Abstract
This study compared different magnetic resonance imaging (MRI) methods with Tl201 single photon emission computerized tomography (SPECT) and the “gold standard” for viability assessment, functional recovery after coronary artery bypass grafting (CABG). Twenty patients (64±7.3 years) with severely impaired left ventricular function (ejection fraction [EF] 28.6±8.7%) underwent MRI and SPECT before and 6 months after CABG. Wall-motion abnormalities were assessed by stress cine MRI using low-dose dobutamine. A segment with a nonreversible defect in Tl201-SPECT and a delayed enhancement (DE) in an area >50% of the entire segment, as well as an end-diastolic wall thickness <6 mm, was defined as nonviable. The mean postoperative EF (n=20) improved slightly from 28.6±8.7% to 32.2±12.4% (not significant). Using the Tl201-SPECT as the reference method, end-diastolic wall thickness, MRI-DE, and stress MRI showed high sensitivity of 94%, 93%, and 84%, respectively, but low specificities. Using the recovery of contractile function 6 months after CABG as the gold standard, MRI-DE showed an even higher sensitivity of 99%, end-diastolic wall thickness 96%, stress MRI 88%, and Tl201-SPECT 86%. MRI-DE showed advantages compared with the widely used Tl201-SPECT and all other MRI methods for predicting myocardial recovery after CABG.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Especially for patients with highly impaired left ventricular function and “chronic myocardial infarction” [1], it is important to have an easy-to-use and reliable method for assessing myocardial viability because the surgeon has to decide whether to perform coronary artery bypass grafting (CABG) or transplantation [2]. The effects of myocardial enhancement after Gd-DTPA administration in chronically infarcted tissue were first described in the early 1980s [3–5], but this has not been widely used for clinical purposes since the publications in the late 1990s [6–8]. Several studies thereafter [9, 10] showed that “delayed enhancement” (DE) can be used to assess myocardial viability. Stress cine imaging has been used for both ischemia induction [11–13] and viability assessment [14, 15] in several studies. Baer et al. also introduced an easy method of assessing viability by simply looking at the rest wall thickness during diastole [8, 16]. These methods have been compared with the “gold standard” of F-18 fluorodeoxyglucose positron emission tomography (FDG-PET) [9, 17] and the widely used and cheaper methods of Tl201 single photon emission computerized tomography (SPECT) [5] and Tc99m sestamibi SPECT [18]. Nagueh et al. [19] were the first to compare the Tl201-SPECT uptake in myocardial tissue with the results of a transmural myocardial biopsy and showed a higher thallium uptake and less interstitial fibrosis in segments with postoperative functional recovery. Besides the gold standard of all imaging modalities for viability assessment, F-18 FDG-PET, there is a more practical approach for assessing the quality of viability evaluation by different imaging modalities: the prediction of functional recovery after CABG surgery. Baer et al. [20] used dobutamine stress MRI to predict contractile recovery of chronically dysfunctional myocardium after successful revascularization. Kim et al. used MRI-DE [21], Kinuuti et al. [22] used F-18 FDG PET, and Qureshi et al. [23] used thallium to predict functional recovery. The aim of this study was to compare the MRI methods of dobutamine stress, end-diastolic wall thickness, MRI-DE, and the widely used scintigraphic method Tl201-SPECT with functional recovery 6 months after surgery.
Materials and methods
Study protocol
The study protocol was approved by the local ethics committee. Twenty patients with triple-vessel coronary artery disease, severely impaired left ventricular function (ejection fraction [EF] <45% measured by MRI), no contraindications to MRI, and the need for CABG surgery were included in the study. Stress MRI and SPECT imaging were performed within the same day. All patients underwent bypass surgery within 1 week after imaging. At 6 months after surgery at the earliest, MR imaging and SPECT imaging were repeated to assess myocardial response to revascularization.
Patients
Informed consent was obtained from all patients before they entered the study. The mean age of the patients was 63.7 (±7.3) years (range 53–81 years). One patient was female, and all others were male. The mean EF of these patients as measured by MRI prior to surgery was 28.6% (±8.7; range 12–44%). Ten patients had a known history of myocardial infarction, and the mean time from last infarction to MR imaging was 16 months (range 12–48 months). In addition to coronary artery disease, patients had diabetes (n=10), hypertension (n=12), and/or hypercholesterolemia (n=13).
