Abstract
Although multimodality therapy for high-grade gliomas is making some improvement in outcome, most patients will still die from their disease within a short time. We need tools that allow treatments to be tailored to an individual. In this study we used diffusion tensor imaging (DTI), a technique sensitive to subtle disruption of white-matter tracts due to tumour infiltration, to see if it can be used to predict patterns of glioma recurrence. In this study we imaged 26 patients with gliomas using DTI. Patients were imaged after 2 years or on symptomatic tumour recurrence. The diffusion tensor was split into its isotropic (p) and anisotropic (q) components, and these were plotted on T2-weighted images to show the pattern of DTI abnormality. This was compared to the pattern of recurrence. Three DTI patterns could be identified: (a) a diffuse pattern of abnormality where p exceeded q in all directions and was associated with diffuse increase in tumour size; (b) a localised pattern of abnormality where the tumour recurred in one particular direction; and (c) a pattern of minimal abnormality seen in some patients with or without evidence of recurrence. Diffusion tensor imaging is able to predict patterns of tumour recurrence and may allow better individualisation of tumour management and stratification for randomised controlled trials.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Malignant gliomas are aggressive tumours that have a poor prognosis. Although metastasis outside of the central nervous system is exceptional, the local infiltration of the brain is a major factor in the failure of current treatment modalities. Recent studies using multimodal therapy that combines surgery, radiotherapy and chemotherapy have demonstrated significant improvements in survival in a minority of patients with glioblastomas [1]. The reasons why a combination of therapies helps a small number of patients require further exploration for patient counselling, adapting the therapeutic cocktail to the individual, and possibly for stratification for randomised controlled trials.
Gliomas are one of the most heterogeneous groups of tumours. There is a marked difference in behavioural and pathological features between tumours of the same histological grade as well as within different regions of the same tumour [2, 3]. This heterogeneity means it is difficult to know if we should consider gliomas as localised tumors or as more widespread diseases [4]. Our clinical experience is that both are seen to differing degrees. Clinicians used to managing glioma patients see some patients with tumours that simply enlarge without extensive invasion, while others infiltrate widely in the surrounding white matter. The development of tools that could determine if an individual tumour has a more localised or a more infiltrative growth pattern might allow a more individualised choice of treatment, such as more aggressive surgery, radiotherapy with localised increase in dose, and local delivery of chemotherapy drugs for localised tumours, or more emphasis on systemic therapies (such as systemic chemotherapy and standard dose radiotherapy with wider margins) for more infiltrative tumours. The ability to combine different modalities based on the particular tumour behaviour and pathology in order to individualise treatment might improve tumour control, while at the same time avoiding the toxicity of therapies unlikely to work. In addition, the identification of different growth patterns might be of value in selecting patients for entry into clinical trials, or stratifying those within large-scale studies.
One key feature of gliomas is their growth and infiltration into white-matter tracts. Diffusion tensor imaging (DTI) is sensitive to subtle disruption of white-matter tracts and can detect abnormalities around gliomas that appear normal on conventional imaging [5, 6]. Newer analytical techniques of the diffusion tensor allow us to differentiate tumours from areas of normal brain infiltrated by glioma cells [7–9], and these methods have been validated using image-guided biopsies of the peritumoural area [10]. There is also evidence that these DTI abnormalities occur before the appearance of obvious tumours on conventional imaging [11].
In this study we aimed to use DTI on initial imaging to identify patterns of white-matter abnormality that can predict patterns of tumour recurrence at a later date in a cohort of glioma patients.
Materials and methods
Patients
Data from patients with gliomas recruited to a series of studies using diffusion tensor imaging carried out in the Wolfson Brain Imaging Centre were used for this study. All studies had been approved by the Local Research Ethics Committee, and all patients had provided informed consent. We included patients with a histologically confirmed diagnosis of glioma who had a DTI study and then had follow-up imaging. Follow-up imaging was performed either more than 2 years after the initial study or at the time of symptomatic recurrence.
