Abstract
The aim of this study was to determine the diagnostic accuracy and frequency of complications of lung biopsy procedures with or without CTF guidance of needle insertion. Records and images of 99 consecutive percutaneous coaxial cutting needle lung biopsy procedures performed on 85 patients were reviewed retrospectively. Fifty-seven and 42 procedures had been done with and without CTF guidance, respectively. Histological results were compared to diagnosis after surgery or after a follow-up period of 12 months. Diagnostic accuracy and the occurrence of pneumothorax and/or bleeding related to the procedures were registered. The level of accuracy of the diagnosis was comparable. The diagnostic accuracy was 96% (50/52) and 95% (34/36) sensitivity 95% (35/37) and 93% (26/28), specificity 100% (15/15) and 100% (8/8) with CTF and conventional CT techniques, respectively. There were fewer post procedure pneumothoraces using the CTF than conventional technique [26% (15/57) vs. 38% (16/42)], but the difference was not statistically significant (P=0.274). The insertion of a chest tube was required in only one (2%) procedure using the CTF technique, while this was needed in four (10%) using the conventional technique. Small or large hemorrhages occurred in 23% of the procedures, with no apparent difference between the two groups. In conclusion, CTF-guided biopsy of lung lesions provides high diagnostic accuracy, comparable to that of conventional CT-guided procedures, with a low rate of complications, even for small tumors.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Computed tomography fluoroscopy (CTF), first introduced by Katada in 1993 [1], has in recent years become a valuable tool for retrieving biopsy specimens from pulmonary masses. CTF provides real-time guidance of the biopsy needle, decreases procedure time and requires fewer needle passes than CT-guided procedures without fluoroscopic guidance [2–6]. CTF has furthermore been shown to significantly reduce radiation doses to the patients, but it exposes the radiologist to radiation, as the operator is in the room at the time [7, 8]. We have used the CTF-technique for thoracic interventions in our daily routine for several years. The purpose of this study was to document and evaluate the diagnostic accuracy and frequency of complications in CT-guided biopsy procedures of pulmonary lesions performed with or without fluoroscopic guidance.
Materials and methods
Patients
From August 2001 to December 2002, 99 percutaneous coaxial cutting needle lung biopsy procedures were performed in 85 consecutive patients in our department. There were 30 women and 55 men (median age 65 years; range 18–87 years). The patients had been admitted to the hospital for the work-up of pulmonary masses suspected of either primary malignant disease or metastasis. All data regarding the procedures were retrieved retrospectively. Fifty-seven procedures were done with CTF guidance, while 42 had been performed with conventional CT technique before the CT fluoroscopy equipment was installed. To achieve material representative of the lung lesion and of diagnostic quality, the CTF procedure had to be repeated once and twice in four and one case, respectively. Four conventional CT-guided procedures had to be repeated once for the same reason. Biopsy procedures were performed during independent sessions on separate days on two distinct tumors in four patients.
Fifty-three (54%) of the lesions were in the right lung, and 45 (46%) were left-sided. Thirty-three (33%) of the lesions were located in the superior lobe, and 57 (58%) were lower lobe lesions. There were 7 (7%) middle lobe lesions. The median lung lesion size was 3.0 cm (range 1.0–8.0 cm). The median distance from the peripheral margin of the lesion to the pleura was 0.0 cm (range 0.0–9.0 cm). No significant difference in size or distance from the lesion to pleura between the two groups could be detected (Table 1).
The clinical course of each patient and the growth rate of pulmonary lesions that were not removed surgically were evaluated for at least 12 months after the biopsy procedures. Categorical data were analyzed with Fischer’s exact test. P-values less than 0.05 were considered statistically significant.
Technical features
Before fluoroscopy equipment for real time guidance was installed in our department in May 2001, all CT-guided lung biopsy procedures were performed without such guidance (conventional CT technique). After the installment, all except two procedures were CTF-guided. Procedures were performed by altogether 19 radiologists from our department. The less experienced radiologists performed the procedure under supervision of a radiologist with more experience. Three of the radiologists each had more than 20 years of radiological and interventional experience. These were considered experienced and performed each 25, 6 and 1 procedure, respectively. The less experienced radiologists performed 67 procedures; the median number of procedures per radiologist was 3.0 (range 1–15). All scans were obtained without intravenous contrast medium. Patients were examined in the prone, supine or lateral decubitus position, depending on the location of the lesion.