MRI protocol
Rest and stress MRI as well as Tl201-SPECT were performed in all patients before surgery. The examinations were repeated 6 months after CABG surgery at the very earliest. An 18-gauge catheter was inserted into the antecubital vein on one side for Gd-DTPA (Magnevist; Schering, Berlin, Germany) and nuclide administration, and another 18-gauge catheter was placed on the other side for dobutamine infusion. Patients were placed in supine position on the table of a 1.5-T imager (Intera, Philips, Best, Netherlands), and imaging was performed using a thorax phased-array surface coil as a receiver.
Blood pressure was monitored before and during dobutamine infusion, and continuous heart rate and pulse oximetry were performed during MR imaging. Furthermore, two-way audio communication and video monitoring of the patient were maintained. Regional systolic wall thickening was monitored with an electrocardiographically gated breath-hold balanced FFE cine-sequence. The imaging parameters for the series were as follows: repetition time (ms)/echo time (ms), 3/1.5; matrix, 126×256; field of view, 240×320 mm; flip angle, 50°; temporal resolution <50 ms and section thickness, 8 mm. Up to 12 contiguous short-axis two-chamber views were acquired during rest and three short-axis views during stress. Furthermore, one long-axis two-chamber and one four-chamber view were acquired during rest and stress examination. Five to ten milligrams of intravenous dobutamine per kilogram of body weight per minute were used for the “low-dose” stress protocol. For “late enhancement” assessment, a T1 weighted 2D gradient echo sequence in breath hold with an inversion prepulse to null viable myocardium was used 10–20 min after the intravenous administration of a double dose (0.2 mmol/kg) of Gd-DTPA (Magnevist; Schering, Berlin, Germany) in the long-axis four- and two-chamber and three short-axis (apical, middle, and basal) two-chamber views. The imaging parameters for the series were as follows: repetition time (ms)/echo time (ms), 7.7/4.5; flip-angle, 25°; matrix, 256×256; field of view, 240×320 mm; and section thickness, 8 mm. The sequence used for viability assessment applied a nonselective inversion pulse after an electrocardiographic trigger. The inversion delay time ranged from 200 to 300 ms and was adjusted in 25-ms steps until a uniform suppression of normal myocardium was attained (Fig. 1).
Example of the inversion delay dependence of the contrast between normal and injured myocardium at three different inversion delays. The long-axis two-chamber view of a patient with a transmural chronic infarction of the thinned inferior wall showed that starting with an inversion delay (TI) of 200 ms, the contrast can be improved between normal and injured myocardium in this patient by increasing the TI in 25-ms steps.
SPECT protocol
SPECT imaging was performed using a Siemens MultiSPECT three-head gamma camera. For viability assessment, Tl201 was administered intravenously at rest before the stress MR examination at a mean dosage of 80 MBq. Rest images were acquired 10 min after the administration. Usually the data acquisition was performed in supine position. If there was a substantial defect at the inferior wall, the examination was repeated in prone position to avoid false positive results due to diaphragm absorption. The acquisition was performed according to the guidelines of the American College of Cardiology/American Heart Association Task Force for Clinical Cardiac Radionuclide Imaging [24].
MR image analysis
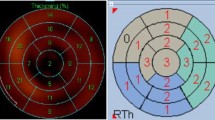
In each patient, the three left ventricular short-axis sections were divided into 12 sectors altogether (Fig. 2), starting at the anterior interventricular groove of the cardiac base in a clockwise fashion using 45°, 90°, and 180° sections. These sectors were analyzed for wall motion during rest and stress using a qualitative scoring system with the following scale:
-
0=dyskinetic
-
1=akinetic
-
2=severely hypokinetic
-
3=hypokinetic
-
4=normal
Wall motion analysis and measurement of the mean end-diastolic wall thickness were done by three experienced examiners (M.F., S.M., H.S.), one with lengthy experience in stress echocardiography (H.S.), by consensus; they were unaware of the patient’s coronary status and whether it was a pre- or postoperative scan. If an akinetic or dyskinetic segment showed hypokinesia with a systolic wall thickening of at least 2 mm [8] during low-dose dobutamine stress, it was considered to be viable; otherwise, it was considered nonviable, as was a segment with a mean end-diastolic wall thickness <6 mm [16].
