Abstract
The aim of the study was to evaluate the potential of new-generation multi-slice computed tomography (CT) scanner technology for the delineation of coronary artery stents in an ex vivo setting. Nine stents of various diameters (seven stents 3 mm, two stents 2.5 mm) were implanted into the coronary arteries of ex vivo porcine hearts and filled with a mixture of an iodine-containing contrast agent. Specimens were scanned with a 16-slice CT (16SCT) machine; (Somatom Sensation 16, Siemens Medical Solutions), slice thickness 0.75 mm, and a 64-slice CT (64SCT, Somatom Sensation 64), slice-thickness 0.6 mm. Stent diameters as well as contrast densities were measured, on both the 16SCT and 64SCT images. No significant differences of CT densities were observed between the 16SCT and 64SCT images outside the stent lumen: 265±25HU and 254±16HU (P=0.33), respectively. CT densities derived from the 64SCT images and 16SCT images within the stent lumen were 367±36HU versus 402±28HU, P<0.05, respectively. Inner and outer stent diameters as measured from 16SCT and 64SCT images were 2.68±0.08 mm versus 2.81±0.07 mm and 3.29±0.06 mm versus 3.18±0.07 mm (P<0.05), respectively. The new 64SCT scanner proved to be superior in the ex vivo assessment of coronary artery stents to the conventional 16SCT machine. Increased spatial resolution allows for improved assessment of the coronary artery stent lumen.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Coronary artery stenting is now the predominant method of non-surgical myocardial revascularisation, with an estimated 475,000 coronary artery stents having been implanted in 2001 in the United States of America [1]. Inhibition of neointimal proliferation by local pharmacological interventions [sirolimus (rapamycin)] is a recent technical development. That clinical experience with sirolimus-eluting coronary stents has shown excellent results in patients with long lesions in small-diameter native vessels, with 3.2% restenosis within the stent versus 34.5% with bare-metal stents at angiographic follow up at 240 days [2]. However, restenosis remains the major limitation of coronary catheter-based intervention, with a 6-month restenosis rate ranging from 11% to 46% with non-drug-eluting stents [3]. These patients could benefit from a non-invasive tool for early detection of in-stent-restenosis to prevent myocardial infarction and thereby improve long-time prognosis.
With the introduction of multi-slice technology combined with fast rotation time and retrospectively electrocardiogram gating, the entire heart can be examined in a single breath hold, while high spatial resolution with sub-millimetre isotropic voxels is maintained [4]. Results from the current generation of 16-slice computed tomography (CT) scanners confirm the role of multi-slice computed tomography (MSCT) coronary angiography in the diagnosis of coronary artery disease (CAD) [5].
Although early results are promising for the detection of CAD, the ability to assess the coronary artery stent lumen with MSCT is still inadequate. Disappointing results were published for a four-slice CT scanner with high-grade lumen narrowing of up to 100% due to artefactual thickening of the stent struts, leading to virtual narrowing of the stent lumen [6]. However, multi-slice computed tomography angiography (MSCTA) appears to be a promising study method for stents greater then 3.5 mm and for those positioned on the proximal segment of the left anterior descending artery [7]. More recent in vitro experiments on a 16DCT system to assess different stent types in terms of stent lumen visualisation revealed that visualisation was largely dependent on the metallic composition and diameter of the particular stent [8]. Promising results have subsequently been obtained with 16-slice CT for the detection of significant in-stent stenosis [9]. Other work has demonstrated the correct diagnosis of an in-stent lumen in 27 of 29 cases after stent placement in 16-slice CT; however, it was exclusively in the left main arteries with comparatively large diameters [10]. Recently, a flat-panel detector with markedly improved spatial resolution proved to be superior for the in vitro assessment of coronary stents when compared with 16-slice CT [11]. Using dedicated edge-enhancing reconstruction kernels (B46) for coronary artery stent imaging the visible stent lumen and the detection of in-stent stenoses was markedly increased [12, 13].
The purpose of our study was to investigate the potential of a new generation of MSCT scanner technology incorporating a 64-slice CT scanner for the depiction of coronary artery stents in an ex vivo setting.
