Abstract
This paper is a feasibility study of magnetic resonance imaging (MRI) of lung perfusion in children with cystic fibrosis (CF) using contrast-enhanced 3D MRI. Correlation assessment of perfusion changes with structural abnormalities. Eleven CF patients (9 f, 2 m; median age 16 years) were examined at 1.5 T. Morphology: HASTE coronal, transversal (TR/TE/α/ST: 600 ms/28 ms/180°/6 mm), breath-hold 18 s. Perfusion: Time-resolved 3D GRE pulse sequence (FLASH, TE/TR/α: 0.8/1.9 ms/40°), parallel imaging (GRAPPA, PAT 2). Twenty-five data sets were acquired after intravenous injection of 0.1 mmol/kg body weight of gadodiamide, 3–5 ml/s. A total of 198 lung segments were analyzed by two radiologists in consensus and scored for morphological and perfusion changes. Statistical analysis was performed by Mantel-Haenszel chi-square test. Results showed that perfusion defects were observed in all patients and present in 80% of upper, and 39% of lower lobes. Normal lung parenchyma showed homogeneous perfusion (86%, P<0.0001). Severe morphological changes led to perfusion defects (97%, P<0.0001). Segments with moderate morphological changes showed normal (53%) or impaired perfusion (47%). In conclusion, pulmonary perfusion is easy to judge in segments with normal parenchyma or severe changes. In moderately damaged segments, MRI of lung perfusion may help to better assess actual functional impairment. Contrast-enhanced 3D MRI of lung perfusion has the potential for early vascular functional assessment and therapy control in CF patients.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cystic fibrosis (CF) is the most common metabolic disorder of autosomal recessive inheritance leading to premature death in the Caucasian population [1]. The genetic defect leads to secretions with high viscosity causing chronic lung infection and airway obstruction [2], followed by continuous deterioration of respiratory function.
Lung function is assessed in patients with CF by pulmonary function tests (PFT). A decrease in forced expiratory volume in 1 s (FEV1) has been shown to be the most important prognostic factor in the course of the disease and the most significant predictor of mortality [3]. Nevertheless, PFTs measure global parameters of lung function and cannot assess regional functional defects.
The regional ventilatory defect causes changes in regional lung perfusion due to the reflex of hypoxic vasoconstriction first described by v. Euler and Liljestrand in 1947 [4]. Only perfused lung areas are able to perform gas exchange. Thus, lung perfusion may serve as an indicator of regional lung function. Lung perfusion can be assessed by radionuclid scintigraphy [5], but is not measured routinely in patients with CF [6, 7].
Recently, contrast-enhanced 3D magnetic resonance imaging (MRI) has been introduced for assessment of lung perfusion [8–11]. This method is based on contrast enhancement of the lung parenchyma during the first pass of a bolus of contrast agent through the pulmonary circulation. Imaging is performed during an inspiratory breath-hold. HASTE sequences have shown good correlation with HRCT and therefore can be used for visualization of the main morphological changes in CF lungs [12].
The aim of this study was to assess the correlation of lung perfusion changes with structural abnormalities in CF patients.
Materials and methods
Eleven patients (9 female, 2 male; median age 16 years, range 11–19) were prospectively examined at 1.5 T (Magnetom Symphony, Siemens Erlangen, Germany) equipped with a high-performance gradient system. None of the patients had clinical symptoms of exacerbation or needed therapy for exacerbation. Three patients had normal lung function [FEV1 median 96% predicted (p), min 86% p, max 99% p]. Six patients showed mild or moderate obstructive lung disease (FEV1 median 69% p, min 55% p, max 76% p) and two patients showed severe obstructive lung disease (FEV1 median 37% p, mean 37% p). For signal reception either a combination of two spine array coils with two four-element body phased-array coils or a six-channel body phased-array coil was used.
The investigation protocol was approved by the local Ethics Committee. Written informed consent was obtained from all patients or their parents after the nature of the procedure had been fully explained.
