Abstract
The purpose of this study was to investigate the possibility of assessing the underlying respiratory disease as well as cardiac function during ECG-gated CT angiography of the chest with 64-slice multidetector-row CT (MDCT). One hundred thirty-three consecutive patients in sinus rhythm with known or suspected ventricular dysfunction underwent an ECG-gated CT angiographic examination of the chest without β-blockers using the following parameters: (1) collimation: 32×0.6 mm with z-flying focal spot for the acquisition of 64 overlapping 0.6-mm slices (Sensation 64; Siemens); rotation time: 0.33 s; pitch: 0.3; 120 kV; 200 mAs; ECG-controlled dose modulation (ECG-pulsing) and (2) 120 ml of a 35% contrast agent. Data were reconstructed: (1) to evaluate the underlying respiratory disease (1-mm thick lung and mediastinal scans reconstructed at 55% of the R-R interval; i.e., “morphologic scans”) and (2) to determine right (RVEF) and left (LVEF) ventricular ejection fractions (short-axis systolic and diastolic images; Argus software; i.e., “functional scans”). The mean heart rate was 73 bpm (range: 42–120) and the mean scan time was 18.11±2.67 s (range: 10–27). A total of 123 examinations (92%) had both lung and mediastinal images rated as diagnostic scans, whereas 10 examinations (8%) had non-diagnostic images altered by the presence of respiratory-motion artifacts (n=4) or cyclic artifacts related to the use of a pitch value of 0.3 in patients with a very low heart rate during data acquisition (n=6). Assessment of right and left ventricular function was achievable in 124 patients (93%, 95% CI: 88–97%). For these 124 examinations, the mean RVEF was 46.10% (±9.5; range: 20–72) and the mean LVEF was 58.23% (±10.88; range: 20–83). In the remaining nine patients, an imprecise segmentation of the right and left ventricular cavities was considered as a limiting factor for precise calculation of end-systolic and end-diastolic ventricular volumes. The mean (±SD) DLP value of the examinations was 279.86 (±117.50) mGy.cm. Assessment of underlying respiratory disease and cardiac function from the same data set was achievable in 92% of the patients with ECG-gated 64-slice MDCT.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Before the advent of fast-scanning multislice CT technology, thoracic CT studies were exclusively used for the morphological assessment of thoracic organs. The introduction of fast rotation speed and dedicated cardiac reconstruction algorithms exploiting the multislice acquisition scheme of the data has opened new possibilities not only for cardiac applications [1–3], but also for chest imaging [4]. The first method for ECG-gated examinations of the entire thorax was introduced by Flohr et al. with four-slice multidetector row CT technology [4]. The proposed technique offered extended volume coverage compared to standard ECG-gated spiral scanning using reconstruction approaches for cardiac applications that were shown to provide more precise morphologic information at the level of major mediastinal vessels. Despite this improvement in image quality, the major impact of fast scanning multislice CT technology was offered by 16-slice multidetector-row CT, which has made possible the integration of cardiac functional information into a diagnostic CT scan of the chest, providing vital information in the management of patients with a wide variety of acute or chronic respiratory disorders [5]. Three approaches were proposed, one investigating cardiac global function during a whole-chest multislice CT acquisition with a 16×1.5-mm collimation to ensure an acceptable breath-hold duration [6], one investigating a two-phase protocol to scan both the entire thorax and cardiac cavities with the highest spatial resolution [7], while the third one evaluated a dedicated cardiac MDCT protocol to assess right ventricular function and mass [8]. Comparing ECG-gated 16-slice multidetector row CT (MDCT) with equilibrium radionuclide ventriculography, these preliminary studies demonstrated the feasibility of such an approach in limited populations of hemodynamically stable patients [6–8].
The recent introduction of 64-slice CT scanners offers further improvement in this concept of integrating morphology and function during CT examinations of the chest. The purpose of the present study was to investigate the possibility of providing morphological and cardiac functional information from an ECG-gated 64-slice MDCT angiogram of the chest in patients referred for the management of a variety of respiratory disorders.
