gestation. Sagittal transvaginal US Doppler with 7.5 MHz probe (a) shows no extension of vessels from the placenta to the myometrium. Sagittal True-FISP (b) and axial HASTE (c) images show irregular border in posterolateral location (arrows) indicative of placenta accreta at the level of S2
Among patients with identified PAD, US underestimated the depth of invasion in 2/12 (17%) cases, which were scored as placenta accreta but proved to be one case of placenta increta and one placenta percreta at the pathology report (Table 2). Overall, US Doppler correlated accurately with surgical pathology in 9/12 cases (75%) of patients with PAD.
MR ruled out and correctly identified the diagnosis of PAD in all cases (Table 2). Moreover, when considering the estimated depth of invasion, it correctly matched pathology findings in all 12 cases. Although MRI and US were not statistically different in identifying patients with PAD (P = 0.74), MR was significantly superior in the assessment of depth of infiltration (P < 0.001). In particular, MRI performed better than US in identifying patients with placenta percreta (sensitivity 100%).
MR accurately characterized the topography of placental invasion in all cases, according to the division into S1 and S2 areas. Significantly, when using US, the topographic diagnosis of adhesiveness was correct in only 9/12 cases, three cases of S2 invasion (two accreta and one increta) being scored as S1 (Table 3).
Therefore, a highly significant difference (P < 0.001) between MRI and US Doppler in evaluating the topography of placenta invasion was observed.
Finally, an almost-perfect interobserver agreement of MRI examinations was found when comparing both the degree of invasion and the evaluation of topographic invasion (weighted k statistic value of 0.85 and 0.92, respectively).
Discussion
The incidence of PAD in its forms—placenta accreta (60–78%), increta (17–20%) and percreta (5–20%) [1]—should increase steadily in the next century due to the increasing number of caesarean sections and maternal age at delivery. There is a need for reliable antenatal diagnosis since PAD that occurs unexpectedly may lead to catastrophic blood loss and related complications and even maternal death. If this condition is identified before delivery a safer surgery can be planned with the availability of blood on the spot, the presence of both a consultant anaesthesiologist and a gynaecology oncology surgeon and with the option for a radiologic occlusion of common iliac arteries to minimize blood loss.
Pelvic US has been the most commonly used imaging modality for the diagnosis of placenta accreta [5, 6, 11].
The findings of PAD have been previously described and include loss of the normal hypoechoic retro placental myometrium zone, thinning or disruption of the hyperechoic uterine serosa-bladder interface, presence of focal exophytic masses, and the presence of lacunae in the placenta; the last one was the most predictive sonographic sign of PAD, with a sensitivity of 79% and a PPV of 92% [11, 12]. Power and color Doppler has been suggested to aid in the diagnosis of placenta accreta because its highlights areas of increased vascularity with dilated blood vessels that cross the placenta and uterine wall [13]. However most of the studies showed that power Doppler did not improve the diagnosis over that achieved by gray-scale sonography alone [14, 15]. Indeed, even if color Doppler US often identifies placenta accreta, it may be falsely positive because of increased maternal vascularity in the space between the myometrium and the bladder probably related to the preparation of the bladder flap and the exposition to blood products.
Altogether, these studies indicated that US had a sensitivity of 82% and specificity of 96.8% in evaluating the presence of PAD [11, 12]. These findings of these are similar to those presented in our study.
The major pitfalls of US findings are: (1) they might not be reliable in cases where the placenta is posterior [16]; (2) no evidence so far published of a precise US estimation of the depth of placenta invasion through the myometrium [15]; (3) the inability to report the precise topography of invasion of the placenta through the myometrium [9].
In the present work, we confirmed all these limitations as a matter of fact: the false-negative case of PAD was evidenced in one patient with posterior placenta and the presence of multiple myomas, two cases of PAD were underestimated with one missed diagnosis of placenta percreta and three S2 cases were incorrectly scored as S1.
The limitations 2 and 3 are particularly important because they might affect both the treatment plan and the prognosis. Placenta increta is usually treated clinically in the same way as placenta accreta, but placenta percreta might result in an extremely difficult surgical technique requiring specific procedures such as aortic clamping or hypogatric legation. Similarly, while S1 PADs are generally of easy access and quick haemostasis, S2 PADs—being irrigated by deeply located vessels—often result in a significantly increased surgical complexity and massive haemorrhage.