Lung morphology was assessed by HASTE MRI (TR/TE/α: 600 ms/28 ms/180°, slice thickness 6 mm, ECG triggered) in inspiratory breath-hold of approximately 18 s, depending on heart frequency and the number of slices needed to cover the whole thorax. Perfusion imaging was performed using a time-resolved 3D GRE pulse sequence (FLASH, TE/TR/α: 0.8/1.9 ms/40°) with parallel imaging (GRAPPA, PAT factor 2). With this sequence, an image data set of the whole lung was acquired within 1.5 s. A total of 25 data sets were acquired continuously beginning after an intravenous injection of 0.1 mmol/kg body weight of gadodiamide (Omniscan, GE Bioscience, Germany) at a rate of 3–5 ml/s. Image data were post-processed by subtraction of the baseline images without contrast from those with maximum contrast, resulting in an image series with the maximum lung perfusion. A total of 198 lung segments (11 patients ×18 segments) were analyzed by two radiologists in consensus for morphological changes (bronchiectasis, bronchial wall thickening, mucus plugging, consolidation) and perfusion defects. The changes were classified as shown in Table 1. Moderate morphological changes were defined as involving less than 50% of the lung segment, severe changes as more than 50%. Perfusion was categorized as normal (homogeneous) or impaired (inhomogeneous, defect).
Statistical analysis of 198 lung segments grouped on a lobular level was performed using the Mantel-Haenszel chi-square test. A P value <0.05 was statistically significant.
Results
MRI was well tolerated by all patients. The image quality was adequate to analyze the images according to the defined criteria.
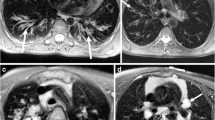
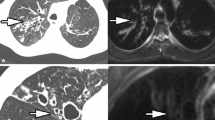
The majority of lung segments showed morphological changes such as bronchiectasis, bronchial wall thickening and mucus plugging. Only 11% of lung segments (n=21) did not show any morphological changes; the majority of lung segments (89%, n=177) were morphologically altered. Of morphologically altered lung segments, 20% (n=35) showed severe morphological changes (Fig. 1a), whereas moderate morphological changes (Fig. 2a) were found in 80% of morphologically altered lung segments (n=142).
a Coronal morphologic imaging (HASTE sequence) of a 17-year-old CF patient with severe morphological changes: peripheral bronchiectasis (1), bronchial wall thickening (2), mucus plugging (3), sacculation with air fluid level (4). The right lung, mainly the right upper lobe, is predominantly involved. b Perfusion imaging (time-resolved 3D GRE pulse sequence with parallel imaging) of the same patient at the comparable slice position showing a large perfusion defect of the right upper lobe (arrow) and inhomogeneous perfusion of the left lung
a Coronal morphologic imaging (HASTE sequence) of a 10-year-old CF patient with moderate morphological changes: central bronchiectasis (1), bronchial wall thickening (2), mucus plugging (3). The right upper lobe and both lower lobes are mainly involved. b Perfusion imaging (time-resolved 3D GRE pulse sequence with parallel imaging) of the same patient at the comparable slice position showing perfusion defects in the right upper lobe and more pronounced defects in the left upper lobe (arrow)
Higher signal intensities of lung perfusion were observed in gravity-dependent lung regions. Segmental perfusion defects were observed in all patients. Homogeneous perfusion was found in 48% of lung segments (n=95), and impaired perfusion in 52% (n=103).
Of the lung segments with a normal lung parenchyma, 86% (18 of 21 segments) showed homogeneous perfusion, with a specifity of 86% (P<0.0001). In lung segments with severe morphological changes, perfusion defects were found in 97% of analyzed lung segments (n=34, P<0.0001). The large group of lung segments with moderate morphological changes (142 segments) showed nearly equal distribution of normal and impaired perfusion: 53% (n=76) of the 142 lung segments had normal perfusion, 47% (n=66) showed perfusion defects. Table 2 summarizes the statistical evaluation of correlation between morphology and lung perfusion.
The local distribution of morphological changes and perfusion defects is shown in Table 3. Morphological defects were found in the whole lung, preferentially in the upper lobes, with defects in 96% (n=53) of upper lung segments. Middle lobe and lingula showed morphological alteration in 86% of segments (n=38), while 85% (n=86) of lower lobe segments were altered. The frequency of severely altered lung segments decreased from upper (32%) to middle (14%) and to lower lobes (11%). Comparable segmental perfusion defects were preferentially located in the upper lung: 80% of the upper lobe segments (44 of 55 lung segments) showed perfusion defects. Lingula and middle lobe segments showed perfusion defects in 45% of the analyzed segments (20 of 44 lung segments). Of the lower lobe segments, 39% (39 of 99 lung segments) had perfusion defects.
