Abstract
Aortic distensibility depending on aortic cross-sectional area changes is an important parameter for the grading of vascular diseases. This study measured aortic area changes by multidetector computed tomography. An image reconstruction algorithm was developed to assess aorta diameter and area as a function of the cardiac cycle with sufficient time resolution along the entire length of the aorta by four-detector row computed tomography. The algorithm was tested on porcine aortic specimens and compared with an optical reference method. The error of the relative vessel area change comparing the two methods was found to be about 3%. Initial tests on patient datasets indicate that clinical application is feasible. The proposed method has the advantage that it can easily be integrated into a modified routine CT angiography study and allows the measurement of aortic cross-sectional area changes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Multidetector-row computed tomography (MDCT) as used for vessel imaging [1, 2] allows very accurate depiction of anatomy; however, functional information is not provided. Vessel elasticity quantified as distensibility is an interesting functional parameter of the vessel wall [3–6]. Several methods have been proposed to achieve this including pulse-wave velocity measurement employing either magnetic resonance velocity mapping [7–11] or Doppler ultrasound [11–14]. Ultrasound methods suffer from inconstant applicability. Magnetic resonance (MR) pulse wave velocity measurements are unreliable in situations with complex flow patterns [9]. It has been shown that MDCT with retrospective electrocardiographic (ECG)-gating provides cardiac images with high temporal resolution [15–17]. Thus the potential for a combined morphology and functional image protocol makes the CT method attractive. In addition, MDCT is readily available and applied in aneurysm follow-up studies [18, 19]. Therefore CT could potentially provide further preventative information in the treatment of a frequent disease in industrial countries.
In this study we demonstrate that aortic wall movements can be visualized with MDCT. The method is based on ECG-gating and a special image reconstruction algorithm to obtain time-resolved images from CT angiography data. We provide experimental validation of this method in a phantom setup and show preliminary results of its application to patient data.
Materials and methods
Our method for measuring aortic area changes was validated using a phantom setup with porcine aortic specimens. In addition, it was tested on angiography datasets from patients undergoing routine MDCT coronary angiography.
Vascular elasticity is quantified as distensibility D [20] which is defined as the relative change in vessel cross-sectional area A that occurs during the cardiac cycle, divided by the corresponding change in blood pressure Δp: D=ΔA/(A0·Δp). A 0 is the minimum vessel area, and ΔA is the difference between maximum and minimum cross-sectional area [21]. While Δp can be determined by sphygmomanometry, the relative change in vessel cross-section ΔA/A0 must be calculated from time-resolved images of the aorta. Below, this value is defined as relative vessel area change=ΔA/A0 100%.
Image acquisition and reconstruction
All experiments were performed using a multidetector-row CT scanner (SOMATOM Volume Zoom, Siemens Medical Solutions, Erlangen, Germany). To reconstruct time resolved CT data retrospective ECG-gating was added to a slightly modified CT angiography protocol. The acquired raw dataset was used to obtain two image sets. First, standard angiography images without ECG synchronization were calculated with the conventional image reconstruction environment of the CT scanner. Second, time-resolved images were calculated with the present ECG-gated algorithm. For this the raw projection data from the CT scanner were transferred to a standard PC via a local network. On this PC retrospective ECG-gating and image reconstruction was performed using programs developed using Matlab (MathWorks, Natick, Mass., USA) and Visual C++ (Microsoft, Redmond, Wash., USA). An algorithm was developed that uses data from several heart beats for image reconstruction [22]. For this scanner such a multiphasic image reconstruction algorithm with sufficient time resolution was commercially not available. The selection of data belonging to a certain heart phase allows the improvement in temporal resolution at the expense of spatial resolution (Fig. 1). Temporal resolution depends on the number of rotations used for interpolation, heart rate, and scanner rotation time [15]. For an interpolation range of four rotations, which was used in this study, temporal resolution ranged between 90 and 250 ms. With a heart rate of 70 bpm, as was used in the phantom experiments (see below), a temporal resolution of 140 ms was obtained. To provide some oversampling 20 time frames per heart cycle are calculated. Images were reconstructed at 0%, 5%, 15%... 95% of the RR interval of the ECG. From this image series vessel area was calculated by simple thresholding by two experienced readers. The threshold value S t , which is a critical value for the determination of the vessel area, was calculated from the mean signal intensity S v in the vessel and the signal intensity in the background S b to S t =(S v +S b )/2.