This wall motion scoring system served as the reference for postoperative functional recovery for all imaging modalities in all patients.
Delayed enhancement was assessed using a multipurpose imaging tool (Amira, version 2.3; Konrad-Zuse-Zentrum, Berlin, Germany; Fig. 3) for absolute quantification of the area of DE. One author (M.G.) performed the DE assessment and was blinded to the rest of the results. The short-axis views of the T1-weighted inversion-recovery gradient-echo images, which were obtained 10–20 min after contrast injection, were used for late enhancement analysis. A region of interest (ROI) was drawn in each segment according to the described 12-segment model (Fig. 2), and care was taken to avoid pixels in the blood pool or epicardial fat. The areas of a signal intensity (SI) of two standard deviations (SD) above the mean were classified as representing the areas with larger extracellular volume, representing scar tissue as previously described by Lauerma et al. [9]. Furthermore, the transmural extent of hyperenhancement was determined and qualitatively assessed as nonviable if the extent of hyperenhanced tissue was more than 50%, according to the results of Kim et al., predicting a low probability of myocardial recovery after revascularization [21] in these segments.
Image segmentation editor of the multimodality imaging software (Amira; Konrad-Zuse-Zentrum, Berlin, Germany) used for assessing the extent of “delayed enhancement” in a patient (same as in Fig. 4) with a transmural infarction (>50%) of the inferior septum (segment 9) and a subendocardial enhancement (<50%) in the anterior wall (segment 6) that shows the segmentation for the midventricular short-axis slice and the area of “delayed enhancement” [Narbe scar]. The area of delayed enhancement was defined by a signal intensity (SI) of more than two standard deviations (SD) above the mean SI of the surrounding myocardium.
Left ventricular ejection fraction
All left ventricular short-axis images were planimetered with a mouse-driven cursor (M.F.), and left ventricular volumes were summed to give the total cavity volume at diastole and systole according to the Simpson rule algorithm. The EF was calculated from the end-diastolic and end-systolic left ventricular volumes.
SPECT image analysis
For assessing the left ventricular volumes and EF (S.M.), the automatic program GS-Quant (Siemens, Erlangen, Germany) was used [25], and for assessing the percentage of maximal nuclide uptake, the Emory Cardiac Toolbox [26], version 1.0 (Siemens and Emory University, Atlanta, GA, USA), was used. The reconstructed images were oriented in the standard short axis, horizontal long axis, and vertical long axis for interpretation and quantification of Tl201 uptake by experienced nuclear cardiologists (H.A., S.M.) unaware of all other data.
Computerized polar maps of the three-dimensional myocardial radioactivity were generated. The 12-segment model comparable to that for MRI (Fig. 2) was used. Myocardial Tl201 activity was determined within an ROI (matrix, 64×64 mm) using a high-resolution collimator and a camera angle of 120°. The activity in each segment was normalized to the segment with the highest uptake. As in the study by Nagueh et al. [19], the absence of a Tl201 uptake defect during rest was considered indicative of viability [23]. Thallium defects during rest of more than 50% of the area of the analyzed segment were classified as nonviable.
Statistical analysis
The sensitivity and specificity as well as the positive and negative predictive values were calculated for all patients using the gated SPECT evaluation of viability assessment as the gold standard (Table 1). Furthermore, for all patients the functional recovery of myocardial wall motion served as the reference for viability to calculate the above-mentioned parameters (Table 2). The statistical significance of these correlations was evaluated using the ANOVA test. A p-value <0.05 was considered statistically significant.
Results
In all patients who were examined by MRI 6 months postoperatively at the earliest (n=20), the mean global function expressed by the left ventricular EF slightly improved from 28.6% (±8.7) to 32.2% (±12.4). The difference was not statistically significant.