Fluoroscopic biopsy interventions were performed with Somatom plus 4 CT (Siemens Medical Systems, Erlangen, Germany) equipped with the CARE-vision software. The CARE vision application includes an in-room monitor with last-image hold, a foot pedal controlling image acquisition and a bedside joystick for moving the patient in and out of the gantry. The exposure parameters were 120 kV, 20 mAs and a slice thickness of 10 mm. To avoid irradiating the operator’s fingers, the co-axial introducer was held and manipulated with a 22-cm surgical forceps during the CT fluoroscopy [1, 2, 5, 9]. The real-time scanning was limited to short glimpses to visualize the position of the advancing needle tip.
Conventional CT-guided biopsy procedures were performed with Somatom plus 4 CT or with HiSpeed CTi single channel scanner (General Electric Medical Systems, Milwaukee, WI, USA). Other than the laser line indicating the axial slice position, there was no in-room guidance equipment. It was not possible to examine the CT images within the examination room so the images had to be viewed on the console next door. Therefore, the physician had to go out of the examination room to evaluate the position of the introducer needle. The co-axial introducer was advanced in small steps to the pulmonary lesion, with interval scans to assess its position.
The puncture area was cleaned with antiseptic solution and then local anesthesia was applied. An incision of the skin was made for needle entrance. With both CT techniques, two 18-G cutting needle (tru-cut) biopsies were taken with an automated biopsy gun guided by the 17-G co-axial introducer. The introducer had been placed in an appropriate position within the lesion during patient breath-hold. A short spiral scan was performed shortly thereafter at the level of the lesion to detect possible complications such as pneumothorax or hemorrhage in all but five procedures. A conventional chest radiograph was taken 2 h later to check for complications. Though this is the normal procedure, it was not performed in ten procedures. There was no chart record of why this was not performed, but no complication or clinical symptoms had been recorded in the clinical journal in these procedures.
Data collection
A biopsy was considered true positive if a histological diagnosis of malignancy had been confirmed at surgery, the clinical course was typical of malignant disease, metastases of the identical malignancy had been detected and/or if the tumor growth rate suggested malignancy during the follow-up period [10, 11]. A procedure provided a true negative result if a benign histological diagnosis had been established with a precise etiology or was confirmed histologically after surgical removal of the mass and if the lesion had disappeared or was reduced or stable in size during the follow-up period of 12 months.
In two procedures, one with CTF, the other with conventional CT guidance, a large pneumothorax occurred just after insertion of the needle. Both procedures had to be terminated before tissue samples were retrieved. In yet another procedure, the pathology report could not be retrieved. The clinical outcome could not be determined after seven procedures because the observation time was less than 12 months. These altogether 11 procedures, were not included in the evaluation of diagnostic accuracy. Lesion size and depth from the pleural surface were recorded from CT images taken during the biopsy procedure. Lesion size was defined as the largest transversal diameter in axial images at the lung window setting (width 1,500 HU; center −500 HU). The depth of the lesion was measured from the pleura surface to the tumor along the planned needle path. The presence of emphysema was based on identification of the following criteria: small and round, or confluent areas of low attenuation; diffuse areas of low attenuation with a paucity of vascular markings; and/or bullae.
Pneumothorax was graded as small if no treatment was needed, moderate if the air could be aspirated by the co-axial introducer at the end of the procedure, or large if the placement of a chest tube was needed [12]. All patients with pneumothorax were followed up with either CT scans or regular chest radiograph. The appearance of haziness, consolidation or fluid along or in relation to the needle track or between pleura layers immediately after the procedure was regarded as hemorrhage [13]. The hemorrhagic lesion was regarded as small if ≤ 3 cm or large if ≥ 3 cm in axial diameter. No hemorrhage produced complication-related symptoms or required therapy such as fluid replacement.