Materials and methods
Ex vivo specimens
Nine ex vivo porcine hearts were examined. Shortly after explantation, the hearts were washed with tap water and prepared for stent implantation. In each heart examined, one stent was implanted in the proximal right coronary artery (RCA) and one in the left anterior descending (LAD) artery. Seven coronary artery stents were of 3 mm diameter, and two stents of 2.5 mm in various lengths (Table 1); one of the latter was a sirolimus-eluting coronary stent. A guide wire (HI-TORQUE Iron Man Guide Wire, Guidant, Santa Clara, Calif., USA) designed for cardiovascular procedures for the selective positioning of the balloon expandable stents systems was used. The balloon-expandable stent systems included an intracoronary stent mounted on the balloon of a delivery catheter. Stents were expanded to a diameter of 3 mm and 2.5 mm by inflation of the balloon catheter to nominal pressure. With this target pressure the nominal width of the stent diameter was achieved according to the manufacturer’s manual. Nominal pressure for the inflation of the balloon catheter was 10 atm for the Bx-Velocity stent and 8 atm and 11 atm for the Syncro Carbostent and for the Cypher select stent, respectively. The coronary arteries were cannulated, and a mixture of an iodine-containing contrast agent (Iomeron 300, Bracco Imaging SpA, Milan, Italy) and ultrasound gel was injected into the ostium of the coronary arteries. In this way, an intraluminal contrast enhancement similar to in vivo CT angiography of the coronary arteries was achieved, with a target attenuation of 250-300 HU within the lumen. Finally, the porcine hearts were laid in a plastic container and placed in the scanner gantry, to simulate the physiological position of the heart and the path of the coronary arteries in vivo.
MSCT parameters and scan protocols
The specimens were examined with a new-generation 64-slice CT system (64SCT, Somatom Sensation 64, Siemens Medical Solutions, Forchheim, Germany), working at an increased rotation rate and applying a z-axis flying focal spot technology, achieving an effective slice-thickness of 0.6 mm. This scanner has an adaptive array detector, which contains detector rows of different sizes in the longitudinal direction. It consists of 40 detector rows, 32 central rows with 0.6 mm collimated slice width; the four outer rows define 1.2 mm collimated slice width. With this protocol, 64 overlapping 0.6 mm slices per rotation are acquired. The total coverage in the z-direction is 28.8 mm. Periodic motion of the focal spot in the longitudinal direction (z-flying focal spot) allows the scanner to double the effective number of simultaneously acquired slices and to improve data sampling along the z-axis.
The gantry rotation time was 330 ms, tube current was 190 mAseff., and tube voltage was 120 kV. Detector collimation was 64 mm×0.6 mm, and spatial resolution was 0.4 mm×0.4 mm×0.4 mm. For image reconstruction a slice-thickness of 0.6 mm was chosen. All datasets were reconstructed using a sharp heart view (B46f) convolution kernel optimised for the assessment of stents.
For comparison purposes, the intact ex vivo hearts were also scanned with a 16-slice CT system (Somatom Sensation 16, Siemens Medical Solutions); the scan parameters were chosen as follows: 16 mm×0.75 mm collimation, tube voltage 120 kV, tube current 190 mAs. For image reconstruction a slice-thickness of 0.75 mm was chosen using the sharp heart view convolution kernel B46f. Table 2 gives a detailed comparison of the imaging parameters used for the 64-slice CT and the 16-slice CT-scanners.
Image analysis
For comparison purposes we excluded the 2.5 mm stents from analysis. From 16SCT as well as 64SCT data sagittal reformations with a slice thickness as thin as possible were reconstructed, resulting in a slice thickness of 0.75 mm for 16SCT and 0.6 mm for 64SCT. All measurements were taken from multiplanar reformations (MPRs). In all ex vivo hearts, coronary artery lumen enhancement (HU, Hounsfield units) was examined both outside and within the stent lumen at the stent entrance and in the centre of the stent, with a 2 mm-diameter region of interest, on both the 16SCT and 64SCT images. Additionally, the inner and outer diameters of each stent were measured manually in the centre and at both ends of the stent, on an offline workstation (Siemens Leonardo, Siemens Medical Solutions, Erlangen, Germany), using curved MPRs.
Statistical analysis
Stent diameters and in-stent densities, as well as densities outside the stent lumen, were compared using an unpaired Student’s t-test (SPSS 12.0, Chicago, Ill., USA).
Results
By application of volume rendering techniques, the heart, including the proximal segments of the coronary artery tree, was visualised. In both the 16SCT images and the 64SCT images metal artefacts, like blooming, were observed. In detail the results are given in Table 3.