Discussion
Cystic fibrosis is caused by mutations of a gene located on the long arm of chromosome 7. The gene codes for the CFTR protein (cystic fibrosis transmembrane regulator protein), which functions as an anion channel. Impaired CFTR function causes disturbances in volume and ion composition of airway surface fluid, leading to bacterial colonization and chronic lung infection [1]. Despite improved understanding of the underlying pathophysiology and the introduction of new therapies, CF is still the most common life-shortening inherited disease in the Caucasian population with an actual median survival probability of 36.8 years in Germany [13].
Chronic lung infection and highly viscous secretions lead to airway obstruction. Due to hypoxic vasoconstriction, consecutive impairment of microcirculation will develop and perfusion defects will result.
The standard for assessment of pulmonary function is the PFT, which is reproducible and broadly available. However, PFTs only reflect a global change in lung function, while regional function remains unassessed. CF is a chronic lung disease with inhomogeneous effects on the lung. This is reflected in the presented cohort: patients had normal lung function or showed mainly mild or moderate obstructive lung disease although morphological changes were found in every patient. Furthermore, the pulmonary destruction process starts at the level of small airways [2, 14, 15]. PFTs are not reliable for measurement of small-airway function, thus assessment of early pulmonary changes is difficult [16].
As mentioned, an additional approach to pulmonary functional evaluation is the assessment of pulmonary perfusion as a part of global lung functionality. Besides ventilatory mechanics and gas diffusion, neither of which was analyzed in the present work, pulmonary perfusion represents the physiological part of lung functionality, enabling gas exchange. Lung perfusion shows a good correlation to disease severity defined by FEV1 and Shwachmann score [7]. Up to now, imaging of pulmonary perfusion has been the domain of scintigraphic methods. Although the radiation exposure of perfusion scintigraphy is relatively low (2–3 mSv), repeated exposure in patients with chronic lung disease such as CF is unfavorable. Therefore perfusion scintigraphy is not used routinely in these patients. Conventional imaging modalities used for follow up in CF patients, such as chest X-ray or high resolution computed tomography (HRCT), are unsuitable for functional information.
In recent years, contrast-enhanced MRI for assessment of lung perfusion was developed [9, 11]. Compared to other organs, the pulmonary circulation time is very short (about 5 s). Therefore MR imaging requires rapid acquisition for peak enhancement visualization. With the introduction of parallel imaging in clinical routine, very fast image acquisition became possible, enabling substantial improvement in temporal and/or spatial resolution, including the visualization of lung perfusion [17]. Using short repetition times as well as partial data acquisition techniques, the acquisition time of the 3D pulse sequence was reduced. This technique enables the acquisition of a 3D-image data set of the whole lung within 1.5 s. Thus, the passage of the contrast media through the lung can be followed and visualized [18].
In this study, contrast-enhanced 3D MRI perfusion was used for the assessment of pulmonary perfusion in patients with CF and was correlated with morphological changes. There was a high prevalence of pathological changes in lung morphology, although these changes were mainly moderate. This is most likely caused by the patients’ age (median: 16 years). Structural lung damage appears early in the course of the disease but is less severe in young patients, progressively worsening with age. Morphological changes and fundamentally severe changes were preferentially located in the upper lobes, which is a typical localization in CF lung pathology. Perfusion defects were found in almost 50% of lung segments, most of them also located in the upper lobes (Table 3).
Higher signal intensities of the lungs were observed in gravity-dependent lung regions, most likely indicating preferential perfusion.
A high correlation between lung morphology and lung perfusion was found in normal lung segments (specificity 86%) and those with severe morphological changes (sensitivity 97%). If the total number of morphologically altered segments with moderate as well as with severe changes is considered, sensitivity decreases.
The large group of lung segments with moderate morphological changes showed a nearly equal distribution between normal and impaired perfusion. Normal lung perfusion indicates normal ventilation, even though moderate structural defects can be detected. Whether impaired perfusion in moderately damaged lung segments is a result of hypoxic vasoconstriction or indicates irreversible lung damage cannot be stated from this study.
Intensified therapy contributes to liquefaction of bronchial mucus and leads to improved airway clearing [19], thus homogenizing ventilation and oxygenation. Under these circumstances, one might expect an improvement in pulmonary perfusion. Otherwise, unchanged perfusion after intensified therapy could be indicative of irreversible lung damage. Thus, follow-up studies of lung perfusion may be helpful to differentiate reversible from irreversible lung changes in CF.