Materials and methods
Population
During a 6-month period (October 2004-March 2005), this prospective study included 133 consecutive inpatients (89 males and 44 females; mean ± SD age: 55.5±15.43 years; range: 18–99) in sinus rhythm who were referred for a CT angiographic examination of the chest. During this period of time, 15 patients with a non-sinusal rhythm were excluded from this study. As ECG-gated CT examination of the entire thorax with 64-slice CT offers the possibility to provide additional information regarding cardiac function, the decision to obtain an ECG-gated CT angiographic study of the entire thorax was approved by our Institutional Review Board and Ethics Committee for patients with known or suspected right ventricular dysfunction; informed consent from patients was not required. In the studied population, CT was indicated for the management of bronchopulmonary (n=102) or pulmonary vascular (n=31) diseases. Underlying bronchopulmonary diseases were chronic obstructive pulmonary disease (n=68) and bronchopulmonary carcinoma treated by chemotherapy with potential cardiac toxicity (n=34). Underlying pulmonary vascular diseases were acute (n=9) or chronic (n=8) pulmonary embolism and primary pulmonary hypertension (n=14). Criteria for exclusion included an irregular heart rate, allergy to iodine contrast media, renal insufficiency (serum creatinine level >120 mmol/l–1), pregnancy, severe respiratory impairment and severe cardiac failure. Owing to the potentially detrimental pulmonary effects of β-blockers in respiratory patients [9], no medication was administered for the reduction of the heart rate during the CT examination.
CT examination
Acquisition parameters
CT examinations were performed with a 64-slice MDCT scanner (Sensation 64, Siemens, Forchheim, Germany). The CT protocol consisted of an ECG-gated acquisition over the entire thorax, obtained in the cranio-caudal direction, with the patients scanned in the supine position and after deep inspiration. The acquisition parameters were as follows: (1) collimation: 32×0.6 mm with z-flying focal spot for the simultaneous acquisition of 64 overlapping 0.6-mm slices; rotation time: 0.33 s; pitch: 0.3; 120 kV; 200 mAs; (2) this examination was systematically obtained with the adjustment of the mAs setting to the patient size (Care Dose 4D; Siemens Medical Solutions, Germany) and also was monitored by ECG tracing (ECG Pulsing, Siemens Medical Solutions, Germany). For ECG-controlled tube current modulation, the tube output is raised to a preset level within every cardiac cycle during a limited interval in the diastolic phase, from which data will be reconstructed for morphological purposes. During the remaining part of the cardiac cycle, the tube output is reduced by up to 80% by a corresponding decrease of the tube current. When employed for coronary CT angiography, this technique has been shown to achieve an overall exposure savings of 30–50% without compromising image quality [10]. In this study, a tube current time product of 200 mAs was used because an acceptable image quality seemed to be achievable with the above-mentioned protocol in the initial patients.
Injection protocol
The injection protocol consisted of the administration of 120 ml of a contrast material with 350 mg of iodine per milliliter (iohexol; Omnipaque 350; Amersham Health, Carrigtohill, Ireland; 300 mg of iodine per ml) administered at the flow rate of 4 ml/s with a single head power injector. The automatic bolus triggering software program available on this CT unit (Care Bolus) was systematically applied with a circular region of interest positioned at the level of the ascending aorta and the threshold for triggering data acquisition was preset at 120 HU.