Principle of retrospective cardiac gating. Upper row Plot of detector angle for each of four detectors as a function of table position z over slice collimation d for a pitch value of 0.66. The first step is to make use of opposite projections, i.e., projections where X-ray tube and detector are interchanged (dashed lines). The same projection angle is available at different heart phases, and each measured projection can be assigned a time within the cardiac cycle where it was acquired. A number of possible data values for each angle are available (circular dots), which differ in spatial distance to the desired reconstruction position zr. Considering the ECG signal, these values also differ with respect to the desired temporal position. These values are filtered so that projections which fit best contribute most to the image
Experimental setup
To test the accuracy of the method a phantom setup was constructed. The phantom provided an artificial pulsatile circulation through ex-vivo porcine aortic specimens (Fig. 2). Water from a reservoir enters a self-constructed pulsatile pump, which consists of a cylinder with two valves and a pneumatically driven piston. The pump system allowed the programming of arbitrary pressure waveforms. An idealized physiological pressure profile with minimum pressure of 80 mmHg and maximum pressure 120 mmHg was used. Flow rate was not measured. The pulsatile flow enters a porcine aortic specimen, which is located inside a water-filled chamber. After passing the specimen the flow passes a hydraulic resistance, which can be changed to set systolic pressure. Before returning to the reservoir it reaches an overflow chamber, which can be adjusted in height to select diastolic pressure.
Schematic representation of the phantom setup, consisting of a computer-controlled pulsatile pump and an aortic specimen, which is located inside a water-filled chamber. The cross-section of the specimen is measured at two locations using two line-CCD cameras. The setup fits onto the patient table of the CT scanner
The cross-sectional measurements were performed at two locations (distance 90 mm) using two line charge-coupled-device (CCD) cameras (DALSA, Waterloo, Ont., Canada) illuminated by a 500-W halogen floodlight. The CCD camera has a 0.1 mm pixel spacing and is read out at a temporal resolution of 0.1 ms. As change in area varies along the length of the specimen, measurement at two locations gives two different change in area values for each specimen. Thus 14 relative change in area values were calculated from the 7 specimens.
To simulate the human aorta specimens of porcine aorta were used. Seven porcine aortas were obtained from a local slaughterhouse 12 h before the experiment. Specimens were cut to a length of 20 cm, and branching vessels were cut and tightly sutured. Pump frequency was set to 70 bpm and pulse pressure to 40 mmHg. The horizontal projections of the line-scan CCD camera underwent a simple threshold algorithm to detect the vessel boundary. Vessel cross-sectional area A was calculated assuming a circular vessel cross-section.
The entire phantom set-up was placed on the patient table of the CT scanner. The specimens were scanned at 120 kVp, 150 mAs, with 2.5 mm collimation, a pitch of 0.375, and a scan length of 25 cm. The collimation of 2.5 mm was used to achieve a reasonable image acquisition time for the length of aorta. The control signal from the driving unit was recorded during the measurement as a substitute for the ECG signal using a separate PC. For each of the seven specimens two positions were analyzed corresponding to the CCD camera locations, resulting in 14 change in area values.
Vessel area was then plotted as a function of time within the cardiac cycle for each method. Furthermore, the relative changes in vessel area as a function of minimum vessel area detected by the CCD camera were plotted against the CT result for all 14 measurements and correlation was calculated. The Bland-Altman [23] test was performed to assess the statistical significance of the degree of concordance of the two methods.