In total 25 patients fulfilled the study inclusion criteria (mean age 44 years, range 23–79; 15 male). Their data are summarised in Table 1. A total of 12 patients had WHO grade IV tumours, 5 had WHO grade III tumours (1 anaplastic astrocytoma, 2 anaplastic oligodendroglioma and 2 anaplastic oligoastrocytoma) and 8 had WHO grade II tumours (6 diffuse astrocytomas, 1 oligodendroglioma and 1 oligoastrocytoma). Of the WHO grade II patients, three patients had not received previous therapy. Of these, one was studied after starting radiotherapy when there was clear evidence of tumour progression with the development of marked enhancement, and the other two progressed rapidly with evidence of recurrence shortly after the end of radiotherapy. The other five patients were studied at the time of tumour transformation into an apparently more aggressive tumour based on imaging findings, but the tumours, as is commonly done in clinical practice, were not re-biopsied.
Of the 25 patients, 22 had evidence of tumour progression and 3 patients with glioblastomas had no evidence of progression. Of the 25, 17 patients had been treated previously with surgery and radiotherapy and were studied at either tumour recurrence (n = 13) or during follow up when they had stable disease (n = 3). Six patients did not receive treatment between the DTI study and imaging to show recurrence. In four cases this was because the patient was undergoing surveillance imaging following surgery and radiotherapy. In others there was evidence of progression before they could start other treatments (especially radiotherapy). Of the remaining patients, eight underwent radiotherapy between the two studies, nine were treated with chemotherapy and two patients were treated with both chemotherapy and radiotherapy.
Imaging details
Patients were imaged at 3 Tesla on a Bruker MedSPEC S300 MR (Bruker BioSpin, Ettlingen, Germany). The diffusion tensor imaging was performed in the axial plane with 4-mm slice thickness and a 1-mm interslice separation using a single-shot, spin echo, echo planar imaging (EPI) diffusion tensor sequence. The sequence evolved slightly over the course of these studies: initially, the sequence had the following parameters: TR 5070 ms, TE 107 ms, FOV 25 × 25 cm, matrix size 128 × 128, providing eight slices. Later studies used a sequence with TR 6,000 ms, TE 100 ms, FOV 20.0 × 20.0 and matrix size of 100 × 100, interpolated to 128 × 128 for reconstruction. This sequence provided 27 slices (whole brain coverage). For both types of DTI sequence, each slice was collected from 12 non-colinear gradient directions. For each direction one T2 (b 0) image and five diffusion gradient-weighted images were collected (with b-values of 318–1,570 s/mm2).
Data reconstruction and DTI analysis
The method of image reconstruction has been previously described [9]. Briefly, processing was performed using an in-house program implemented in MATLAB (The MathWorks, Natick, MA), following the method proposed by Basser [12]. For each voxel, the eigenvalues (λ1, λ2, λ3) were computed, and then used to calculate the isotropic component, p, and the anisotropic component of the diffusion tensor, q. These were calculated on a voxel-by-voxel basis to produce maps of both the p and q components.
These maps were coregistered to the T2-weighted images using VTK CSIG (Computational Imaging Sciences Group, Kings College, London, UK) [13]. Regions of interest were drawn around the area of obviously reduced anisotropy on the q map, and this was superimposed on the p map where another region was drawn around the isotropic abnormality. The pattern of the differences between the two maps was examined. This was compared to imaging performed at time of recurrence. Patterns of DTI abnormality were defined as diffuse if the p abnormality exceeded the q abnormality by more than 0.5 cm in all directions, localised if the p abnormality exceeded the q abnormality by more than 0.5 cm in one particular direction, and a minimal pattern if the p abnormality was similar to the q abnormality.