Image reconstruction
Each data set was reconstructed: (1) to evaluate the underlying respiratory disease and (2) to determine right and left ventricular ejection fractions. Consequently, two series of images were systematically generated. The first series of images, i.e., the “morphologic scans,” consisted of 1-mm-thick contiguous transverse CT scans of the entire thorax, viewed in both the mediastinal (window width, 450 HU; window center, 50 HU; soft reconstruction kernel) and lung parenchymal (window width, 1,600 HU; window center, -600 HU; high-spatial-frequency algorithm) window settings. Whereas 1-mm-thick scans were used for clinical purposes, 3-mm-thick scans were also reconstructed for the purpose of image quality analysis. Diagnostic scans were systematically reconstructed at 55% of the R-R intervals; this interval was considered as an acceptable compromise in order to generate cardiac motion-free images of the chest in the diastolic phase [11]. The second series of images was created to calculate the right (RVEF) and left (LVEF) ventricular ejection fractions and will be referred to as “functional scans.” It consisted of 5-mm-thick, contiguous, 2D images of the cardiac cavities along their short axis; reconstructions were done at 10% increments from 0–90% of the cardiac cycle with a soft-reconstruction kernel (B20) on the scanner’s workstation (Navigator). The choice of the temporal window for reconstruction of systolic and diastolic images was based on the identification of the maximal systolic contraction and diastolic relaxation phases on the short-axis test images. Delineation of right ventricular endocardial contours was performed manually, whereas delineation of left ventricular endocardial contours was assessed using a semi-automated tool. Calculation of cardiac functional parameters was performed using the manufacturer’s software dedicated to the analysis of cardiac function (Syngo Argus software; 2004 version; Siemens Medical Solutions) on a commercially available console (Leonardo workstation; Siemens Medical Solutions). The analysis software provided calculation of right ventricular end-diastolic and end-systolic volumes by summing the volumes of all short-axis slices (Simpson method), RV stroke volume (RV end-diastolic volume minus RV end-systolic volume) and RV ejection fraction (RV stroke volume divided by RV end-diastolic volume times 100%). The images of selected reconstruction windows were transferred to an off-line computer workstation (Leonardo workstation; Siemens Medical Solutions) for post-processing in a standardized fashion. To improve the temporal resolution and to avoid motion artifacts on each series of images, the multi-segmental image reconstruction algorithm (utilizing raw data from up to two cardiac cycles for a heart rate above 72 bpm) was systematically used for generating images in each case (Adaptive Cardio-Volume algorithm, release VA 70; Siemens Medical Solutions).
CT parameters evaluated
The CT imaging protocol was supervised by two readers (DD; RS) with 6 and 3 years of experience in MDCT angiography of the chest at the time of initiation of this study. They determined by consensus the quality of diagnostic scans and calculated right and left ventricular function as further detailed.
Morphologic scans
Lung (center, -600 HU; width, 1,600 HU) and mediastinal (center, 50 HU; width, 350 HU) images were analyzed to determine whether the investigated acquisition and reconstruction protocols could affect their diagnostic value. Two parameters of the acquisition protocol could theoretically interfere with the image quality, namely the low pitch value of 0.3, which was potentially responsible for respiratory-motion artifacts due to the duration of data acquisition, and the use of an ECG-controlled tube-current modulation that requires a regular cardiac rhythm during data acquisition to produce high-quality diastolic images. The influence of the acquisition protocol was thus evaluated by searching for respiratory-motion artifacts (i.e., blurring or doubling of bronchopulmonary structures) and ECG-pulsing-induced artifacts (i.e., grainy images suggestive of the reconstruction of images during the phase of reduced tube current) on lung images that were rated as altering or not altering their diagnostic value. Any other category of artifacts, if present, was systematically recorded.
With regard to the reconstruction protocol, the arbitrary choice of the window reconstruction at 55% of the R-R interval might not correspond to the optimal temporal window to generate motion-free images of cardiovascular structures for every examination. The influence of this reconstruction parameter was investigated by the search for cardiac-motion artifacts on mediastinal images at the level of major mediastinal vessels (i.e., blurring or doubling of the wall of the pulmonary trunk, right and left main pulmonary arteries and ascending aorta), cardiac borders (i.e., displacement of the right and left heart borders), completed by an assessment of the four cardiac chambers, where diagnostic image quality was defined by a sharp delineation between the cardiac cavity lumen and wall. The quality of mediastinal images was thus rated as diagnostic or non-diagnostic, the latter score corresponding to the presence of artifacts of sufficient severity to alter their diagnostic value.
For each examination, the overall image quality was assessed as follows: (1) examinations with distinct anatomic detail and no artifacts as well as examinations with clear anatomic detail and the presence of artifacts that did not affect the diagnostic accuracy of the examination were classified as diagnostic; (2) examinations with obscured anatomic details, a distinct increase in noise and extensive blurring that were not sufficient for diagnosis were classified as non-diagnostic. For each examination, the breath-hold duration and the patient’s heart rate during quiet breathing before the start of scanning were systematically recorded as well as the dose-length product (DLP).
