Abstract
To assess the feasibility of contrast-enhanced ultrasound (CEUS) of the thyroid gland and to evaluate the potential of this method for characterising solitary thyroid nodules.18 patients affected by solitary thyroid nodules (size range: 0.6 to 3.6 cm; mean: 1.8 cm) confirmed by surgery (nine papillary carcinomas, four follicular carcinomas, three hyperplasias, one follicular adenoma and one Plummer’s adenoma) underwent pulse inversion US at low M.I. (0.06 to 0.08) after i.v. injection of a 2.4-mL bolus of SonoVue. Baseline echogenicity and the dynamic enhancement pattern of each nodule, in comparison with adjacent thyroid parenchyma, were assessed. Signal intensity values on grey-scale images were also calculated at baseline, 30 s, 60 s and 120 s after SonoVue administration. Following administration of SonoVue, malignant nodules showed absent (4 out of 13), faint dotted (4 out of 13) and diffuse (5 out of 13) contrast enhancement, in this last case inhomogeneous (4 out of 5 cases) or homogeneous (1 out of 5). Benign nodules showed diffuse contrast enhancement, both homogeneous (3 out of 5) and heterogeneous (2 out of 5). Quantitative data have confirmed subjective findings, but CEUS never modified precontrast analysis. CEUS of thyroid gland is a feasible technique, but overlapping findings seem to limit the potential of this technique in the characterization of thyroid nodules.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
High-resolution ultrasound (HRUS) and color power Doppler allow for the detailed depiction of the thyroid and provide functional information about the vascularization of normal and diseased gland [1]. Since HRUS is increasingly being used for the evaluation of both the thyroid gland and the neck, a dramatic increase in the detection rate of non-palpable thyroid nodules is registered, up to nearly 70% [2–5]. Consequently, thyroid incidentalomas have become a real emerging epidemic and their management a conundrum [6]. Many thyroid incidentalomas are of little clinical significance since thyroid cancer is an uncommon malignancy, representing 1.5% of all new cancers and 0.23% of all cancer deaths in 2001 in the United States [6]. Furthermore, surgery- or autopsy-based studies reported that occult thyroid cancer is more common than clinical apparent thyroid cancer, up to 36% in some countries, thus demonstrating the slight biological significance of many of these tumors [7, 8].
Recently, new ultrasound techniques, such as pulse inversion (PI) harmonic imaging, have been developed that are extremely sensitive to non-linear effects of US interaction with microbubble contrast agents [9]. Some studies, carried out with a first-generation air-based contrast agent (SH U 508A), have analysed time/intensity curves in the characterization of solitary thyroid nodules, not providing conclusive data [10, 11]. SonoVue is a new, second-generation, stabilized microbubble preparation containing sulphur hexafluoride. This latter has low solubility, is isotonic and does not contain antigenic potential gas [12]. An early report showed that SonoVue may enable the identification of some specific contrast-enhancement patterns in different focal liver lesions [13–15].
The objective of this study was to assess the feasibility of CEUS of the thyroid gland and to evaluate the potential of this method for characterizing solitary thyroid nodules.
Materials and methods
Study population
From September 2003 to July 2004 18 consecutive patients (10 women, 8 men; age range: 26 to 66 years, mean: 44.3 years) who were scheduled to undergo thyroidectomy for a previously diagnosed solitary thyroid nodule (size range: 0.6 to 3.6 cm; mean: 1.8 cm) were enrolled in this prospective study and underwent CEUS of the thyroid gland.
Ethical Committee permission was obtained and all patients gave their full informed consent before the CEUS. The procedure followed was in accordance with the Declaration of Helsinki [16].
Baseline US
Scanning was performed by one experienced radiologist (more than 15 years in thyroid US imaging and more than 5 years in CEUS) blinded to the final diagnosis with a commercially available scanner (HDI 5000, ATL, Bothell, WA, USA) using an electronically focused near-field linear array transducer with a 7- to 12-MHz bandwidth and provided with real-time compound spatial imaging (SonoCT) and pulse inversion (PI) imaging software. A color Doppler study of the thyroid gland was performed with a pulse repetition frequency of 1200, whereas power Doppler was used to detect slow flow. The amplifier gain was raised individually until random color noise appeared and then slightly lowered. The wall filter was set low (100 Hz). Once set, US parameters remained unchanged in each patient.