Follow-up imaging and assessment of recurrence patterns
Follow-up imaging was performed on clinical grounds at 1.5 Tesla (Signa CV/I, GE, Milwaukee, WI). Imaging consisted of a minimum of an axial T2-weighted fast spin echo sequence (TR 6,000 ms, effective TE 106 ms, FOV 22.0 × 16.0, matrix 320 × 256, with a slice thickness of 6.0 mm with 1.0 mm gap), and a pre- and post-gadolinium-enhanced T1-weighted spin echo sequence (TR 500 ms, TE 9 ms, FOV 22.0 × 22.0, matrix 256 × 224, with a slice thickness of 6.0 mm with 1.0 mm gap). The image used for this study was the one taken at the time of initial clinical progression as defined by the patient developing features of clinical deterioration when the oncologist was considering further therapy. These images were examined by experienced neuroradiologists, and the direction of abnormality on T2-weighted images at tumour recurrence was compared to the pre-treatment T2-weighted images to assess pattern of recurrence. The assessor was blinded to the result of the DTI data. In cases where the area increased in all directions, this was referred to as a diffuse recurrence. Where the signal increased in a particular direction, this was referred to as localised recurrence. The minimal recurrence patterns exhibited little difference between the two sets of images.
Results
Examples of tumour recurrence patterns are shown in Figs. 1, 2 and 3. The mean interval between studies (±SE) was 406 ± 102 days for WHO grade II tumours, 200 ± 65 days for WHO grade III tumours and 219 ± 64 days for WHO grade IV tumours. Only nine patients had a further MRI study; this includes the three patients with stable disease. Of the remainder, 10 had no further imaging as their clinical state deteriorated rapidly on recurrence and 6 had a CT study only.
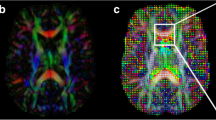
An example of a diffuse pattern of glioma growth. This 46-year-old man (patient 12) was diagnosed with a WHO grade III anaplastic oligodendroglioma 4 years prior to the study, when he had been treated with surgery and radiotherapy. He was imaged before starting chemotherapy (a). DTI study showed a p abnormality (shown in red) that was larger than the q abnormality (shown in green) in all directions (b). He received four cycles of temozolomide and initially remained clinically well, progressing after completing the fourth cycle. At recurrence there was a diffuse increase in the size of the enhancing lesion on T2-weighted imaging (c)
a–c An example of a localised growth pattern in a 32-year-old man (patient 17) with a right parietal glioblastoma previously treated with surgical excision and post-operative radiotherapy. He was studied with DTI at the time of recurrence. The images show a T2-weighted image of the initial tumour (a), a map of the isotropic component of the DTI (p, effectively the mean diffusivity) (b), and a T2-weighted image performed after tumour progression on PCV chemotherapy (c). These images have been coregistered with the isotropic diffusion tensor abnormality (shown in green) and anisotropic tensor abnormality (shown in red) superimposed. There is an area posteriorly where the isotropic abnormality is greater than the anisotropic abnormality. Follow-up imaging with tumour progression shows an overt tumour growing in a localised pattern in the area predicted by DTI
An example of minimal progression in a 37-year-old woman (patient 19) who had been diagnosed with an occipital glioblastoma 4 years previously. She had undergone surgical resection followed by radiotherapy, but relapsed 2 years later. She was then managed with repeat resection, three cycles of PCV chemotherapy, and was retreated with radiotherapy (45 Gy in 30 fractions). She was studied with DTI 2 years after finishing the second course of radiotherapy. a The T2-weighted abnormality and b a map of the isotropic component of the diffusion tensor with the isotropic abnormality (shown in green) and anisotropic components (shown in red) outlined. There is little difference between either of these abnormalities and the T2-weighted abnormality. c A gadolinium-enhanced T1-weighted abnormality performed 2.5 years after the previous study. It shows no evidence of tumour progression. The patient remains well
Diffuse pattern of DTI abnormality
A diffuse pattern of DTI abnormality was seen in 12 patients. The mean interval between studies in this group was 261 ± 75 days. This included six WHO grade II tumours, and three each of the WHO grade III and WHO grade IV tumours. Of these 12, 10 had either had no surgical procedure before treatment or just a biopsy. Approximately half (5 of 12) had been treated with radiotherapy. In all of these patients the tumour recurrence showed a generalised increase in the size of the tumour. An example is shown in Fig. 1.
In 7 of 12 patients, the gadolinium enhancement mirrored the changes in T2-weighted abnormality. Of the other five patients, one subsequently showed a diffuse increase in enhancement.