Functional scans
Factors affecting the calculation of the right (RVEF) and left (LVEF) ejection fractions were systematically recorded, including: (1) the level of enhancement within the right and left ventricular cavities, measured after positioning a ROI over the almost complete ventricular cavity; (2) the sharpness of the delineation process of the right and left endocardial contours; when graded as imprecise, the cause for the difficulty in delineation was systematically recorded. The right ventricular endomyocardial contours were manually delineated on short-axis images of the right ventricle at end diastole and end systole. The left ventricular myocardial contours were delineated using a semi-automated tool. Window settings were adapted for each examination to achieve the best contrast between the myocardium and blood (on average, the window width was set at 800 HU and the window level at 140 HU). Image quality of functional scans was subsequently rated as sufficient or otherwise for quantitative analysis. The time needed to provide functional information, i.e., the post-processing duration, was systematically recorded.
Statistical analysis
Statistical analysis was performed with commercially available software (SAS Institute, Cary, N.C., 25513). Results were expressed as means, standard deviations and ranges for continuous variables and as frequencies, percentages and 95% confidence intervals for categorical variables.
Results
Characteristics of the population scanned
The mean (±SD) heart rate of the studied population before data acquisition was 73.10±16.12 bpm (range: 42–120). The mean scan time was 18.11±2.67 s (range: 10–27). The mean (±SD) DLP value of the examinations was 279.86 (±117.50) mGy.cm (range: 97–857). The mean body mass index (BMI) of the population scanned was 25.63 (±4.42; range: 14.86–40.23).
Image quality of the morphologic scans
Both the lung and mediastinal images of 123 examinations [92%; 95% confidence interval (CI): 87–96%) were rated as diagnostic, without any impairment of the diagnostic accuracy of these examinations. Cardiac cavities were considered assessable in 122 examinations (92%; 95% CI: 86–96%). The overall quality of mediastinal images reconstructed during a diastolic phase is illustrated in Figs. 1 and 2. Ten examinations (8%) had lung and/or mediastinal images with artifacts altering their diagnostic value. Two categories of artifacts were identified in these examinations: (1) severe respiratory-motion artifacts in four examinations (only detectable on lung images) and (2) the cyclic presence of noisy and blurred images, exclusively depicted on CT scans of six patients with a heart rate below 60 bpm during data acquisition (mean heart rate: 50 bpm), which altered the diagnostic value of both the lung and mediastinal images.
A 46-year-old male referred for worsening of dyspnea in the clinical context of chronic obstructive pulmonary disease (COPD) (170 cm; 68 kg; 46 bpm). Transverse CT scans (a–d) reconstructed during a diastolic phase of the cardiac cycle (window width: 350 HU; window level: 50 HU). Note the high quality of vascular enhancement as well as the absence of motion artifacts at the level of the aortic wall (double arrow, a; large arrow, b), pulmonary trunk (small arrow; b) and cardiac borders (arrowheads, d)
A 72-year-old male referred for pulmonary hypertension (169 cm; 75 kg; 64 bpm). Transverse CT scans (a–d) reconstructed during a diastolic phase of the cardiac cycle (window width: 640 HU; window level: 60 HU). Note the lack of motion artifacts on cardiovascular structures as well as the sharp delineation between the right and left ventricular cavity lumen and wall (arrows, c and d)
A detailed description of artifacts observed on lung images is provided in Table 1. Table 2 summarizes the frequency of cardiac-motion artifacts on major mediastinal vessels and cardiac borders.
Functional scans
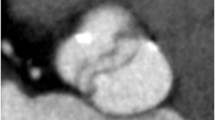
Table 3 summarizes the mean attenuation values measured within the right and left ventricular cavities in the population scanned. The level of enhancement was rated as homogeneous in all cases for the left ventricle and in 110 cases for the right ventricle (Fig. 3). In 13 cases, high-contrast streak artifacts were observed within the right ventricle, resulting in a level of enhancement that was rated as non-homogeneous. Delineation of the endocardial contours of the right and left ventricles was achievable in all but nine examinations, which showed marked irregularities of the cardiac contours because of frequent extrasystolic beats during data acquisition (n=9) and/or high-contrast streak artifacts within the right ventricle (n=2), hampering precise delineation of the endocardial contours. Image noise observed on the systolic short-axis images of right and left ventricular cavities was not found to affect ventricular segmentation (Fig. 4). Assessment of right and left ventricular function was achievable in 124 patients (93%). For these 124 examinations, the mean RVEF was 46.10% (±9.5; range: 20–72) and the mean LVEF was 58.23% (±10.88; range: 20–83).