Results
Diagnostic accuracy
Eighty-eight of 99 procedures (89%) provided histological material representative of the lesion and of sufficient quality for histological diagnosis as deemed by the pathologist. Twenty-three (26%) lesions were benign and 65 (74%) were malignant at the final diagnosis. Of the lesions deemed true negative, there were four lesions that regressed and three that were confirmed benign by operation. Three had a specific pathological diagnosis (sarcoidosis and tuberculosis), and all were controlled at least a year. One lesion, considered to be rheumatoid lung affection, was observed for 36 months. Finally, there were 12 lesions with a benign histology, observed for at least 12 months (range 12–19; mean 14; median 15 months) that had the typical radiological features of round atelectases (n=10) and pulmonary aspergillosis (n=2). In all, 84 lung lesions were correctly diagnosed, yielding an overall diagnostic accuracy rate of 95%.
Of 52 CTF procedures, two provided a false-negative result. No false-positive result was seen with this technique. Therefore, the sensitivity, specificity, positive and negative predictive values for a diagnosis of malignancy at CTF were 95, 100, 100 and 88%, respectively. No significant difference in the diagnostic accuracy with and without fluoroscopic guidance could be found (Table 2). Procedures providing false-negative results (n=4) were performed on lesions significantly smaller than the size of the lesions providing accurate histological diagnosis [true-positive or true-negative results; n=85; mean size 1.7 (SD 0.6) vs. 3.5 cm (SD 1.7); (P=0.017)]. The diagnostic accuracy of procedures performed by experienced and inexperienced radiologists was comparable (96 vs. 95%, respectively; P=1.000). The mean distance from the lesion to the pleura was not significantly different in procedures providing a false-negative result compared to those that provided true-positive or negative results (1.2 vs. 1.0 cm; P=0.897).
Complications
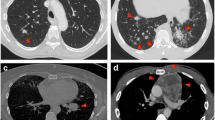
The frequency of pneumothorax using CTF guidance was not significantly different from that experienced using conventional technique [26% (15/57) vs. 38% (16/42) of procedures, respectively; P=0.274]. Of CTF procedures complicated by a pneumothorax (n=15), 11 were regarded as mild (Fig. 1a), three moderate and only one severe (Fig. 1b), requiring the insertion of a chest tube. Of procedures performed without CTF (n=16), 12, 1 and 4 were regarded as mild, moderate and severe, respectively. Regardless of the procedure, the risk of a severe pneumothorax was significantly higher if the lesion was completely surrounded by aerated lung (Fig. 2) [(17% (8/47) vs. 2% (1/49) moderate and severe pneumothoraxes; P=0.015; 11% (5/47) vs. 0% (0/49) chest tube insertions; P=0.025]. We did not see an independent increase in the pneumothorax rate with increasing distance from the pleura.Hemorrhage occurred in 21 of 94 procedures (22%), of which 6 (7%) were regarded as large (Fig. 3).
The risk of hemorrhage was significantly higher if the needle had traversed aerated lung than if not [34% (16/47) vs. 11% (5/44); P=0.013]. No major hemorrhage occurred in the procedures performed in lungs where the lesion abutted the pleura. The mean lesion size of procedures complicated by hemorrhage was significantly smaller than lesions of procedures without this complication (2.6 vs. 3.6 cm; P=0.019). The presence of emphysema did not appear to increase the risk of complications.
Discussion
CTF guidance represents an important technological development, enabling real-time imaging of percutaneous intervention of pulmonary lesions inaccessible with other techniques such as ultrasound guidance or transbronchial biopsy. Compared to conventional CT-guided procedures, CTF guidance allows continuous monitoring of the needle towards the target lesion, thus controlling for respiratory movement. CTF is faster and requires fewer needle passes than the conventional CT-guided technique [2–6]. Our study demonstrated that CTF-guided procedures of lung lesions can be performed with high diagnostic accuracy comparable to that achieved with a more conventional technique. The high level of diagnostic accuracy achieved with and without CTF was comparable to that reported previously, ranging from 83% to 95% [6, 13–18].
Intuitively, CTF-guided procedures may yield higher accuracy rates than the conventional technique, especially with regard to small lesions subjected to respiratory motion. However, we were not able to document an improved diagnostic accuracy after the introduction of CTF in our department. This may partly be because conventional CT-guided percutaneous lung biopsy is a well-established method with an already high success rate, providing little room for improvement [6]. Biopsies can now be attempted in lesions previously considered impractical for intervention [2]. One great advantage of CT fluoroscopy may be that this method substantially decreases procedure time and may therefore be cost effective. Unfortunately, procedure times were not regularly registered in our department during the study period. However, we experienced that the procedures were performed rapidly. Patients were not randomized to CTF or conventional guidance in our retrospective study. However, procedures were performed on lesions comparable with regard to size and distance from the pleura.