Localised pattern of DTI abnormality
Localised DTI abnormality was seen in eight patients. The mean interval between studies in this group was 287 ± 62 days, and there was no significant difference between the interval for this group and the diffuse group. This group included six WHO grade IV tumours and one each of WHO grade II and III gliomas. Of the eight, six had previously been treated with surgical resection and all but two had received radiotherapy before the DTI study. In three of these patients, there were abnormalities in both the p (isotropic) and q (anisotropic) components in regions with normal T2-weighted signal. In the other patients, the isotropic abnormality (p) was larger than the anisotropic component (q) within areas of abnormal T2-weighted signal. In all cases further tumour regrowth occurred in the direction in which the p abnormality exceeded the q abnormality. An example is shown in Fig. 2.
In half these patients, the gadolinium-enhanced abnormality mirrored the changes in T2-weighted abnormality. Three of the other patients subsequently showed a diffuse increase in enhancement on later imaging.
Minimal pattern of DTI abnormality
The isotropic abnormality (p) was similar to the anisotropic component (q) in five patients. One of these patients subsequently progressed in a diffuse growth pattern within 4 months of imaging. This patient had a large bihemispheric WHO grade III anaplastic oligoastrocytoma that had infiltrated most of the surrounding white matter on initial imaging. One patient who had a recurrence 3 years after biopsy and radiotherapy for a WHO grade III anaplastic astrocytoma also showed a minimal abnormality on DTI. After imaging she underwent PCV chemotherapy, and although she has definite evidence of tumour progression, remains alive and well 2.5 years after imaging. In a further three patients with WHO grade IV glioblastomas, there was no evidence of tumour recurrence or growth after radiotherapy. The patients have remained free of disease progression over 2.5, 3 or 5 years of follow up. An example is shown in Fig. 3.
Misclassified patients
Table 2 describes the effect of various factors on the ability of DTI to predict recurrence patterns. In total, three patients (12%) were misclassified using this technique. They included one WHO grade II astrocytoma, and two WHO grade III patients (one an anaplastic astrocytoma and one an anaplastic oligoastrocytoma). All three patients had received radiotherapy prior to the DTI study (compared to 13 in the correctly classified group). Two patients were treated after the DTI study, one was retreated with radiotherapy, and one was started on PCV chemotherapy.
Discussion
By splitting the diffusion tensor into its isotropic and anisotropic components it is possible to define two regions around a tumour: immediately around the central part of the tumour is an area with reduced anisotropy which corresponds to gross tumour, and surrounding this area is a region of increased isotropic but normal anisotropic diffusion which correlates with tumour infiltration. These findings have been previously verified using image-guided biopsies [10], where this technique was shown to correctly identify tumour infiltration in 19 out of 20 cases. This compared to conventional gadolinium-enhanced T1-weighted MR, which could only identify the extent of infiltration in 11 out of the 20 cases, and T2-weighted MR, which could correctly identify 12 out of the 20 cases.
We have identified three patterns of growth from the initial DTI study, namely a diffuse abnormality that corresponds to generalised tumour growth in most cases, a more localised abnormality that predicted further tumour growth in that direction, and a minimal abnormality that might be a predictor of improved survival and slower tumour growth. In this study we have shown that, using these techniques, we can identify patterns of tumour recurrence.
Understanding the pattern of glioma recurrence might allow us to identify the appropriate treatment for the individual patient. There is evidence that gliomas can be considered as both local and systemic diseases. Hochberg and Pruitt were able to show that most gliomas recurred within 2 cm of the original tumour [14]. This was later confirmed on MRI by Albert et al. who showed that 80% of glioma recurrences emerged from areas of enhancement [15]. Yet biopsy studies suggest that gliomas extend beyond the limits seen on imaging [16–18], and cells with the characteristics of gliomas can be cultured in tissue taken over 4 cm from the obvious tumour edge [19].