A 53-year-old female referred for follow-up of occluded pulmonary arteriovenous malformations (150 cm; 57 kg; 59 bpm). a High-quality short-axis image of the ventricular cavities reconstructed during a diastolic phase of the cardiac cycle. b Short-axis image of the ventricular cavities reconstructed during a systolic phase of the cardiac cycle. Note the increased image noise compared to a, secondary to the decrease of the tube current during the systolic phase. The overall image quality is fully compatible with precise ventricular segmentation
Duration of data analysis
Reconstruction of morphologic scans in the diastolic phase, selection of end-systolic and end-diastolic phases for functional evaluation and post-processing of images for assessment of right and left ventricular function took 30 min per patient.
Discussion
To date, and to the best of our knowledge, this is the first study demonstrating that morphologic information of underlying respiratory disease as well as cardiac functional information can be efficiently obtained in patients undergoing ECG-gated CT angiographic examinations of the chest with 64-slice CT. The prerequisite for such an approach was to demonstrate that the scanning protocol did not alter the overall image quality. Evaluating 133 consecutive patients in sinus rhythm, we observed that 123 examinations (92%) had both lung and mediastinal images rated as diagnostic scans, enabling the acquisition of high-quality images of major mediastinal vessels and cardiac chambers on diastolic images. According to the literature dedicated to cardiac and coronary artery CT imaging, the optimal phase of the cardiac cycle for the generation of motion-free images mainly depends on the patient’s heart rate. At heart rates below 65 bpm, the mid to late diastole is a prolonged phase of relative immobility, while at higher heart rates, this phase becomes much shorter as opposed to late systole and early diastole, which remain as hypokinetic windows [11]. Investigating a non-selected population with a mean heart rate of 73 bpm who did not receive specific medication to reduce the heart rate, we chose a 55% image reconstruction window, corresponding to the phase of early diastole. With this reconstruction interval window, we observed a low frequency of artifacts at the level of the central pulmonary arteries (observed in 2 to 7% of the patients) and aortic wall motion (17% of the cases). Moreover, cardiac chambers were rated as analyzable in 122 examinations (92%), resulting from the concurrent presence of motion-free cardiac images and homogeneous enhancement of the cardiac chambers. This improvement in image quality is expected to influence daily practice as assessment of the heart in the course of thoracic CT examination can influence the clinical management of patients [12]. Whereas this arbitrary reconstruction window was not able to suppress all cardiac-motion artifacts on morphologic scans, our results suggest that this average value is an acceptable compromise in routine clinical practice, allowing an independent reconstruction of CT images without medical supervision. In cases of unsatisfactory image quality, the alternative would be to adjust image reconstruction to each patient’s ECG curve and heart rate, which is always possible with retrospective ECG-gated MDCT scanning. ECG-gated examinations of the chest require scanning of patients with a low pitch compared to non-ECG-gated examinations and thus require patients to hold their breath for a longer period of time. In our study, the breath-hold duration was in the order of 18 s, which was responsible for respiratory-motion artifacts altering the diagnostic value of lung images in four cases (3%). This low frequency of severe respiratory-motion artifacts suggests that an ECG-gated acquisition of the entire thorax obtained with a pitch of 0.3 is well tolerated by patients with underlying respiratory disease. This pitch value was found to be responsible for the cyclic presence of blurry and noisy images in six patients with a low heart rate. Although well-adapted for patients with a heart rate above 60 bpm, this table feed was too fast for these patients. Consequently, there were anatomical regions that were not covered by a detector row during each phase of the cardiac cycle, and the system had to perform interpolations to cover these gaps, resulting in blurry and noisy images. Apart from two patients with a heart rate below 50 bpm before the examination who should have been scanned with a lower pitch (i.e., 0.2), it should be emphasized that the low heart rate observed in the remaining four patients was not predictable prior to CT scanning. As previously reported in the literature, a bradycardia can be seen after deep inspiration in some patients, resulting in suboptimal image quality [13].