A precontrast cine clip lasting approx. 10 s was acquired for each lesion and digitally stored as raw data in a PC-based workstation connected to the US unit via a standard Ethernet link.
Contrast-enhanced US
The US contrast agent used in the present study was SonoVue (Bracco, Milan, Italy) injected intravenously as a bolus at a 2.4-mL dose, followed by 5 mL of normal saline flush, using a 20- or 22-gauge peripheral intravenous cannula [17]. A low frame rate (5 Hz) and a very low mechanical index (MI=0.05–0.08) were used. Since bubble disruption is strictly related to depth and focalization of a US beam, focus was always placed deeper than the nodule being examined in order to minimize microbubble disruption. Once set, US parameters remained unchanged in each patient.
Each exam lasted about 5 min following bolus injection.
Three postcontrast cine clips lasting approx. 15 s were acquired for each lesion, 10 to 25 s, 45 to 60 s and 110 to 125 s respectively after contrast agent administration and digitally stored as raw data on a PC-based workstation connected to the US unit via a standard Ethernet link.
Image analysis
Subjective evaluation
Digital cine clips stored on the US unit were reviewed retrospectively on screen by two independent experienced radiologists not involved in the US scanning and blinded to the final diagnosis. Cine clips were presented in a random order, and the readers were asked to evaluate in consensus each cine clip for nodule location, size (measured in three diameters), echogenicity (hyper-, iso- or hypoechoic), echotexture (solid, cystic or mixed), margin (well defined, irregular or blurred) and the presence of hyperechoic spots (coarse calcifications or microcalcifications) in all detected nodules [18]. In particular, in a grey-scale US study malignant features were considered: (a) punctate microcalcification defined as hyperechoic spots less than 2 mm with or without acustic shadowing, (b) a marked hypoechoic pattern (more hypoechoic than surrounding strap muscle) without posterior shadowing, (c) irregular or microlobulated margin, (d) a shape that is taller than it is wide [19].
The presence and pattern of blood flow by means of color power Doppler was also evaluated and classed as follows: type I, absence of blood flow; type II, perinodular and absent intranodular blood flow; and type III, marked intranodular blood flow [20]. Since a study reported the intranodular vascular pattern as an independent risk factor of malignancy, for the purpose of this study the presence of intranodular blood flow (pattern III) was also considered suspicious for malignancy [21].
Readers subjectively assessed in consensus lesion contrast enhancement. Lesions with predominantly higher, similar or lower echogenicity compared with that of the adjacent thyroid parenchyma were defined as hyperechoic, isoechoic or hypoechoic respectively. The contrast-enhancement pattern was classified as absent, that is, with no difference in enhancement between the lesion before and the lesion after microbubble contrast agent injection; dotted, that is, with tiny separate spots of enhancement distributed throughout the lesion; or diffuse, that is, with homogeneous or heterogeneous enhancement of the entire lesion.
Readers were also asked to define each lesion as benign or malignant on the basis of B-mode only, B-mode and CD, and CEUS respectively. Both readers used in consensus a five-point scale to grade diagnostic confidence: Grade 1 meant definitely benign; grade 2, probably benign; grade 3, indeterminate; grade 4, probably malignant; and grade 5, definitely malignant.
Statistical evaluation by means of the chi-square and Fisher exact tests was performed. The significance level was set at p less than 0.05.
Objective evaluation
Since raw data were stored on a PC-based workstation, the same sets of images selected for reader assessment were also evaluated off-line by means of a dedicated software (HDI Lab, ATL, Bothell, WA, USA) [22, 23]. Signal intensity values on grey-scale images were calculated at baseline and 10 to 25 s, 45 to 60 s and 110 to 25 s after SonoVue administration by positioning operator-defined regions of interest (ROI) of the same size (5 mm2). The ROIs were positioned within the nodule in the adjacent thyroid parenchyma and in the ipsilateral common carotid artery at the same depth.
All calculations of the signal intensity values were performed using a linear scale, and the results were transformed into logarithmic scale [logarithmic intensity=10×log10 (linear intensity)] to reduce the wide range of variation of the measured values and match the intensity differences that are perceivable by the human eye as different grey levels. The transformed values were presented as average±standard deviation (SD).
A T-Student test was performed to assess whether or not measured signal intensity differences were statistically significant (p< 0.05).
Standard of reference
In all patients the final diagnosis was made by means of histological analysis of resected specimens.