Current treatment modalities, and especially radiotherapy, try to deal with both problems. Radiotherapy treatment volumes are planned to include the whole tumour (gross tumour volume, GTV) and then expanded in all directions to produce a generous margin designed to include microscopic infiltration (clinical target volume, CTV). For glioblastomas, this margin is typically 2.5 cm around the edge of the gross tumour. A further 0.5-cm margin is applied to account for positioning and set-up errors (the planning target volume, PTV). Data on the tolerance of the normal brain to radiotherapy suggest that a total dose of 60 Gy given in 30 fractions has approximately a 5% risk of producing radiation necrosis within 5 years of treatment [20]. Yet this dose is not sufficient to control the tumour, since virtually all patients with high-grade gliomas treated to 60 Gy recur within the central high-dose area [21, 22]. Dose escalation studies suggest that doses of the order of 90 Gy can control tumour growth, at the expense of radiation necrosis developing in about a third of patients [23]. There have been recent attempts to improve tumour localisation using imaging techniques, for example MR spectroscopy [24], diffusion tensor MRI [25] and PET imaging [26]. These studies have shown that these techniques can be incorporated into radiotherapy planning and that they can reduce the size of the CTV. In turn this should allow dose escalation without increasing the risk of radiation necrosis in areas of normal brain [25].
Clinical experience suggests that tumours of the same grade can behave differently; some simply increase in size, while others are highly infiltrative. If we can predict potential recurrence patterns we could individualise therapy to that patient. This applies both to the choice of treatment, and the specific details of that treatment, such as radiotherapy treatment volumes. Patients with diffuse tumours, for example, would need treating with more widespread therapy. This could entail radiotherapy with wide margins, systemic chemotherapy or the local application of drugs that diffuse over large distances. More localised patterns of recurrence might be suitable for more aggressive surgical resection including integrating advanced imaging techniques (e.g. MR spectroscopy) into image guidance systems to determine the tumour margin more accurately, more targeted radiotherapy, possibly using an intensity modulated technique [25], and more directed local therapies to the areas likely to lead to recurrence. The development of convection-enhanced delivery allows the local administration of various drugs directly into the peritumoural area without the problems of penetration of the blood-brain barrier. If the sites of likely tumour spread and hence the likely recurrence is known, these therapies could be directed to these regions.
Other studies have also found that tumour imaging can predict recurrence. Using early gadolinium-enhanced conventional MRI, Ekinci et al. showed that the presence of nodular enhancement predicted recurrence [27]. Only 1 of their 16 patients exhibiting thin linear enhancement within 24 h of surgery recurred within the year. Pirzkall et al. showed that MR spectroscopic (MRS) abnormalities could predict sites of new contrast enhancement in high-grade gliomas and could correlate with the time to onset of new contrast enhancement [24]. Studies using imaging techniques to look at glioma outcomes have shown that glioblastomas exhibiting enhancement, multifocality, extensive oedema or satellite lesions have a worse prognosis [28]. Imaging studies suggest that gliomas with increased perfusion [29, 30] and higher metabolic rate as determined by FDG PET [29] or 11C-methionine PET [31] have a poorer prognosis.
In this study we have used the T2-weighted signal as our measure of tumour recurrence. There is a commonly held view that the high signal on T2-weighted images represents oedema. This is not completely true as numerous biopsy studies have shown that the infiltrating tumour margin extends at least as far as the T2-weighted abnormality. Johnson studied tumour spread in post-mortem brains and found that the tumour margin correlated well with the area of T2-signal abnormality [32]. In a study that used serial stereotactic biopsies on 40 patients with gliomas, Kelly et al. found histological evidence of tumours in areas with a normal T1 signal in 16% of biopsies and a normal T2 signal in only 4% of biopsies [18]. They concluded that tumour cell infiltration extends at least as far as the abnormal T2 signal in high- and low-grade gliomas. Animal models of developing tumours suggest that areas of increased T2-weighted signals are the first markers of tumour development and precede T1 signal changes [33]. In most cases we found that the gadolinium enhancement mirrored these T2-weighted changes. It must be remembered that the gadolinium enhancement is due to contrast leakage through a neovasculature that occurs later in the development of the tumour. As a result it is not surprising that these changes in enhancement occur later than the changes in the T2-weighted signal which appear to be due to changes as a result of tumour infiltration.