The second objective of this study was to investigate the assessability of cardiac function from the same data set as that used for morphologic assessment of the underlying respiratory disease. In our population, cardiac function of the right and left ventricle was assessable in 124 patients (93%). The main limiting factor, observed in nine patients, was the presence of marked irregularities of cardiac contours due to frequent extrasystolic beats during data acquisition that hampered the precise delineation of the endocardial contours. This limitation could be overcome by the administration of β-blockers to the patients before scanning, but this requires a careful selection of patients referred from pulmonology departments. As recently pointed out in the literature, the anticipated beneficial cardiovascular effects of a β-blocker must be weighed against the anticipated detrimental pulmonary effects of β-blockers in patients with COPD, i.e., effects on FEV1, airway responsiveness and response to additional beta2-agonists, especially when non-selective beta-blockers are used [9]. Because of these potential complications, we favored the practical approach of an ECG-gated examination of the chest without specific medication, and this was found to provide high-quality examinations in the vast majority of the population scanned. In two cases, an additional limitation for precise segmentation of the right ventricular cavity was the presence of high-contrast streak artifacts within this cardiac chamber. These artifacts could be eliminated by an optimization of the injection protocol, namely the use of a double body injector with a systematic saline flush of the iodinated contrast column, which was not applied in our study. Because assessment of cardiac function requires a segmentation of the right and left ventricular cavities on systolic and diastolic images, we analyzed the influence of image quality on systolic short-axis scans owing to the systematic use of ECG-gated dose modulation during data acquisition. This technical adjustment allows the nominal tube output to be applied only during the diastolic phase of the cardiac cycle, at which time images are likely to be reconstructed, while the tube output is reduced for the rest of the cardiac cycle. The use of this dose modulation system was not shown to affect the ability of CT to assess cardiac function as the level of noise in images reconstructed from the systolic phase was never found to affect the segmentation process, and thus the assessment of cardiac function. Because the overall radiation dose delivered during retrospectively ECG-gated acquisitions is higher than that delivered during non ECG-gated acquisitions, it was mandatory to apply the dose reduction tools available on the CT equipment used. We combined two approaches, namely an automatic tube current adaptation to the patient’s size and anatomic shape, and the use of ECG-gated dose modulation, which has been shown to reduce patient’s exposure by 40–50% when applied for coronary calcium scoring [10]. Our results show that morphological and functional information could be obtained with lower DLP values than those recommended for standard non-ECG-gated examinations of the chest. In our study, the mean DLP value of CT examinations was 279.86 mGy.cm, which is below the European reference value of 650 mGy.cm for non-ECG-gated CT examinations of the chest [14]. An additional benefit of this protocol is to provide high quality coronary artery imaging from the same data set. These results are reported separately [15] and confirm the clinical value of such an approach with ECG-gated 64-slice MDCT technology. It should be noticed that the upper limit of body weight of the population scanned was 117 kg. Whereas the investigated protocol remained compatible with a diagnostic CT image quality, the scanning parameters should be optimized to scan obese patients.
This study suffers from several limitations. Firstly, we exclusively included patients with a regular sinus rhythm owing to the decision to avoid any administration of β-blockers prior to CT scanning. Consequently, our protocol cannot be recommended for every respiratory patient for whom morphological and functional information is worth obtaining. Secondly, we did not correlate the results of cardiac function obtained with MDCT with a reference method. However, the software used in the present study was similar to that used in a previous investigation with a 16-slice MDCT scanner in which cardiac function assessed with CT was correlated with equilibrium radionuclide ventriculography [7]. Two recent studies have confirmed good correlations between MDCT and radionuclide ventriculography in the evaluation of right ventricular volume and mass [8] as well as in the evaluation of biventricular ejection fraction [6] with ECG-gated 16-slice CT. These results are in agreement with those from Lembcke et al. [16]. Comparing 8- and 16-detector row CT scans with magnetic resonance imaging in a population of 25 patients prior to cardiac surgery, these authors found that multislice CT was an accurate and reliable noninvasive technique for evaluating right ventricular measurements. As reported in our results, reconstruction of diastolic morphologic scans, selection of end-systolic and end-diastolic phases for functional assessment and post-processing for the assessment of right and left ventricular function took 30 min per patient. The time needed to provide morphological and functional information could be shortened by means of recent software developments that allow direct reconstruction of double oblique short axis slices from image raw data that then can be loaded to dedicated software tools for image segmentation. In conclusion, our results suggest that ECG-gated 64-MDCT angiography of the chest enables a combined assessment of morphology and function in patients with regular sinus rhythm.