Results
Histology revealed 13 malignant tumors (9 papillary carcinomas and 4 follicular carcinomas; size range: 0.6 to 3.6 cm; mean: 1.5 cm) and 5 benign lesions (3 hyperplasias, 1 follicular adenoma and 1 Plummer’s adenoma; size range: 1.7 to 3.5 cm; mean: 2.6 cm).
Unenhanced US
Table 1 shows baseline sonographic features of thyroid nodules. 18 out of 18 nodules presented with at least one sonographic feature suspected of malignancy, such as microcalcification (n=6), a marked hypoechoic pattern (n=7), irregular or microlobulated margins (n=9), a taller than wide shape (n=6) or the presence of intranodular blood flow (n=10). In particular, 13 out of 18 nodules showed at least a B-mode sign of malignancy (5 out of 18 of which had a CD pattern III), whereas the remaining 5 out of 18 nodules did not show any B-mode sign of malignancy but presented a CD pattern III.
At unenhanced grey-scale US, 6 out of 18 nodules were considered to be definitely malignant, 7 out of 18 probably malignant, 3 out of 18 probably benign and 2 out of 18 definitely benign (Table 2). When adding color Doppler analysis, all three “probably benign” nodules were included in the “indeterminate” class, a nodule previously diagnosed as “probably malignant” was moved into the “definitely malignant” class, and the two “definitely benign” nodules were considered “probably benign” (Table 2).
Contrast-enhanced US
No adverse events or side effects were recorded during or immediately after the injection of SonoVue.
Overall, 4 out of 18 nodules (mean diameter: 0.9 cm) showed no appreciable contrast enhancement after SonoVue administration. Four out of 18 nodules (mean diameter: 1.3 cm) showed a faint contrast enhancement with a dotted appearance but remained hypoechoic in comparison with the adjacent thyroid parenchyma. Ten out of 18 nodules (mean diameter: 2.3 cm) showed a diffuse contrast-enhancement pattern. These observations were confirmed by quantitative analysis, which showed a statistically significant increase in echogenicity (>1.25 dB; p<0.005) in nodules (10 out of 18) assessed as contrast-enhancing at subjective evaluation, whereas a statistically insignificant increase in echogenicity (<0.32 dB) was evidenced in the remaining nodules (Table 3).
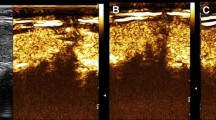
Malignant nodules showed absent (4 out of 13) (Fig. 1), faint dotted (Fig. 2) (4 out of 13) and diffuse contrast-enhancement (5 out of 13), in this latter case both inhomogeneous (4 out of 5 cases) (Fig. 3) and homogeneous (1 out of 5). Benign nodules showed diffuse contrast enhancement, homogeneous in 3 out of 5 cases and heterogeneous in the remaining two (Fig. 4) (Table 2).
a–c a Baseline axial image in a 32-year-old man shows an isoechoic nodule sized 1 cm (arrow) in the right lobe of the thyroid. b Color Doppler reveals a perinodular flow (pattern II). c On the axial image obtained in the arterial phase (25 s after SonoVue injection) the lesion shows lack of contrast enhancement (arrow); note the strong enhancement of the ipsilateral carotid artery (C): Papillary carcinoma (case # 9 of Tables 1 and 2)
a–c a Baseline axial image in a 38-year-old man shows a markedly hypoechoic nodule sized 1.3 cm (arrow) in the right lobe of the thyroid. b Color Doppler reveals a perinodular flow (pattern II). c On the axial image obtained in the arterial phase (25 s after SonoVue injection) the lesion shows a faint dotted contrast enhancement (arrow) (C: ipsilateral carotid artery): papillary carcinoma (case # 8 of Tables 1 and 2)
a–c a Baseline axial image in a 33-year-old woman shows a 2-cm inhomogeneous hypoechoic nodule (arrow) in the right lobe of the thyroid (C: ipsilateral carotid artery). b Color Doppler reveals both perinodular and intranodular flow (pattern III). c On the axial image obtained in the arterial phase (25 s after SonoVue injection) the lesion shows diffuse but inhomogeneous contrast enhancement: follicular carcinoma (case # 4 of Tables 1 and 2)
a–c a Baseline sagittal image in a 51-year-old woman shows a 3.5-cm inhomogeneous hypoechoic nodule in the rigth lobe of the thyroid. b Color Doppler reveals both perinodular and intranodular flow (pattern III). c On the axial image obtained in the arterial phase (25 s after SonoVue injection) the lesion shows diffuse but inhomogeneous contrast enhancement (C: ipsilateral carotid artery): Plummer’s adenoma (case # 3 of Tables 1 and 2)
All ten nodules with type III CD pattern revealed a diffuse contrast enhancement at CEUS - both homogeneous (4 out of 10) or heterogeneous (6 out of 10) - whereas all eight nodules showing a type II CD pattern revealed no (4 out of 8) or faint dotted (4 out of 8) contrast enhancement at CEUS.