Not surprisingly, false-negative biopsies were retrieved more often from small than from larger lung lesions in our study, in accordance with a previous study by Yeow et al. [17]. This indicates that sampling is technically more demanding in small lesions. Since tumor necrosis may be present in larger lesions [18], we tried to sample tissue from the peripheral zone of lesions, which resulted in a high success rate. In this study, we did not restrict the procedures included to those performed by experienced interventional radiologists, but evaluated all the procedures performed in the period, assessing the routine in our department. We could not detect any difference with regard to the diagnostic accuracy or rate of complications in experienced versus inexperienced radiologists performing the procedures. It is possible that the more complicated interventions were reserved for the more experienced radiologists, regardless of the use of fluoroscopy. None the less, we experienced a steep learning curve for CTF-guided intervention as reported previously by several authors [14, 17, 19].
CTF-guidance gave fewer pneumothoraces than conventional techniques in our study. This may be because CTF requires fewer needle passes than conventional techniques, as experienced by Froelich et al. [2–5, 20]. Also, CTF improves safety because the pneumothorax can be detected immediately [2, 3, 21]. By early detection, three (5%) moderate pneumothoraces were converted to mild pneumothoraces at the end of the procedures using CTF in our study. Pneumothorax appeared in approximately one third of the procedures, but only a minority required insertion of a chest tube. This was well within the range of previously published frequencies of pneumothoraces (12 to 42% of procedures) [12–14, 19, 22, 23] and chest tube placements (0 to 14% of procedures) [12, 13, 19, 21, 23, 24]. The operator should be especially alert with regard to pneumothorax if the needle has to transverse lung tissue before entering the lesion [13, 17, 21, 25].
The rate of hemorrhage was comparable with CTF and conventional CT technique. As shown in previous studies, small lesion size and long distance to the lesion increase the risk of bleeding [12, 13]. When taking a biopsy from a small lesion, the cutting needle often includes a portion of aerated lung, which offers less tamponading effect than solid tissue. To reach a deep tumor, the needle will cross more aerated lung and more pulmonary vessels, which increase in size with distance to pleura, increasing the risk of large pulmonary hemorrhages.
In conclusion, CTF-guided percutaneous biopsy of pulmonary masses provides high diagnostic accuracy, comparable to that of conventional CT-guided procedures, and can be performed safely, even on small tumors.
References
Katada K, Kato R, Anno H, Ogura Y, Koga S, Ida Y, Sato M, Nonomura K (1996) Guidance with real-time CT fluoroscopy: early clinical experience. Radiology 200:851–856
Daly B, Templeton PA (1999) Real-time CT fluoroscopy: evolution of an interventional tool. Radiology 211:309–315
Froelich JJ, Ishaque N, Regn J, Saar B, Walthers EM, Klose KJ (2002) Guidance of percutaneous pulmonary biopsies with real-time CT fluoroscopy. Eur J Radiol 42:74–79
Kirchner J, Kickuth R, Laufer U, Schilling EM, Adams S, Liermann D (2002) CT fluoroscopy-assisted puncture of thoracic and abdominal masses: a randomized trial. Clin Radiol 57:188–192
Silverman SG, Tuncali K, Adams DF, Nawfel RD, Zou KH, Judy PF (1999) CT fluoroscopy-guided abdominal interventions: techniques, results, and radiation exposure. Radiology 212:673–681
Gianfelice D, Lepanto L, Perreault P, Chartrand-Lefebvre C, Milette PC (2000) Value of CT fluoroscopy for percutaneous biopsy procedures. J Vasc Interv Radiol 11:879–884
Carlson SK, Bender CE, Classic KL, Zink FE, Quam JP, Ward EM, Oberg AL (2001) Benefits and safety of CT fluoroscopy in interventional radiologic procedures. Radiology 219:515–520