As lung perfusion is an indirect but seemingly very sensitive parameter of lung function, one could expect that early functional changes can be assessed by this technique. Both considerations are subject to further studies.
The technique of pulmonary perfusion with contrast-enhanced 3D MR as described is restricted to a quantitative or semi-quantitative assessment (“indicator dilution method”) because of the lack of proportional linearity in signal intensity and contrast media concentration. This method enables the calculation of regional pulmonary blood flow, regional pulmonary blood volume and mean transit time [20].
Up to now, imaging techniques used for assessment of CF have been based on morphological changes. The Chrispin Norman Score [21] is used for assessment of chest X-ray, the Bhalla [22] or Helbich scores [23] for CT. Both of them show strong correlation with lung function. The main disadvantage of these procedures is the need for repeated examinations and thus a high radiation exposure during a patient’s lifetime. With MRI, morphological [12, 24] as well as functional [11, 25, 26] assessment of the lung is possible for the first time with a radiation-free method.
Studies are required to further assess the importance of lung perfusion for characterization of CF lung disease, and its potential for early detection and therapy control.
Conclusion
In CF patients, impaired ventilation causes perfusion defects due to hypoxic vasoconstriction. While pulmonary perfusion is easy to judge in segments with normal parenchyma or severe changes, this is not possible in mildly damaged segments. Here, MR lung perfusion measurements may help to better assess actual functional impairment.
Contrast-enhanced 3D MRI of lung perfusion is feasible in CF patients. This radiation-free method allows for a functional evaluation of CF lungs. By detecting perfusion defects even in moderately morphologically altered segments, contrast-enhanced 3D MRI lung perfusion appears to be a very sensitive and highly promising method for early vascular functional assessment and therapy control in patients with CF.
References
Davis PB, Drumm M, Konstan MW (1996) Cystic fibrosis. Am J Respir Crit Care Med 154(5):1229–1256
Gibson RL, Burns JL, Ramsey BW (2003) Pathophysiology and management of pulmonary infections in cystic fibrosis. Am J Respir Crit Care Med 168(8):918–951
Kerem E, Reisman J, Corey M, Canny GJ, Levison H (1992) Prediction of mortality in patients with cystic fibrosis. N Engl J Med 326(18):1187–1191
v. Euler US, Liljestrand G (1947) Observations on the pulmonary arterial blood pressure in the cat. Acta Phys Scand 12:301–320
Fazio F, Wollmer P (1981) Clinical ventilation-perfusion scintigraphy. Clin Physiol 1(4):323–337
Fauroux B, Itti E, Pigeot J, Isabey D, Meignan M, Ferry G, Lofaso F, Willemot JM, Clement A, Harf A (2000) Optimization of aerosol deposition by pressure support in children with cystic fibrosis: an experimental and clinical study. Am J Respir Crit Care Med 162(6):2265–2271
Itti E, Fauroux B, Pigeot J, Isabey D, Clement A, Evangelista E, Harf A, Meignan M (2004) Quantitative lung perfusion scan as a predictor of aerosol distribution heterogeneity and disease severity in children with cystic fibrosis. Nucl Med Commun 25(6):563–569
Hatabu H, Gaa J, Kim D, Li W, Prasad PV, Edelman RR (1996) Pulmonary perfusion: qualitative assessment with dynamic contrast-enhanced MRI using ultra-short TE and inversion recovery turbo FLASH. Magn Reson Med 36(4):503–508
Hatabu H, Tadamura E, Levin DL, Chen Q, Li W, Kim D, Prasad PV, Edelman RR (1999) Quantitative assessment of pulmonary perfusion with dynamic contrast-enhanced MRI. Magn Reson Med 42(6):1033–1038
Uematsu H, Levin DL, Hatabu H (2001) Quantification of pulmonary perfusion with MR imaging: recent advances. Eur J Radiol 37(3):155–163