CEUS never modified precontrast analysis (B-mode+CD US) in any case.
Discussion
Approximately 4 to 7% of the general population have palpable thyroid nodules, but both autopsy data and nodules found incidentally on US suggest a true prevalence of up to 70%, many of which are less than 1 cm in diameter and non-palpable [2–5]. In clinical practice HRUS is increasingly becoming an extension of a thyroid physical examination, and it is conceivable that the number of referrals to endocrinologists for incidentally discovered thyroid nodules will also dramatically increase, raising some concern about how to manage a real epidemic [6]. Cost/effectiveness issues are emphasized by the very low rates of both malignancy among thyroid nodules and death from thyroid cancer and by the long-term outcome of benign thyroid nodules, which tend to remain benign for a long time [24].
The usefulness of both grey-scale US and color Doppler findings in predicting malignancy of thyroid nodules is still controversial, especially when compared with fine-needle biopsy [25, 26]. Several studies have addressed the issue of the relative risk of malignancy of both grey-scale and color Doppler findings in non-palpable thyroid nodules, suggesting that probably no single pattern by itself should be used as a definite criterion of malignancy, since a considerable overlap of characteristic tissue in benign and malignant lesions was found [21, 27–29]. In particular, even if a given work was unable to demonstrate any value for US findings as predictors of malignancy [30], other and more recent studies suggest a predictive role for irregular or blurred margins, microcalcifications, marked hypoechogenicity and taller-than-average shape [19].
In our series, using the B-mode sonographic criteria described above, 12 out of 18 nodules were assessed as probably/definitely malignant, and all of them were confirmed to be malignant by surgery. These malignant nodules showed a type II CD pattern in 8 out of 12 cases and a type III CD pattern in the remaining 4 cases. Interestingly, other authors have reported that thyroid carcinomas might show poor vascularization [10].
Using the same B-mode criteria, 6 out of 18 lesions were considered to be probably/definitely benign, whereas histology demonstrated one follicular carcinoma, one follicular adenoma, one Plummer’s adenoma, and three hyperplasias. Note that all these lesions showed a type III CD pattern. According to previously published data, this latter finding confirms again that color Doppler patterns, taken by themselves, should not be considered as highly predictive for malignancy [29].
Some studies exploited the introduction of first-generation air-based contrast agent (SH U 508A, Levovist) with color power Doppler techniques to characterize solitary thyroid nodules. In one study, focusing on time/intensity curves, thyroid carcinomas showed a significantly earlier arrival time of Levovist vs. nodular hyperplasic benign nodules and adenomas [11]. By contrast, other authors observed no difference in the time of appearance of the contrast enhancement but discovered regular and monophasic washout curves more frequently in the benign lesions and irregular and polyphasic mostly in the malignant ones [10].
Second-generation microbubble-based contrast agents, such as SonoVue, allow the radiologist to perform continuous imaging at low acoustic power, providing an easier and more accurate depiction of tumour vascularity, especially when considering microcirculation, not assessable by means of color power Doppler techniques [31–35]. In our series, all nodules with a type III CD pattern revealed a diffuse contrast enhancement at CEUS, whereas all nodules showing a type II CD pattern revealed absent or dotted contrast enhancement at CEUS. This correspondence between CD and CEUS patterns may explain why, in our experience, CEUS did not change precontrast diagnostic confidence. None of the contrast-enhancement patterns observed in our series was specific for malignancy, but malignant nodules showed mainly absent or faint dotted vascularization, whereas benign lesions showed only diffuse vascularization, either homogeneous or inhomogeneous. Nevertheless, this subjective finding, confirmed by our quantitative data, could very well be related to the size of the lesion rather than to the histology, since, on average, in our series nodules measuring less than 1 cm showed mainly absent vascularization, those between 1 and 2 cm revealed faint dotted contrast enhancement and, finally, nodules with a diameter larger than 2 cm presented diffuse contrast enhancement, irrespective of the histology. It might be hypothesized that in smaller, even malignant, nodules, the neoangiogenetic vascular bed is not yet completely developed in comparison with the uncontrolled cell proliferation typical of cancer [36].