There are a number of limitations of our study. The main problem is that this is a retrospective study of a heterogeneous group of patients that were selected for other studies. Table 3 shows the numbers of patients that were treated previously with some assessment as to the success of treatment. Most of the patients had already been treated with surgery and radiotherapy and were studied at either recurrence or with stable disease. Although we excluded patients who underwent surgical resection as this could alter the location of white-matter tracts, we have included patients that received radiotherapy or chemotherapy. We doubt, however, that either radiotherapy or chemotherapy will alter the pattern of imaging and clinical relapse since we found similar patterns in the eight patients that had not received any treatment and the treated patients who were imaged at recurrence. Since most patients treated with radiotherapy recur within the treated volume, this treatment is not likely to alter the pattern of recurrence [21, 22]. It is interesting to note, however, that all three patients misclassified by DTI had received radiotherapy prior to the DTI study.
The majority of patients with high-grade gliomas fail to respond to chemotherapy. Those that do respond initially progress later in the same location, suggesting that this treatment will have little effect on the recurrence pattern. The final treatment received by 19 of 25 patients was dexamethasone. This is known to alter the diffusion properties around the tumour, but has its main effect on the isotropic measures of diffusion rather than anisotropic diffusion [34]. This would suggest that dexamethasone would uniformly reduce the isotropic abnormality around the tumour and thus underestimate the extent of the tumour infiltration. An image-guided biopsy study has shown that this is not the case [10].
It is clear that the diffuse and localised groups may describe different groups of patients. There were lower grade tumours in the diffuse group who had at most been biopsied, whereas the localised group had more glioblastomas that were largely resected before receiving radiotherapy. It was not the aim of this study to look at the clinical factors that were involved in patterns of tumour recurrence, rather to see if DTI could predict these patterns. As a result, we feel that the effects of heterogeneity are less important for the reporting of the results of this study.
One possible interpretation of the differences between the diffuse and localised groups would be the timing of imaging. It is quite possible that if the diffuse group were imaged sooner they would demonstrate an initial localised recurrence pattern. Similarly, the localised group might go on to develop a diffuse recurrence pattern. At the time of this study it was our usual practice to image patients only at the time of clinical progression. In other words, the timing of the repeat imaging was determined by clinical factors which we would expect to be similar in both groups. In fact, using this time point, the interval between studies for the diffuse and localised groups was not significantly different. Future studies are clearly needed to confirm these findings in a larger cohort of patients of similar tumour grade treated in a more uniform fashion and studied prospectively with a standard follow-up pattern.
Conclusion
This study has shown that the use of diffusion tensor imaging can predict patterns of tumour recurrence. By looking at the patterns of either tumour infiltration or occult tumour not seen on conventional MR sequences, it is possible to classify tumours into three categories: those with a diffuse abnormality that predicts generalised increase in tumour size; a localised abnormality that predicts more localised recurrence; and in a few patients, a limited abnormality that appears to predict a better prognosis. Use of these techniques may allow individualisation of treatments and has the advantage over molecular markers that the data are acquired pre-operatively so they can direct surgical treatments, guide biopsies to the relevant parts of a tumour and direct local chemotherapy treatments before tissue samples are available. A larger, prospective study based on a more homogeneous cohort of high-grade glioma patients is planned to investigate this further.