Study on patient CT angiography datasets
The method was also tested on scans of two patients who had been enrolled in a previous heart study with cardiac CT for coronary angiography (protocol: 120 kVp, 150 mAs, 1 mm collimation, pitch 0.375) for which ethics approval and informed consent had been properly obtained [24]. We selected a dataset from one 64 year-old patient who had obvious atherosclerotic changes (i.e., vessel wall calcification and clinical symptoms of angina pectoris) and from a 17 year old patient requiring a CT for a pulmonary vein abnormality who otherwise was not known to have any cardiovascular disease.
While the standard angiography images were reconstructed on the scanner console, the CT raw data were used to obtain a second, time-resolved image set. This allowed analyzing vessel area changes in the upper part of the descending thoracic aorta covered by the cardiac scan. Images were reconstructed and processed as described above. The patient datasets were reconstructed using the same protocol as for the phantom study. Blood pressure was not measured.
Results
The ECG-gated CT images from the phantom measurement showed good contrast between the contrast-medium inside the vessel and the surrounding water (Fig. 3a). Data from four rotations were used for ECG gating. With a table feed of 0.375×10 mm=3.75 mm per rotation, this gives a range of 15 mm in z, from which the data are taken. Additionally, the collimation of 2.5 mm broadens the slice profile so that the effective z-axis resolution is approx. 17.5 mm. Vessel area was determined in all samples using a threshold segmentation. The threshold was set to the mean value between vessel and background levels. From these values the relative area as a function of minimum area was calculated (Fig. 3b). The corresponding data from the CCD camera (Fig. 3c) allowed reliable detection of the edge of the vessel. A plot of vessel area as a function of time within the cardiac cycle indicates that there is a good agreement between the methods for this measurement (Fig. 3d). Considering the pulse pressure of 40 mmHg a mean distensibility of (2.7±0.9)×10−5 Pa−1 was calculated from the CT data over all 14 measurements, while the distensibility was (2.8±1.0)×10−5 Pa−1 with the optical method. The standard deviations are indicated as error bounds with the mean values.
Processing steps required to calculate vessel area, shown for one example measurement. The reconstructed CT images (a) are segmented using a simple gray-scale threshold (b). From the data of the line-scan CCD camera (c), vessel area is calculated from horizontal projection of the vessel assuming a circular cross-section. The resulting area vs. time curves for both methods area is shown (d); solid line CCD data; dashed line CT data. Relative area is defined as area relative to the minimum cross-sectional area
To assess the degree of agreement between the two methods the relative changes in vessel area detected by the CCD camera were plotted against the CT result for all 14 measurements in Fig. 4a. The line that represents perfect correlation (y=x) is included for reference. An excellent correlation between the two methods was found, as is described by the linear correlation coefficient R2=0.96.
Furthermore, the Bland-Altman analysis was performed where the difference between the two methods was plotted as a function of the mean (Fig. 4b). The mean of the differences (shown as a solid line) is approx. 0, indicating that there is no substantial bias between the two methods. The lines corresponding to the mean plus and minus two standard deviations are shown as dashed lines. These values of the relative vessel cross-sectional area change lie at approx. 0.03 above and below the mean difference. Since 95% of the differences are expected to fall between these limits, the CT measurement does not deviate by more than ±3% from the CCD value.
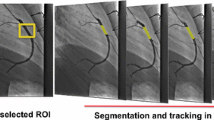
The CT method was applied to two patient datasets (Fig. 5). CT cross-sections are shown through the thoracic aorta during both diastole and systole for a young patient without aortic disease and a 64-year-old patient with coronary heart disease. The corresponding plot of the relative vessel area vs. time within the cardiac cycle shows reduced vessel cross-sectional area change in the patient with coronary heart disease compared to the young patient.