Our study had limitations. The inclusion of patients already scheduled for surgery of a nodule suspected of being malignant may explain the high incidence of cancer in our series. These inclusion criteria have led us to obtain pathological proof, but in clinical practice the incidence of cancer is by far lower, and thus further studies with larger series are needed. This is of particular relevance in countries with high incidence of multinodular goitre, where cost/efficacy issues are also raised. Another potential limit of this study is that comparison of baseline and CEUS images could be, at least in part, system dependent; hence further studies with different equipment should be considered. Furthermore, the exams were evaluated by consensus. A separated, blinded revision would have allowed testing interobserver concordance and objectivity of CEUS-based characterization.
In conclusion, our experience demonstrates that CEUS of thyroid gland is a feasible technique, but up to now overlapping findings have seemed to limit the potential of this technique in the characterization of thyroid nodules.
References
Solbiati L, Osti V, Cova L, Tonolini M (2001) Ultrasound of thyroid, parathyroid glands and neck limph nodes. Eur Radiol 11:2411–2424
Brander A, Viikinkoski P, Nickels J, Kivisaari L (1991) Thyroid gland: US screening in a random adult population. Radiology 181:683–687
Ezzat S, Sarti DA, Cain DR, Braunstein GD (1994) Thyroid incidentalomas. Prevalence by palpation and ultrasonography. Arch Intern Med 154:1838–1840
Wiest PW, Hartshorne MF, Inskip PD, Crooks LA, Vela BS, Telepak RJ, Williamson MR, Blumhardt R, Bauman JM, Tekkel M (1998) Thyroid palpation versus high-resolution thyroid ultrasonography in the detection of nodules. J Ultrasound Med 17:487–496
Tan GH, Gharib H, Reading CC (1995) Solitary thyroid nodule. Comparison between palpation and ultrasonography. Arch Intern Med 155:2418–2423
Ross DS (2002) Nonpalpable thyroid nodules-managing an epidemic. J Clin Endocrinol Metab 87:1938–1940
Wang C, Crapo LM (1997) The epidemiology of thyroid disease and implications for screening. Endocrinol Metab Clin North Am 26:189–218
Martinez-Tello FJ, Martinez-Cabruja R, Fernandez-Martin J, Lasso-Oria C, Ballestin-Carcavilla C (1993) Occult carcinoma of the thyroid. A systematic autopsy study from Spain of two series performed with two different methods. Cancer 71:4022–4029
Burns PN, Wilson SR, Simpson DH (2000) Pulse inversion imaging of liver blood flow: improved method for characterizing focal masses with microbubble contrast. Invest Radiol 35:58–71
Argalia G, De Bernardis S, Mariani D et al (2002) Ultrasonographic contrast agent: evaluation of time intensity curves in the characterisation of solitary thyroid nodules. Radiol Med 103:407–413
Spiezia S, Farina R, Cerbone G et al (2001) Analysis of color Doppler signal intensity variation after levovist injection: a new approach to the diagnosis of thyroid nodules. J Ultrasound Med 20:223–231
Schneider M, Arditi M, Barrau M et al (1995) BRI: a new ultrasonographic contrast agent based on sulphur hexafluoride-filled microbubbes. Invest Radiol 30:451–457
Quaia E, Calliada F, Bertolotto M et al (2004) Characterization of focal liver lesions with contrast-specific US modes and a sulfur hexafluoride-filled microbubble contrast agent: diagnostic performance and confidence. Radiology 232:420–430
Bartolotta TV, Midiri M, Quaia E et al (2005) Liver haemangiomas undetermined at grey-scale ultrasound: contrast-enhancement patterns with SonoVue and pulse-inversion US. Eur Radiol 15:685–693
Bartolotta TV, Midiri M, Quaia E et al (2005) Benign focal liver lesions: spectrum of findings on SonoVue-enhanced pulse-inversion ultrasonography. Eur Radiol 15:1643–1649