The role of MRI in the diagnosis of PAD is somewhat equivocal or limited [16, 17] because US is more accessible to patients and physicians, and most importantly because only a modest benefit from MRI was reported along with a low sensitivity for diagnosing PAD [20]. Lax et al. [19] found that, even in normal cases, the interface between the myometrium and the placenta was often indistinct. Recently, it was suggested that the superiority of MRI over ultrasonography in the diagnosis of PAD is not statistically significant. However, in this study the diagnosis of placenta accreta also referred to placenta increta and percreta [7]. Altogether, these studies were retrospective, limited to a few cases and lacked pathologic correlation [18, 21].
Possible exceptions to this evidence are represented by populations of patients with a high risk of abnormal placenta implantation that could increase the sensitivity and specificity of MRI. For instance, it was reported that MRI is better than US in posterior location of the placenta or with placenta previa [22, 23].
The emerging picture from a review of the literature does not substantiate a role of MRI as a screening tool for PAD, with the exception of particular isolated cases.
Recently, Kim et al. [6], in a small retrospective study, reported the advantage of either True FISP) or HASTE to reduce fetal and maternal artifact motions, thereby improving the anatomical evaluation of the uteroplacental interface. They proposed the analysis of the intensity of the inner, middle and outer layer of this interface and suggested that the obliteration of the inner layer without other abnormalities can depict the condition of PAD.
In our MRI imaging protocol we were able to clearly visualize the uteroplacental interface in all normal cases and to obtain unequivocal images related to the presence and degree of PAD. The MRI finding of focal thinning and irregularity or disruption of the myometrium represents placenta accreta (Fig. 2), the presence of foci of hyper signal within the myometrium represents placenta increta (Fig. 3), whereas transmural extension of abnormal signal intensity through the myometrium and irregularity of the bladder represents placenta percreta (Fig. 4).
MRI has another potential benefit compared with US in that it provides a larger field of view, thereby granting an easier evaluation of the topography of placenta invasion and classification of S1 PAD and S2 PAD.
In our series of 12 cases of PAD, the MRI upgraded the diagnosis in one case of placenta percreta and correctly identified three cases of S2 PAD missed by US that were all treated with total hysterectomy, with an overall dramatic contribution to the preoperative planning of these patients.
Based on these findings, at our institution, we resort to MRI not only to optimize the US diagnostic accuracy in cases of suspicious or inconclusive PAD but also to use it as a staging technique in all PAD patients in order to plan surgery under the safest possible conditions to preserve the uterus with the lowest grade of complications [24].
As an additional result, we found that, while US is strongly operator dependent, MRI has a very good interobserver agreement that can allow a reproducible evaluation of placenta abnormalities.
With the newest generation of MRI units and with technological advances such as parallel MRI, the speed of image acquisition and the spatial resolution can increase, raising three-dimensional imaging (3D T2-weighted single-shot sequences and 3D True FISP) and real-time sequences possible for placental imaging.
In conclusion, we confirmed that pelvic US is highly reliable to diagnose or exclude the presence of PAD and found MRI to be an excellent tool for the staging and topographic evaluation of PAD. We feel that the introduction of MRI as a routine exam in cases of suspected PAD will fulfil the obstetrician’s main concern of an accurate diagnosis in order to organize the most appropriate and safest surgical procedure.