a Images of the thoracic aorta of a 17-year-old patient (A, upper row) without cardiac disease are shown. Only the two time frames with minimum (diastole) and maximum (systole) distension were selected for display. Taking into account that the 50% change in area corresponds to only a 22% change in diameter, it can be seen that there is a significant difference in vessel cross-sectional area between diastole and systole. Dotted line Contours. Lower row (patient B) A dataset from the thoracic aorta of a patient with known cardiovascular disease. There is hardly a vessel area change visible between the two images. b Relative vessel area change is plotted vs. time within the cardiac cycle using data from all time frames of patient A (dashed line) and patient B (solid line) The time profile showed normal pulsatile vessel area change for patient A and greatly reduced pulsatile change in vessel area in patient B. Relative area is defined as area relative to the minimum cross-sectional area
Discussion
This study demonstrates that it is possible to calculate change in area from a dataset that is originally acquired for CT angiography using a special ECG-gated image reconstruction algorithm. The development of a dedicated offline algorithm for this CT scanner was necessary to obtain sufficient time resolution. Such algorithms are now implemented in newer scanners. We tested the accuracy of this method using an experimental setup with porcine aortic specimens and measured change in area with an optical reference method. In 14 measurements a mean distensibility value of 2.7×10−5 Pa−1 was found, applying an idealized physiological pressure profile (80–120 mmHg). The values correspond to those reported in the literature for elderly human subjects. For example, Mohiaddin et al. [11] using pulse wave velocity measurements found flow velocity increasing linearly with age, from 4.3 m/s (age 10–19 years) to 7.2 m/s (age 50–59 years), which corresponds to a distensibility from 5.2×10−5 Pa−1 in young subjects up to 1.8×10−5 Pa−1 in the elderly.
There is a good agreement between the CT method and the reference method, and Bland-Altman analysis shows that the error in relative vessel area change can be expected to be about 3%. The results are comparable to distensibility values of the abdominal aorta found in healthy elderly human subjects [11], which indicates that the porcine aorta is a reasonable model especially for the age group of patients with aortic diseases.
Relative aortic cross-sectional area changes found in young human subjects can be as high as 50%; however, they can be as low as a few percent in older subjects and in those with diseased vessels [14]. This somewhat limits the sensitivity of distensibility measurements: a relative area change of 5% is hardly detectable by this method. The relative error of the area measurement was found to be 3%. This sets a lower limit for the vessel cross-sectional area changes that can be detected by this method. Even with this limitation this method may be useful in detecting substantial distensibility changes related to aortic disease.
There are some limitations of our phantom study. The optical setup that we used was only able to measure a horizontal projection of the aortic diameter, thus a circular cross-section had to be assumed, which may have introduced some systematic error. Furthermore, it was not tested how accuracy depends on heart rate. As temporal resolution of ECG-gated CT depends on heart rate [15], it is expected that accuracy of change in area measurement also changes with heart rate. However, the temporal resolution of 140 ms used in our study is a typical value for a retrospective multiphase ECG-gating algorithm.
In the phantom study it was possible to determine vessel cross-sectional area by a simple threshold segmentation. In patient images this process is more difficult, and manual segmentation may be an obstacle in using this method in larger patient studies. Algorithms for fully automated vessel segmentation will help to make clinical application practicable. In this pilot study only a few variables were explored in the experimental setup. The leverage of other variables was not examined, such as different degrees of atherosclerosis (there is no atherosclerosis in pigs), different heart rates, and various pulse pressures.
In our study we used a 2.5 mm collimation to cover the entire length of aorta. Recent developed scanners (16-to 64-row detector CTs) allow smaller collimation. In patient examination online ECG-related tube current modulation (ECG pulsing) maintains radiation exposure acceptably [25]. Secondly, improved rotation speed will increase temporal resolution, which is expected to result in more accurate determination of aortic change in area [26]. Application of the proposed method to two patient datasets demonstrates that changes due to age and vascular disease can be detected using CT. This indicates that CT measurements in cross-sectional area change are feasible in patients.