References
Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJB, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352:987–996
Coons SW, Johnson PC, Shapiro JR (1995) Cytogenetic and flow cytometry DNA analysis of regional heterogeneity in a low grade human glioma. Cancer Res 55:1569–1577
Paulus W, Peiffer J (1989) Intratumoral histologic heterogeneity of gliomas. A quantitative study. Cancer 64:442–447
Shapiro WR (1999) Current therapy for brain tumors: back to the future. Arch Neurol 56:429–432
Price SJ, Burnet NG, Donovan T, Green HA, Pena A, Antoun NM, Pickard JD, Carpenter TA, Gillard JH (2003) Diffusion tensor imaging of brain tumours at 3T: a potential tool for assessing white matter tract invasion? Clin Radiol 58:455–462
Provenzale JM, McGraw P, Mhatre P, Guo AC, Delong D (2004) Peritumoral brain regions in gliomas and meningiomas: investigation with isotropic diffusion-weighted MR imaging and diffusion-tensor MR imaging. Radiology 232:451–460
Witwer BP, Moftakhar R, Hasan KM, Deshmukh P, Haughton V, Field A, Arfanakis K, Noyes J, Moritz CH, Meyerand ME, Rowley HA, Alexander AL, Badie B (2002) Diffusion-tensor imaging of white matter tracts in patients with cerebral neoplasm. J Neurosurg 97:568–575
Field AS, Alexander AL, Wu YC, Hasan KM, Witwer B, Badie B (2004) Diffusion tensor eigenvector directional color imaging patterns in the evaluation of cerebral white matter tracts altered by tumor. J Magn Reson Imaging 20:555–562
Price SJ, Pena A, Burnet NG, Jena R, Green HA, Carpenter TA, Pickard JD, Gillard JH (2004) Tissue signature characterisation of diffusion tensor abnormalities in cerebral gliomas. Eur Radiol 14:1909–1917
Price SJ, Jena R, Burnet NG, Hutchinson PJ, Dean AF, Pena A, Pickard JD, Carpenter TA, Gillard JH (2006) Improved delineation of glioma margins and regions of infiltration using diffusion tensor imaging: an image-guided biopsy study. AJNR Am J Neuroradiol 27(9):1969–1974
Price SJ, Pena A, Burnet NG, Pickard JD, Gillard JH (2004) Detecting glioma invasion of the corpus callosum using diffusion tensor imaging. Br J Neurosurg 18:391–395
Basser PJ, Mattiello J, LeBihan D (1994) Estimation of the effective self-diffusion tensor from the NMR spin echo. J Magn Reson B 103:247–254
Hartkens T, Rueckert D, Schnabel JA, Hawkes DJ, Hill DLG (2002) VTK CISG registration toolkit: an open source software package for affine and non-rigid registration of single- and multimodal 3D images. BVM2002, Leipzig 56:185, http://sunsite.informatik.rwth-aachen.de/Publications/CEUR-WS/Vol-56/185.pdf
Hochberg FH, Pruitt A (1980) Assumptions in the radiotherapy of glioblastoma. Neurology 30:907–911
Albert FK, Forsting M, Sartor K, Adams HP, Kunze S (1994) Early postoperative magnetic resonance imaging after resection of malignant glioma: objective evaluation of residual tumor and its influence on regrowth and prognosis. Neurosurgery 34:45–60
Burger PC, Heinz ER, Shibata T, Kleihues P (1988) Topographic anatomy and CT correlations in the untreated glioblastoma multiforme. J Neurosurg 68:698–704
Burger PC, Dubois PJ, Schold SC Jr, Smith KR Jr, Odom GL, Crafts DC, Giangaspero F (1983) Computerized tomographic and pathologic studies of the untreated, quiescent, and recurrent glioblastoma multiforme. J Neurosurg 58:159–169
Kelly PJ, Daumas-Duport C, Kispert DB, Kall BA, Scheithauer BW, Illig JJ (1987) Imaging-based stereotaxic serial biopsies in untreated intracranial glial neoplasms. J Neurosurg 66:865–874
Silbergeld DL, Chicoine MR (1997) Isolation and characterization of human malignant glioma cells from histologically normal brain. J Neurosurg 86:525–531
Emami B, Lyman J, Brown A, Coia L, Goitein M, Munzenrider JE, Shank B, Solin LJ, Wesson M (1991) Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys 21:109–122