SPECT as the gold standard
Using SPECT as the reference method for viability assessment, we were able to include 20 patients in the evaluation for a total of 240 segments. Out of these 240, 56 segments were preoperatively defined as nonviable by SPECT using the mentioned criteria, 32/240 segments by MRI delayed enhancement, 20/240 by the mean end-diastolic wall thickness, and 57/240 segments by MRI wall motion analysis. Although the examinations were repeated in prone position if no thallium uptake was obvious in the inferior wall, the majority of patients (62.5%) with nonviable areas assessed by SPECT had their “scar tissue” in the inferior wall, 25% in the anterior wall, and 12.5% in the lateral wall. MRI-DE showed a sensitivity for viability assessment of 93% and a low specificity of 39%. The positive predictive value was 83%, and the negative predictive value was 65%. The MRI wall motion analysis using low-dose dobutamine stress showed a lower sensitivity of 84% but a higher specificity of 50%. The positive predictive value for this MR method of assessing viability was 85%; the negative predictive value was 49% (Table 1). The mean end-diastolic wall thickness as a criterion for viability showed a sensitivity of 94%, with the lowest specificity of all evaluated methods, 36%. The positive predictive value was 83%, and the negative predictive value was 65%.
Functional recovery after revascularization as the gold standard
A complete examination with MRI and gated SPECT at 6 months at the earliest after complete surgical revascularization could be performed in all 20 patients. A total of 240 segments could be evaluated, and 34/240 segments did not show postoperative functional recovery after complete revascularization. MRI-DE assessed 32/240 segments as nonviable, MRI wall motion 57/240, end-diastolic wall thickness 20/240, and gated SPECT 56/240. Therefore, we found the highest sensitivity for MRI-DE with the highest negative predictive value for functional recovery of 99% each, indicating that we found only two false negative segments in the postoperatively examined group that were assessed as nonviable by MRI-DE and showed functional recovery 6 months after complete revascularization. In comparison with MRI-DE, the MRI end-diastolic wall thickness, wall motion analysis, and gated SPECT showed a lower sensitivity of 96%, 88%, and 86%, respectively, and an even lower negative predictive value of 57%, 56%, and 44%, respectively (Table 2).
Delayed enhancement (DE)
The postoperative extent of DE was only evaluated qualitatively as in other studies [21] and did not show a significant difference from the preoperative extent. The assessment of the segments as viable or nonviable did not change. In most patients, DE showed only a subendocardial (66%) extent.
In only a few patients was a transmural extent obvious within the segment (34%). The total area of scar tissue of the three evaluated short-axis slices, representing the left ventricle, was also calculated from the DE images and showed a mean area of 21.7% (±15.4) of the evaluated segments. Therefore, a slight inverse correlation with an r-value of 0.46 was found between the total extent of DE representing scar tissue and the postoperative EF. Furthermore, an inverse correlation (r=0.64) was also found between the thallium uptake within the segments and the extent of DE within the analyzed segments (Fig. 5).
Discussion
Especially in patients with “end-stage” coronary artery disease, preoperative evaluation of myocardial viability is of great significance [2, 27] because it predicts the patient’s postoperative outcome and is therefore important in deciding which patients should be treated by bypass surgery and which should undergo orthotopic heart transplantation or pharmacological treatment alone. Myocardial viability can be assessed noninvasively by different imaging modalities including the widely used nuclear cardiological methods such as PET [3], thallium, and technetium SPECT [5, 17, 18]; the cheapest method with the highest availability, stress echocardiography; or different MRI methods, which include wall motion analysis, wall thickness, perfusion imaging, and tissue characteristics [8–10]. The method favored in assessing myocardial viability in MRI up until now is low-dose dobutamine stress [11–13, 15, 20], which is used analogously to low-dose dobutamine stress echocardiography for viability assessment. Baer et al. [16] showed in comparison to F-18 FDG PET that low-dose dobutamine MRI in combination with the evaluation of end-diastolic wall thickness has a high sensitivity (88%) and specificity (87%) for predicting myocardial viability as defined by F-18 FDG PET (more than 50% uptake of the maximum uptake in a region with normal wall motion). Since the publications of Ramani et al. [6] and Kim et al. [7, 21], the easily performed method of DE has become more and more popular [9, 10]. The reason for this evolution was the development of new MRI sequences that could substantially improve the signal intensity and contrast-to-noise ratios [8, 28], thereby improving the delineation of infarcted regions tenfold [21]. The most striking advantage of MRI-DE compared with other imaging modalities is that it shows very precisely the transmural extent of viable myocardium (Fig. 4). An approach less often used for viability assessment was favored by Lauerma et al. [9]: first-pass perfusion. First-pass perfusion analysis has recently been used mostly for detecting myocardial ischemia [29–31]. In combination with dobutamine stress MRI, Lauerma et al. favored a multimodality approach and not the single-method approach with DE, which Kim et al. [7, 21] prefer.