Assessment of lumen enhancement outside and within the stent lumen
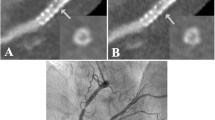
No significant differences in vessel lumen enhancement between the 16SCT and 64SCT images were found outside the stent lumen: 265±25 HU and 254±16 HU (P=0.33), respectively. For both scanners, density measurements inside the stent lumen were significantly higher than the lumen densities outside the stent, owing to beam-hardening artefacts and partial volume effects. However, CT densities measured from the 64SCT images were significantly lower than those measured from 16SCT images (367±36 HU versus 402±28 HU, P<0.05). In general, MSCT datasets showed better visibility of the stent lumen and superior delineation of stent struts when 64SCT was used than when 16SCT was used (Figs. 1, 2, 3).
Maximum intensity projection images (slice thickness 2mm) of the contrast agent-filled right coronary artery demonstrate excellent visibility of the stent lumen (Sorin Biomedica Cardio; stent diameter 3.0 mm, length 15 mm), comparing 16SCT (a) with 64SCT (b). Sharper delineation of stent material in 64SCT can be observed as a result of significantly higher spatial resolution. Note the air bubble after refilling with contrast medium (arrow, Fig. 1b)
MSCT datasets after stent placement in the LAD (Bx-Velocity, Cordis Langenfeld; stent diameter 2.5 mm, length 33 mm). The multiplanar reformation of the MSCT dataset shows better visibility of the stent lumen when 64SCT was used (b) than when 16SCT was used (a). Air bubbles remain in the coronary artery lumen after filling with contrast medium (arrow in a and b)
Assessment of inner and outer stent diameters
64SCT images showed less artificial lumen narrowing than did 16SCT images.
Inner stent diameters as measured from 16SCT images were 2.68±0.08 mm versus 2.81±0.07 mm when compared with 64SCT (P<0.05). Outer stent diameters as measured from 16SCT and 64SCT images were 3.29±0.06 mm versus 3.18±0.07 mm (P<0.05), respectively.
In general, 64DCT images showed less artefactual lumen narrowing than 16SCT images did.
Discussion
Coronary computed tomography angiography (CTA) is a promising method for non-invasive imaging of the coronary arteries and could replace conventional angiography for selected applications, e.g. exclusion of coronary artery disease (CAD). Follow-up examinations of patients after coronary artery stent placement is an important clinical issue in daily cardiology routine, owing to high restenosis rates of up to 46% during the first 6 months in non-drug-eluting stents. Early detection of in-stent restenosis is crucial to circumvent myocardial ischaemia and resulting arrhythmias and to improve long-term outcome. A non-invasive alternative for the evaluation of stented coronary arteries would be highly desirable.
In vivo and in vitro studies of coronary artery stents, using a four-slice MSCT scanner, have been reported by several authors. Depending on the stent type and the metallic composition, artefactual lumen narrowing of 62% to 100% was observed [6]. Maintz et al. [14] reported frequent false-positive diagnoses of high-grade in-stent stenoses due to artificial thickening of the stent struts when they used a four-slice MSCT scanner. The use of 16-slice CT with a dedicated sharp kernel for image reconstruction improved visualisation of the coronary artery stent lumen and detection of in-stent stenoses has been demonstrated [8]. Beam hardening and streak artefacts are presumably responsible for this limitation. Recently, Mahnken et al. [11] demonstrated the influence of improved spatial resolution, using a prototype flat-panel detector scanner. The improved z-axis resolution yielded superior in-stent visibility.
The 64-slice scanner evaluated in this study makes use of a periodic motion of the focal spot in the longitudinal direction (z-flying focal spot) to double the number of simultaneously acquired slices with the intention of improving longitudinal resolution. With this technique, 64 overlapping 0.6 mm slices per rotation are acquired. It should be explored whether these technical accomplishments are associated with improvements in clinical applications.
The aim of the study was to test the performance of this new-generation CT scanner for visualisation of coronary artery stents and vessel lumen in comparison with 16-slice CT-angiography in an ex vivo setting. The use of 0.6 mm slice-width with z-flying focal spot allowed for a markedly improved evaluation of the in-stent lumen, compared with 0.75 mm slice width. The present study demonstrates the impact of improved spatial resolution on the visibility of the stent lumen. In general, in-stent CT densities derived from the 64SCT images were significantly lower than densities measured on the 16SCT images, as a result of reduced blooming artefacts. In addition, 64SCT showed markedly less artificial lumen narrowing and the assessable stent lumen became more visible, when compared with 16SCT. Moreover, our results are in good agreement with earlier in vitro studies showing the influence of superior spatial resolution on the delineation of coronary artery stents [8, 11].