Lee SW, Fraass BA, Marsh LH, Herbort K, Gebarski SS, Martel MK, Radany EH, Lichter AS, Sandler HM (1999) Patterns of failure following high-dose 3-D conformal radiotherapy for high-grade astrocytomas: a quantitative dosimetric study. Int J Radiat Oncol Biol Phys 43:79–88
Oppitz U, Maessen D, Zunterer H, Richter S, Flentje M (1999) 3D-recurrence-patterns of glioblastomas after CT-planned postoperative irradiation. Radiother Oncol 53:53–57
Fitzek MM, Thornton AF, Rabinov JD, Lev MH, Pardo FS, Munzenrider JE, Okunieff P, Bussiere M, Braun I, Hochberg FH, Hedley-Whyte ET, Liebsch NJ, Harsh GR (1999) Accelerated fractionated proton/photon irradiation to 90 cobalt gray equivalent for glioblastoma multiforme: results of a phase II prospective trial. J Neurosurg 91:251–260
Pirzkall A, Li X, Oh J, Chang S, Berger MS, Larson DA, Verhey LJ, Dillon WP, Nelson SJ (2004) 3D MRSI for resected high-grade gliomas before RT: tumor extent according to metabolic activity in relation to MRI. Int J Radiat Oncol Biol Phys 59:126–137
Jena R, Price SJ, Baker C, Jefferies SJ, Pickard JD, Gillard JH, Burnet NG (2005) Diffusion tensor imaging: possible implications for radiotherapy treatment planning of patients with high-grade glioma. Clin Oncol 17:581–590
Grosu AL, Weber WA, Riedel E, Jeremic B, Nieder C, Franz M, Gumprecht H, Jaeger R, Schwaiger M, Molls M (2005) L-(methyl-11C) methionine positron emission tomography for target delineation in resected high-grade gliomas before radiotherapy. Int J Radiat Oncol Biol Phys 63:64–74
Ekinci G, Akpinar IN, Baltacioglu F, Erzen C, Kilic T, Elmaci I, Pamir N (2003) Early-postoperative magnetic resonance imaging in glial tumors: prediction of tumor regrowth and recurrence. Eur J Radiol 45:99–107
Pope WB, Sayre J, Perlina A, Villablanca JP, Mischel PS, Cloughesy TF (2005) MR imaging correlates of survival in patients with high-grade gliomas. AJNR Am J Neuroradiol 26:2466–2474
Mineura K, Sasajima T, Kowada M, Ogawa T, Hatazawa J, Shishido F, Uemura K (1994) Perfusion and metabolism in predicting the survival of patients with cerebral gliomas. Cancer 73:2386–2394
Cao Y, Tsien CI, Nagesh V, Junck L, Ten Haken R, Ross BD, Chenevert TL, Lawrence TS (2006) Clinical investigation survival prediction in high-grade gliomas by MRI perfusion before and during early stage of RT. Int J Radiat Oncol Biol Phys 64:876–885
De Witte O, Goldberg I, Wikler D, Rorive S, Damhaut P, Monclus M, Salmon I, Brotchi J, Goldman S (2001) Positron emission tomography with injection of methionine as a prognostic factor in glioma. J Neurosurg 95:746–750
Johnson PC, Hunt SJ, Drayer BP (1989) Human cerebral gliomas: correlation of postmortem MR imaging and neuropathologic findings. Radiology 170:211–217
Sathy BN, Hsu Y-H, Jang T, Recht L, Chang C (2006) Development and evolution of glioma after ENU exposure: a model of malignant transformation of low grade gliomas. Proc Intl Soc Mag Reson Med 14:613
Sinha S, Bastin ME, Wardlaw JM, Armitage PA, Whittle IR (2004) Effects of dexamethasone on peritumoural oedematous brain: a DT-MRI study. J Neurol Neurosurg Psychiatry 75:1632–1635
Acknowledgements
We wish to thank Dr. Andrew Dean for help with neuropathology. Imaging costs were covered by a Technology Foresight Award. S.J.P. was supported by the New and Emerging Applications of Technology (NEAT) Programme from the Department of Health and by the Samuel Scott of Yews Research Fellowship from the Royal College of Surgeons of England. R.J. was supported by an unrestricted educational grant from Siemens Oncology Care Systems, USA.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Price, S.J., Jena, R., Burnet, N.G. et al. Predicting patterns of glioma recurrence using diffusion tensor imaging. Eur Radiol 17, 1675–1684 (2007). https://doi.org/10.1007/s00330-006-0561-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-006-0561-2