From left to right: the short-axis two-chamber views (a) and long-axis two-chamber (b) reconstruction of the Tl201-SPECT (top images) and MRI-DE (bottom images, c, d) of a patient with a transmural infarction of the inferior septal wall and right ventricle (c, arrows) and an anteroseptal subendocardial infarction (d, arrow), which can be better delineated by MRI-DE than by Tl201-SPECT (same patient as in Fig. 3)
The reason for these results might be that they did not quantify, or at least qualitatively describe, the extent of the area with DE as Kim et al. [21] did. In this study we first describe a quantitative single-use approach to predict functional recovery of dysfunctional myocardial segments after revascularization by MRI-DE in comparison not to the so-called gold standard of noninvasive viability assessment—F-18 FDG PET [3] but to the more often used scintigraphic methods Tl201-SPECT [32, 33] and low-dose dobutamine stress MRI. In a metaanalysis, vom Dahl et al. [32] showed that Tl201-SPECT (n=237) achieved a similar positive predictive value for predicting myocardial recovery after revascularization: 66% compared with 67% for F-18 FDG PET (n=306). Nevertheless, the negative predictive value for the PET studies was obviously higher at 88% compared with Tl201-SPECT at 77%. Using a hybrid PET protocol with Tc99m MIBI and F-18 FDG PET, the positive predictive value of PET could be increased to 82%. In this protocol, a match of perfusion and metabolic defect reflected irreversible myocardial dysfunction and therefore nonviable myocardium. Nevertheless, in this special group with patients with highly impaired left ventricular function, scintigraphic methods showed only a low negative predictive value compared with the MRI methods (Table 2). Arnese et al. [34] and Kinuuti et al. [22] found similar results for Tl201-SPECT and F-18 FDG PET, respectively. The low-dose dobutamine stress MRI could also serve as a comparison with stress echocardiography because both methods use the same pathophysiological approach to assess myocardial viability. If we compare stress MRI with the gold standard for viability assessment, functional recovery after revascularization, it shows a similar sensitivity and positive predictive value to Tl201-SPECT, but a higher specificity and a low but higher negative predictive value compared with SPECT. We could assume that with stress echocardiography we would have found similar results.
Consistent with Baer et al. [8, 16], we also found a very high sensitivity for predicting myocardial viability with the analysis of end-diastolic wall thickness, 96% (Baer et al., 92%), but a very low specificity of only 35% (56% resp.), the lowest of all examined methods for viability assessment in this study.
In our study we found an inverse correlation (Fig. 5) between the thallium uptake and the DE within a segment. This may be a hint that in areas with destructed sarcolemma due to necrosis or replacement of myocardial cells, we found the phenomenon of DE.
That the area with DE does not represent stunned myocardium was already described by Kim et al. [7] in an animal model. In our study we could exclude stunned myocardium because we examined the patients postoperatively at the earliest 6 months after total revascularization. Kim et al. [21] were the first to describe the likelihood of functional recovery after revascularization in relation to the transmural extent of DE. They found a mean of 41% (±14) of transmural extent in regions with no improvement in contractile function after revascularization. Therefore, we chose a cut-off value of >50% of transmural extent within the segment for MRI-DE as the criterion for nonviability. In agreement with the results of Kim et al., we found an improvement after revascularization in only two segments with an extent of DE of >50%. In both segments, the extent was, at 55%, only slightly above the chosen cut-off. We did not evaluate other cut-off values because we found a good positive and negative predictive value for nonviability with this approach. Further studies with more patients and a quantitative approach to assess the extent of DE are necessary to define cut-off values, but these will probably be close to that used in this study. We can conclude that MRI-DE showed at least a sensitivity and specificity as well as a positive and negative predictive value comparable to Tl201-SPECT for assessing myocardial viability in patients with highly impaired left ventricular function.