World Medical Association Declaration of Helsinki. Ethical principles for medical research involving human subjects. Available at: http://www.wma.net/e/policy/pdf/17c.pdf. Accessed 1 September 2003
Leen E, Angerson WJ, Yarmenitis S et al (2002) Multi-centre clinical study evaluating the efficacy of SonoVue (BR1), a new contrast agent in Doppler investigation of focal hepatic lesions. Eur J Radiol 41:200–206
Solbiati L, Livraghi T, Ballarati E, Ierace T, Crespi L (1996) Thyroid gland. In: Solbiati L, Rizzatto G, Charboneau JW (eds) Ultrasound of superficial structures. Churchill-Livingstone, Edinburgh, pp 48–85
Kim EK, Park CS, Chung WY et al (2002) New sonographic criteria for recommending fine-needle aspiration biopsy of nonpalpable solid nodules of the thyroid. Am J Roentgenol 178:687–691
Lagalla R, Caruso G, Novara V, Cardinale AE (1993) Flowmetric analysis of thyroid diseases: hypothesis on integration with qualitative color-Doppler study. Radiol Med 85:606–610
Papini E, Guglielmi R, Bianchini A et al (2002) Risk of malignancy in nonpalpable thyroid nodules: predictive value of ultrasound and color-Doppler features. J Clin Endocrinol Metab 87:1941–1946
Bertolotto M, Dalla Palma L, Quaia E, Locatelli M (2000) Characterization of unifocal liver lesions with pulse inversion harmonic imaging after Levovist injection: preliminary results. Eur Radiol 10:1369–1376
Bartolotta TV, Midiri M, Scialpi M, Sciarrino E, Galia M, Lagalla R (2004) Focal nodular hyperplasia in normal and fatty liver: a qualitative and quantitative evaluation with contrast-enhanced ultrasound. Eur Radiol 14:583–591
Kuma K, Matsuzuka F, Yokozawa T, Miyauchi A, Sugawara M (1994) Fate of untreated benign thyroid nodules: results of long-term follow-up. World J Surg 18:495–498
Marqusee E, Benson CB, Frates MC et al (2000) Usefulness of ultrasonography in the management of nodular thyroid disease. Ann Intern Med 133:696–700
Gritzmann N, Koischwitz D, Rettenbacher T (2000) Sonography of the thyroid and parathyroid glands. Radiol Clin North Am 38:1131–1145
Solbiati L (1998) Thyroid gland. In: James EM (ed) Diagnostic ultrasound. Mosby, St Louis, pp 703–729
Kats JF, Kane RA, Reyes J, Clarke MP, Hill TC (1984) Thyroid nodules: sonographic-pathologic correlation. Radiology 151:741–745
Rago T, Vitti P, Chiovato L et al (1998) Role of conventional ultrasonography and color flow-Doppler sonography in predicting malignancy in ‘cold’ thyroid nodules. Eur J Endocrinol 138:41–46
Hagag P, Strauss S, Weiss M (1988) Role of ultrasound-guided fine-needle aspiration biopsy in evaluation of nonpalpable thyroid nodules. Thyroid 8:989–995
Schneider M (1999) SonoVue, a new ultrasound contrast agent. Eur Radiol 9(Suppl.3):S347–S348
Leen E (2001) The role of contrast-enhanced ultrasound in the detection of focal liver lesions. Eur Radiol 11(Suppl. 3):E27–E34
Lencioni R, Cioni D, Bartolozzi C (2002) Tissue harmonic and contrast-specific imaging: back to gray scale in ultrasound. Eur Radiol 12:151–165
Solbiati L, Tonolini M, Cova L, Goldberg N (2001) The role of contrast-enhanced ultrasound in the detection of focal liver lesions. Eur Radiol 11(Suppl. 3):E15–E26
Albrecht T, Oldenburg A, Hohmann J et al (2003) Imaging of liver metastases with contrast-specific low-MI real-time ultrasound and SonoVue. Eur Radiol 13(Suppl 3):79–86
Passe TJ, Bluemke DA, Siegelman SS (1997) Tumor angiogenesis: tutorial on implications for imaging. Radiology 203:593–600
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bartolotta, T.V., Midiri, M., Galia, M. et al. Qualitative and quantitative evaluation of solitary thyroid nodules with contrast-enhanced ultrasound: initial results. Eur Radiol 16, 2234–2241 (2006). https://doi.org/10.1007/s00330-006-0229-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-006-0229-y