Froelich JJ, Wagner HJ (2001) CT-fluoroscopy: tool or gimmick? Cardiovasc Intervent Radiol 24:297–305
Kato R, Katada K, Anno H, Suzuki S, Ida Y, Koga S (1996) Radiation dosimetry at CT fluoroscopy: physician’s hand dose and development of needle holders. Radiology 201:576–578
Erasmus JJ, McAdams HP, Connolly JE (2000) Solitary pulmonary nodules: part II. Evaluation of the indeterminate nodule. Radiographics 20:59–66
Erasmus JJ, Connolly JE, McAdams HP, Roggli VL (2000) Solitary pulmonary nodules: part I. Morphologic evaluation for differentiation of benign and malignant lesions. Radiographics 20:43–58
Yeow KM, Su IH, Pan KT, Tsay PK, Lui KW, Cheung YC, Chou AS-B (2004) Risk factors of pneumothorax and bleeding: multivariate analysis of 660 CT-guided coaxial cutting needle lung biopsies. Chest 126:748–754
Yeow KM, See LC, Lui KW, Lin MC, Tsao TC, Ng KF, Liu HP (2001) Risk factors for pneumothorax and bleeding after CT-guided percutaneous coaxial cutting needle biopsy of lung lesions. J Vasc Interv Radiol 12:1305–1312
Hirose T, Mori K, Machida S, Tominaga K, Yokoi K, Adachi M (2000) Computed tomographic fluoroscopy-guided transthoracic needle biopsy for diagnosis of pulmonary nodules. Jpn J Clin Oncol 30:259–262
Yamagami T, Iida S, Kato T, Tanaka O, Toda S, Kato D, Nishimura T (2003) Usefulness of new automated cutting needle for tissue-core biopsy of lung nodules under CT fluoroscopic guidance. Chest 124:147–154
Yamagami T, Iida S, Kato T, Tanaka O, Nishimura T (2003) Combining fine-needle aspiration and core biopsy under CT fluoroscopy guidance: a better way to treat patients with lung nodules? Am J Roentgenol 180:811–815
Yeow KM, Tsay PK, Cheung YC, Lui KW, Pan KT, Chou AS (2003) Factors affecting diagnostic accuracy of CT-guided coaxial cutting needle lung biopsy: retrospective analysis of 631 procedures. J Vasc Interv Radiol 14:581–588
Tsukada H, Satou T, Iwashima A, Souma T (2000) Diagnostic accuracy of CT-guided automated needle biopsy of lung nodules. AJR Am J Roentgenol 175:239–243
Muehlstaedt M, Bruening R, Diebold J, Mueller A, Helmberger T, Reiser M (2002) CT/fluoroscopy-guided transthoracic needle biopsy: sensitivity and complication rate in 98 procedures. J Comput Assist Tomogr 26:191–196
Gianfelice D, Lepanto L, Perreault P, Chartrand-Lefebvre C, Milette P (2000) Effect of the learning process on procedure times and radiation exposure for CT fluoroscopy-guided percutaneous biopsy procedures. J Vasc Interv Radiol 11:1217–1221
Yamagami T, Nakamura T, Iida S, Kato T, Nishimura T (2002) Management of pneumothorax after percutaneous CT-guided lung biopsy*. Chest 121:1159–1164
Covey AM, Gandhi R, Brody LA, Getrajdman G, Thaler HT, Brown KT (2004) Factors associated with pneumothorax and pneumothorax requiring treatment after percutaneous lung biopsy in 443 consecutive patients. J Vasc Interv Radiol 15:479–483
Montaudon M, Latrabe V, Pariente A, Corneloup O, Begueret H, Laurent F (2004) Factors influencing accuracy of CT-guided percutaneous biopsies of pulmonary lesions. Eur Radiol 14:1234–1240
Saji H, Nakamura H, Tsuchida T, Tsuboi M, Kawate N, Konaka C, Kato H (2002) The incidence and the risk of pneumothorax and chest tube placement after percutaneous CT-guided lung biopsy*: the angle of the needle trajectory is a novel predictor. Chest 121:1521–1526
Haramati LB, Aviram G (2002) What constitutes effective management of pneumothorax after CT-guided needle biopsy of the lung? Chest 121:1013–1015
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Heck, S.L., Blom, P. & Berstad, A. Accuracy and complications in computed tomography fluoroscopy-guided needle biopsies of lung masses. Eur Radiol 16, 1387–1392 (2006). https://doi.org/10.1007/s00330-006-0152-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-006-0152-2