Compared to the established noninvasive distensibility measurements methods that employ MR and ultrasound imaging, the proposed CT method has several advantages. The method requires a modified routine CT angiography examination with additional recording of an ECG signal during the scan and the subsequent reconstruction and postprocessing. Meanwhile such algorithms with adequate time resolution are commercially available for newer scanners so there will be no need of a dedicated offline reconstruction algorithm as for our experiment. Thus the relative change in area value is obtained from the same CT scan which typically lasts less than 1 min, and there is no need for an additional examination or additional X-ray dose. As CT angiography is routinely performed for many aortic diseases [27], the presented method fits well into a clinical examination protocol. We are convinced that this will make it possible to study cross-sectional area change in larger groups of patients with an indication for CT angiography with little additional effort. Aortic distensibility can then be calculated from estimated aortic pressure using brachial arterial pressure; however, the measurement of blood pressure may lead to further inaccuracy of distensibility determination. We suggest the estimation of aortic pressure using brachial arterial pressure obtained by the auscultatory method and believe it to be equal to systemic blood pressure in the abdominal aorta.
It is well known that the arterial pressure waves undergo transformation with more prominent peaks the further the waves have traveled from the heart. Thus the peak pressure in the brachial artery is higher than in the aorta. However, there are indications that these differences in aortic and brachial pressures are reduced at older ages [28]. Comparing intra-arterial pressure in the abdominal aorta and brachial blood pressure by the auscultatory method, Imura et al. [29] found that the pulse pressure is approx. 10% less by the auscultatory method. Recalculation of their data shows a slight underestimation of stiffness by the auscultatory method. Although a small systematic error of obtained pressure values exist in patient studies, the error is consistent, and therefore the distensibility measurements is valid, and comparisons between individuals are possible. One example could be the investigation of patients with aortic aneurysms, where distensibility might be a relevant factor for the predication of the risk for rupture [30].
The proposed method will benefit from the ongoing improvements in CT technology [31]. Firstly, with an increased number of simultaneously measured slices it will be easier to combine ECG-gating with sufficient volume coverage [26]. Secondly, improved rotation speed will increase temporal resolution, which is expected to result in more accurate determination of aortic change in area.
In summary, measurement of aortic cross-sectional area change using CT with ECG-gating is feasible. It can simply be integrated into modified clinical routine scanning protocols so that the value of pulsatile change in cross-sectional area can be analyzed in future patient studies.
References
Kopp AF, Kuttner A, Trabold T et al (2003) Cardiac and vascular MDCT: thoracic imaging. Eur Radiol 13(Suppl 5):M73–M81
Willoteaux S, Lions C, Gaxotte V et al (2004) Imaging of aortic dissection by helical computed tomography (CT). Eur Radiol 14:1999–2008
Laurent S, Katsahian S, Fassot C et al (2003) Aortic stiffness is an independent predictor of fatal stroke in essential hypertension. Stroke 34:1203–1206
Herrington DM, Kesler K, Reiber JC et al (2003) Arterial compliance adds to conventional risk factors for prediction of angiographic coronary artery disease. Am Heart J 146:662–667
Willens HJ, Davis W, Herrington DM et al (2003) Relationship of peripheral arterial compliance and standard cardiovascular risk factors. Vasc Endovasc Surg 37:197–206
Hirai T, Sasayama S, Kawasaki T et al (1989) Stiffness of systemic arteries in patients with myocardial infarction. A noninvasive method to predict severity of coronary atherosclerosis. Circulation 80:78–86
Urchuk SN, Plewes DB (1995) A velocity correlation method for measuring vascular compliance using MR imaging. J Magn Reson Imaging 5:628–634