References
Wu S, Kocherginsky M, Hibbard JU (2005) Abnormal placentation: twenty-year analysis. Am J Obstet Gynecol 192:1458–1461
Miller DA, Chollet JA, Goodwin TM (2007) Clinical risk factors for placenta previa-placenta accrete. Am J Obstet Gynecol 177:210–214
Zelop CM, Harlow BL, Frigoletto FD Jr, Safon LE, Saltzman DH (1993) Emergency peripartum hysterectomy. Am J Obstet Gynecol 168:1443–1448
O’Brien JM, Barton JR, Donaldson ES (1996) The management of placenta percreta: conservative and operative strategies. Am J Obstet Gynecol 175:1632–1638
Chou MM, Ho ESC, Lee YH (2000) Prenatal diagnosis of placenta previa accreta by transabdominal color Doppler ultrasound. Ultrasound Obstet Gynecol 15:28–35
Kim JA, Narra VR (2004) Magnetic resonance imaging with true fast imaging with steady-state precession and half-Fourier acquisition single-shot turbo spin-echo sequences in cases of suspected placenta accreta. Acta Radiol 45:692–698
Warshak CR, Eskander R, Hull AD, Scioscia AL, Mattrey RF, Bernirschke K, Resnik R (2006) Accuracy of Ultrasonography and Magnetic Resonance Imaging in the diagnosis of placenta accreta. Obstet Gynecol 108:573–581
Finberg HJ, Williams JW (1992) Placenta accreta: prospective sonographic diagnosis in patients with placenta previa and prior cesarean section. J Ultrasound Med 11:333–339
Palacios Jaraquemada JM, Bruno CH (2005) Magnetic resonance imaging in 300 cases of placenta accreta: surgical correlation of new findings. Acta Obstet Gynecol Scand 84:716–724
Bernirschke K, Kaufmann P (2000) Pathology of the human placenta, 4th edn. Springer, New York
Comstock CH, Love JJ, Bronsteen RA, Lee W, Vettraino IM, Huang RR et al (2004) Sonographic detection of placenta accreta in the second and third trimesters of pregnancy. Am J Obstet Gynecol 190:1135–1140
Ito T, Katagiri C, Ikeno S, Takahashi H, Nagata N, Terakawa N (1999) Placenta previa increta penetrating the entire thickness of the uterine myometrium: ultrasonographic and magnetic resonance imaging findings. J Obstet Gynaecol Res 25:303–307
Chou MM, Tseng JJ, Ho ES (2002) The application of three-dimensional color power Doppler ultrasound in the depiction of abnormal uteroplacental angioarchitecture in placenta previa percreta. Ultrasound Obstet Gynecol 19:625–627
Lerner JP, Deane S, Timor-Trisch IE (1995) Characterization of placenta accreta using transvaginal sonography and color Doppler imaging. Ultrasound Obstet Gynecol 5:198–201
Comstock CH (2005) Antenatal diagnosis of placenta accreta: a review. Ultrasound Obstet Gynecol 26:89–96
Levine D, Barnes PD, Edelman RR (1999) Obstetric MR imaging. Radiology 211:609–617
Maldijan C, Adam R, Pelosi M, Pelosi MI, Rudell RD, Maldijan J (1999) MRI appearance of placenta percreta and placenta accreta. Magn Reson Imaging 17:965–971
Levine D, Hulka CA, Ludmir J, Li W, Edelman RR (1997) Placenta accreta:evaluation with color Doppler US, power Doppler US, and MR imaging. Radiology 205:773–776
Lax A, Prince MR, Mennitt KW, Schwebach JR, Budorick NE (2007) The value of specific MRI feautures in the evaluation of suspected placental invasion. Magn Reson Imaging 25:87–93
Lam G, Kuller J, McMahon M (2002) Use of magnetic resonance imaging and ultrasound in the antenatal diagnosis of placenta accreta. J Soc Gynecol Investig 9:37–40
Taipale P, Orden MR, Berg M, Manninen H, Alafuzoff I (2004) Prenatal diagnosis of placenta accreta and percreta with Ultrasonography, Color Doppler, and Magnetic Resonance Imaging. Obstet Gynecol 104:537–540
Thorp JM Jr, Councell RB, Sandridge DA, Wiest HH (1992) Antepartum diagnosis of placenta previa percreta by magnetic resonance imaging. Obstet Gynecol 80:506–508
Bakri YN, Rifai A, Legarth J (1993) Placenta previa-percreta: magnetic resonance imaging findings and methotrexate therapy after hysterectomy. Am J Obstet Gynecol 169:213–214
Chan JK, Morrow J, Manetta A (2003) Prevention of ureteral injuries in gynecologic surgery. Am J Obstet Gynecol 188:1273–1277
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Masselli, G., Brunelli, R., Casciani, E. et al. Magnetic resonance imaging in the evaluation of placental adhesive disorders: correlation with color Doppler ultrasound. Eur Radiol 18, 1292–1299 (2008). https://doi.org/10.1007/s00330-008-0862-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-008-0862-8