References
Ohnesorge B, Flohr T, Becker CR, Kopp AF, Knez A, Baum U, Klingenbeck-Regn K, Reiser MF (2000) Cardiac imaging by means of electronically gated multisection spiral CT: initial experience. Radiology 217:564–571
Knez A, Becker CR, Leber A, Ohnesorge B, Reiser MF, Haberl R (2000). Nonivasive assessment of coronary artery stenoses with multidetector helical computed tomography. Circulation 101: 221–222
Flohr T, Ohnesorge B (2001) Heart-rate adaptive optimization of spatial and temporal resolution for ECG-gated multislice spiral CT of the heart. J Comput Assist Tomogr 25:907–923
Flohr T, Prokop M, Becker CR, Schoepf UJ, Kopp AF, White RD, Schaller S, Ohnesorge B (2002) A retrospectively ECG-gated multislice spiral CT scan and reconstruction technique with suppression of heart pulsation artifacts for cardiothoracic imaging with extended volume coverage. Eur Radiol 12:1497–1503
Oldershaw P (1992) Assessment of right ventricular function and its role in clinical practice. Br Heart J 68:12–15
Coche E, Vlassenbroeck A, Roelants V, D’Hoore W, Verschuren F, Goncette L, Maldague B (2005) Evaluation of biventricular ejection fraction with ECG-gated 16-slice CT: preliminary findings in acute pulmonary embolism in comparison with radionuclide ventriculography. Eur Radiol 15:1432–1440
Delhaye D, Remy-Jardin M, Teisseire A, Hossein-Foucher C, Leroy S, Duhamel A, Remy J (2005) Estimation of right ventricular ejection fraction by multidetector row CT: comparison with equilibrium radionuclide scintigraphy. AJR (in press)
Kim TH, Ryu YH, Hur J, Kim SJ, Choi BW, Kim Y, Kim HJ (2005) Evaluation of right ventricular volume and mass using retrospective ECG-gated cardiac multidetector computed tomography: comparison with first-pass radionuclide angiography. Eur Radiol 15:1987–1993
Van der Woude H, Zaagsma J, Postma DS, Winter TH, van Hulst M, Aalbers R (2005) Detrimental effects of beta-blockers in COPD. A concern for nonselective beta-blockers. Chest 127:818–824
Jakobs TF, Becker CR, Ohnesorge B, Flohr T, Suess C, Schoepf UJ, Reiser MF (2002) Multislice helical CT of the heart with retrospective ECG gating: reduction of radiation exposure by ECG-controlled tube current modulation. Eur Radiol 12:1081–1086
Hertzog C, Abolmaali N, Balzer JO, Baunach S, Ackerman H, Dogan S, Britten MB, Vogl TJ (2002) Heart-rate-adapted image reconstruction in multidetector-row cardiac CT: influence of physiological and technical prerequisite on image quality. Eur Radiol 12:2670–2678
Bruzzi J, Remy-Jardin M, Delhaye D, Teisseire A, Khalil C, Remy J (2005) When, why and how to examine the heart during thoracic CT imaging. AJR (in press)
Mao SS, Oudiz RJ, Bakhsheishi H, Wang SJ, Brundage BH (1996) Variation of heart rate and electrocardiograph trigger interval during ultrafast computed tomography. Am J Card Imag 10:239–243
EC99 (1999) European guidelines on quality criteria for computed tomography. Report EURO 16262 EN. Luxembourg 69–78
Delhaye D, Remy-Jardin M, Salem R, Teisseire A, Khalil C, Delannoy-Deken V, Duhamel A, Remy J (2005) Coronary imaging quality in routine ECG-gated multidetector CT examinations of the entire thorax: preliminary experience with a 64-slice CT system. European Radiology submitted for publication (2006)
Lembcke A, Dohmen PM, Dewey M, Klessen C, Elgeti T, Hermann KG, Konertz W, Hamm B, Kivelitz DE (2005) Multislice computed tomography for preoperative evaluation of right ventricular volumes and function: comparison with magnetic resonance imaging. Ann Thorac Surg 79:1344–1351
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Salem, R., Remy-Jardin, M., Delhaye, D. et al. Integrated cardio-thoracic imaging with ECG-Gated 64-slice multidetector-row CT: initial findings in 133 patients. Eur Radiol 16, 1973–1981 (2006). https://doi.org/10.1007/s00330-006-0157-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-006-0157-x