Fink C, Puderbach M, Bock M, Lodemann KP, Zuna I, Schmahl A, Delorme S, Kauczor HU (2004) Regional lung perfusion: assessment with partially parallel three-dimensional MR imaging. Radiology 231(1):175–184
Puderbach M, Eichinger M, Ley S, Fink C, Plathow C, Gahr J, Wiebel M, Tuengerthal S, Schmahl A, Mueller FM, Kauczor HU (2004) Visualisation of parenchymal lung changes in patients with cystic fibrosis (CF) - MRI versus HRCT. J Cyst Fibros 3(Suppl 1):54
Stern M, Sens B, Wiedermann B, Busse O, Damm G, Wenzlaff P (2005) Qualitätssicherung Mukoviszidose, Überblick über den Gesundheitszustand der Patienten in Deutschland 2004. Verlag Wissenschaftliche Scripten, Zwickau
Tiddens HA (2002) Detecting early structural lung damage in cystic fibrosis. Pediatr Pulmonol 34(3):228–231
Lindstrom M, Camner P, Falk R, Hjelte L, Philipson K, Svartengren M (2005) Long-term clearance from small airways in patients with cystic fibrosis. Eur Respir J 25(2):317–323
Barnes PJ (2004) Small airways in COPD. N Engl J Med 350(26):2635–2637
Fink C, Bock M, Puderbach M, Schmahl A, Delorme S (2003) Partially parallel three-dimensional magnetic resonance imaging for the assessment of lung perfusion-initial results. Invest Radiol 38(8):482–488
Ley S, Fink C, Puderbach M, Plathow C, Risse F, Kreitner KF, Kauczor HU (2004) Contrast-enhanced 3D MR perfusion of the lung: application of parallel imaging technique in healthy subjects. Rofo 176(3):330–334
Rosenfeld M, Gibson RL, McNamara S, Emerson J, Burns JL, Castile R, Hiatt P, McCoy K, Wilson CB, Inglis A, Smith A, Martin TR, Ramsey BW (2001) Early pulmonary infection, inflammation, and clinical outcomes in infants with cystic fibrosis. Pediatr Pulmonol 32(5):356–366
Fink C, Ley S, Risse F, Eichinger M, Zaporozhan J, Buhmann R, Puderbach M, Plathow C, Kauczor HU (2005) Effect of inspiratory and expiratory breathhold on pulmonary perfusion: assessment by pulmonary perfusion magnetic resonance imaging. Invest Radiol 40(2):72–79
Chrispin AR, Norman AP (1974) The systematic evaluation of the chest radiograph in cystic fibrosis. Pediatr Radiol 2:101–106
Bhalla M, Turcios N, Aponte V, Jenkins M, Leitman BS, McCauley DI, Naidich DP (1991) Cystic fibrosis: scoring system with thin-section CT. Radiology 179(3):783–788
Helbich TH, Heinz-Peer G, Eichler I, Wunderbaldinger P, Gotz M, Wojnarowski C, Brasch RC, Herold CJ (1999) Cystic fibrosis: CT assessment of lung involvement in children and adults. Radiology 213(2):537–544
Abolmaali N, Schmidt H, Anjorin A, Posselt H-G, Vogl TJ (2002) Chrispin-Norman-score and Bhalla-score of patients with cystic fibrosis: Comparative study of chest radiographs and MR-Imaging. Eur Radiol 12 [Congress Suppl]:227
Vonk-Noordegraaf A, van Wolferen SA, Marcus JT, Boonstra A, Postmus PE, Peeters JW, Peacock AJ (2005) Noninvasive assessment and monitoring of the pulmonary circulation. Eur Respir J 25(4):758–766
Ley S, Puderbach M, Fink C, Eichinger M, Plathow C, Teiner S, Wiebel M, Muller FM, Kauczor HU (2005) Assessment of hemodynamic changes in the systemic and pulmonary arterial circulation in patients with cystic fibrosis using phase-contrast MRI. Eur Radiol 15(8):1575–1580
Acknowledgement
This study was supported by Forschungsgemeinschaft Mukoviszidose (Mukoviszidose e.V.): S06/04.
Author information
Authors and Affiliations
Corresponding author
Additional information
Monika Eichinger and Michael Puderbach contributed equally to this work.
Rights and permissions
About this article
Cite this article
Eichinger, M., Puderbach, M., Fink, C. et al. Contrast-enhanced 3D MRI of lung perfusion in children with cystic fibrosis—initial results. Eur Radiol 16, 2147–2152 (2006). https://doi.org/10.1007/s00330-006-0257-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-006-0257-7