Hardy CJ, Bolster BD Jr, McVeigh ER et al (1996) Pencil excitation with interleaved fourier velocity encoding: NMR measurement of aortic distensibility. Magn Reson Med 35:814–819
Boese JM, Bock M, Schoenberg SO et al (2000) Estimation of aortic compliance using magnetic resonance pulse wave velocity measurement. Phys Med Biol 45:1703–1713
Itskovich VV, Kraft KA, Fei DY (2001) Rapid aortic wave velocity measurement with MR imaging. Radiology 219:551–557
Mohiaddin RH, Yang GZ, Burger P et al (1992) Automatic enhancement, animation, and segmentation of flow in peripheral arteries from MR phase-shift velocity mapping. J Comput Assist Tomogr 16:176–181
Wright JS, Cruickshank JK, Kontis S et al (1990) Aortic compliance measured by non-invasive Doppler ultrasound: description of a method and its reproducibility. Clin Sci (Lond) 78:463–468
Hansen F, Mangell P, Sonesson B et al (1995) Diameter and compliance in the human common carotid artery-variations with age and sex. Ultrasound Med Biol 21:1–9
Wilson KA, Hoskins PR, Lee AJ et al (2000) Ultrasonic measurement of abdominal aortic aneurysm wall compliance: a reproducibility study. J Vasc Surg 31:507–513
Kachelriess M, Ulzheimer S, Kalender WA (2000) ECG-correlated image reconstruction from subsecond multi-slice spiral CT scans of the heart. Med Phys 27:1881–1902
Boese JM, Bahner ML, Albers J et al (2000) Optimizing temporal resolution in CT with retrospective ECG gating. Radiologe 40:123–129
Bahner ML, Boese JM, Wallschläger H et al (1999) Spiral CT of the heart with retrospective ECG-gating. Electromedica 67:37–41
Abada HT, Sapoval MR, Paul JF et al (2003) Aneurysmal sizing after endovascular repair in patients with abdominal aortic aneurysm: interobserver variability of various measurement protocols and its clinical relevance. Eur Radiol 13:2699–2704
Catalano C, Napoli A, Fraioli F et al (2003) Multidetector-row CT angiography of the infrarenal aortic and lower extremities arterial disease. Eur Radiol 13(Suppl 5):M88–M93
Nollen GJ, Westerhof BE, Groenink M et al (2004) Aortic pressure-area relation in Marfan patients with and without beta blocking agents: a new non-invasive approach. Heart 90:314–318
Nichols WW (1990) Properties of the arterial wall. In: Nichols WW, O’ Rourke MF (eds) McDonald’s blood flow in arteries. Edward Arnold, London, p 96
Boese JM, Bahner ML, Albers J et al (2001) An improved algorithm for multi-slice cardiac CT imaging with high temporal resolution (abstract). Radiology 221:457
Bland JM, Altman DG (1986) Statistical methods for assessing agreement between two methods of clinical measurement. Lancet I:307–310
Albers J, Boese JM, Vahl CF et al (2003) In vivo validation of cardiac spiral computed tomography using retrospective gating. Ann Thorac Surg 75:885–889
Poll LW, Cohnen M, Brachten S et al (2002) Dose reduction in multi-slice CT of the heart by use of ECG-controlled tube current modulation (“ECG pulsing”): phantom measurements. Rofo Fortschr Geb Rontgenstrahlen Neuen Bildgeb Verfahr 174:1500–1505
Flohr T, Ohnesorge B, Bruder H et al (2003) Image reconstruction and performance evaluation for ECG-gated spiral scanning with a 16-slice CT system. Med Phys 30:2650–2662
Hahmann M, Richter GM, Schuhmacher H et al (2001) Post-traumatic dissection of the abdominal aorta. Radiologe 41:590–594
O’Rourke MF (1990) Aging, high blood pressure and disease in humans. In: Nichols WW, O’ Rourke MF (eds) McDonald’s blood flow in arteries. Edward Arnold, London, p 450
Imura T, Yamamoto K, Kanamori K et al (1986) Non-invasive ultrasonic measurement of the elastic properties of the human abdominal aorta. Cardiovasc Res 20:208–214
Hahmann M, Boese JM, Leitermann D et al (2001) Measurement of aortic elasticity using ECG-gated multidetector-row CT: an ex-vivo experiment (abstract). Radiology 221:33
Wintersperger B, Jakobs T, Herzog P et al (2005) Aorto-iliac multidetector-row CT angiography with low kV settings: improved vessel enhancement and simultaneous reduction of radiation dose. Eur Radiol 15:334–341
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ganten, M., Boese, J.M., Leitermann, D. et al. Quantification of aortic elasticity: development and experimental validation of a method using computed tomography. Eur Radiol 15, 2506–2512 (2005). https://doi.org/10.1007/s00330-005-2857-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-005-2857-z