There were some technical limitations to our study. The ex vivo setting with porcine hearts and contrast media-filled coronary arteries was used to simulate conditions comparable to in vivo CT-angiography. However, these results were obtained without any cardiac and respiratory motion. Sixteen-slice CT systems using retrospective ECG gating and breath-hold examinations usually result in minimal motion artefacts. Our experiments were obtained excluding the influence of motion artefacts, and the impact on the increased temporal resolution of the new scanner generation was not examined in this ex vivo study. Moreover, the use of curved MPRs for quantitative analysis may provide invalid results, as the results of this kind of analysis depend on the course of the centreline. Finally, it has to be stated clearly that our experiments present initial results from a small ex vivo study.
Despite the favourable results of this in vitro study, clinical trials in patients with coronary artery stents, and correlation with catheter angiography, will be required to elucidate the potential of new MSCT technology for the assessment of stent patency.
Conclusion
In conclusion, MSCT scanning with 64 sub-millimetre slices represents a further increase in spatial resolution. In the technical note presented here, the superior spatial resolution allowed for a markedly improved evaluation of the in-stent lumen, raising hopes that the reliable assessment of patients after coronary interventions and stent placement might become feasible with 64SCT. Future studies will have to evaluate this potential in an in vivo setting, comparing 64SCTA data with conventional coronary angiography.
References
American Heart Association (2004) Heart and stroke statistics—update
Popma JJ, Leon MB, Moses JW, et al (2004) Quantitative assessment of angiographic restenosis after sirolimus-eluting stent implantation in native coronary arteries. Circulation 110:3773–80
Antoniucci D VR, Santoro GM, et al (1998) Restenosis after coronary stenting in current clinical practice. Am Heart J 135:510–518
Flohr T, Stierstorfer K, Raupach R, Ulzheimer S, Bruder H (2004) Performance evaluation of a 64-slice CT system with z-flying focal spot. Rofo Fortschr Geb Rontgenstr Neuen BildgebVerfahr 176:1803–1810
Ropers D, Baum U, Pohle K, et al (2003) Detection of coronary artery stenoses with thin-slice multi-detector row spiral computed tomography and multiplanar reconstruction. Circulation 107:664–666
Maintz D JK, Wichter T, et al (2003) Imaging of coronary artery stents using multislice computed tomography: in vitro evaluation. Eur Radiol 13:830–835
Ligabue G, Rossi R, Ratti C, Favali M, Modena MG, Romagnoli R (2004) Noninvasive evaluation of coronary artery stents patency after PTCA: role of multislice computed tomography. Radiol Med (Torino) 108:128–137
Mahnken AH, Buecker A, Wildberger JE, et al (2004) Coronary artery stents in multislice computed tomography: in vitro artifact evaluation. Invest Radiol 39:27–33
Schuijf JD, Bax JJ, Jukema JW, et al (2004) Feasibility of assessment of coronary stent patency using 16-slice computed tomography. Am J Cardiol 94:427–430
Gilard M, Cornily JC, Rioufol G, et al (2005) Noninvasive assessment of left main coronary stent patency with 16-slice computed tomography. Am J Cardiol 95:110–112
Mahnken AH, Seyfarth T, Flohr T, et al (2005) Flat-panel detector computed tomography for the assessment of coronary artery stents: phantom study in comparison with 16-slice spiral computed tomography. Invest Radiol 40:8–13
Seifarth H, Raupach R, Schaller S, et al (2005) Assessment of coronary artery stents using 16-slice MDCT angiography: evaluation of a dedicated reconstruction kernel and a noise reduction filter. Eur Radiol 15:721–726
Maintz D, Seifarth H, Flohr T, et al (2003) Improved coronary artery stent visualization and in-stent stenosis detection using 16-slice computed-tomography and dedicated image reconstruction technique. Invest Radiol 38:790–795
Maintz D, Grude M, Fallenberg EM, Heindel W, Fischbach R (2003) Assessment of coronary arterial stents by multislice-CT angiography. Acta Radiol 44:597–603
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rist, C., Nikolaou, K., Flohr, T. et al. High-resolution ex vivo imaging of coronary artery stents using 64-slice computed tomography—initial experience. Eur Radiol 16, 1564–1569 (2006). https://doi.org/10.1007/s00330-006-0186-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-006-0186-5