In comparison with the gold standard of functional recovery after revascularization, it even shows advantages compared with the more complicated and time-consuming techniques of low-dose dobutamine stress and Tl201-SPECT in assessing functional recovery. In addition, no radiation exposure is necessary.
Limitations of the study
We did not use the gold standard for viability assessment F-18 FDG PET. However, this may be of minor importance because recently published data [17] have shown advantages of MRI-DE even compared with F-18 FDG PET. Until now only a small group of patients has been examined postoperatively, and only patients with severely impaired left ventricular function were included. The group is, therefore, preselected and not comparable with other cohorts in which SPECT, low-dose dobutamine stress MRI, end-diastolic wall thickness, or stress echocardiography showed better results. With our gamma camera, no attenuation correction (AC) could be performed. Recent studies [35] have shown that Tl201-SPECT without AC may underestimate the extent of tissue viability, as in our study, and this may explain the slightly lower sensitivity compared with F-18 FDG PET, in which AC is a routinely performed procedure. To improve our results without AC, we repeated all SPECT imaging in prone position if a substantial defect in the inferior wall was obvious. Furthermore, AC does not show only benefits because it also produces an increase in background scatter, which may lead to overestimation of viability in these segments [36]. We did not use the same threshold (>5.5 mm) for viability as Baer et al. [16] did for end-diastolic wall thickness. We used 6 mm for practical reasons but received even higher sensitivities as described by Baer et al. The specificity would have been even lower for this method if we had used the lower threshold of >5.5 mm.
References
Kaul TK, Agnihotri AK, Fields BL, Riggins LS, Wyatt DA, Jones CR (1996) Coronary artery bypass grafting in patients with an ejection fraction of twenty percent or less. J Thorac Cardiovasc Surg 111:1001–1012
Hausmann H, Topp H, Siniawski H, Holz S, Hetzer R (1997) Decision-making in end-stage coronary artery disease: revascularization or heart transplantation? Ann Thorac Surg 64:1296–1301; discussion 1302
Wesbey GE, Higgins CB, McNamara MT, Engelstad BL, Lipton MJ, Sievers R, Ehman RL, Lovin J, Brasch RC (1984) Effect of gadolinium-DTPA on the magnetic relaxation times of normal and infarcted myocardium. Radiology 153:165–169
Eichstaedt HW, Felix R, Dougherty FC, Langer M, Rutsch W, Schmutzler H (1986) Magnetic resonance imaging (MRI) in different stages of myocardial infarction using the contrast agent gadolinium-DTPA. Clin Cardiol 9:527–535
de Roos A, Doornbos J, van der Wall EE, van Voorthuisen AE (1988) MR imaging of acute myocardial infarction: value of Gd-DTPA. Am J Roentgenol 150:531–534
Ramani K, Judd RM, Holly TA, Parrish TB, Rigolin VH, Parker MA, Callahan C, Fitzgerald SW, Bonow RO, Klocke FJ (1998) Contrast magnetic resonance imaging in the assessment of myocardial viability in patients with stable coronary artery disease and left ventricular dysfunction. Circulation 98:2687–2694
Kim RJ, Fieno DS, Parrish TB, Harris K, Chen EL, Simonetti O, Bundy J, Finn JP, Klocke FJ, Judd RM (1999) Relationship of MRI delayed contrast enhancement to irreversible injury, infarct age, and contractile function. Circulation 100:1992–2002
Sandstede JJ (2003) Assessment of myocardial viability by MR imaging. Eur Radiol 13:52–61
Lauerma K, Niemi P, Hanninen H, Janatuinen T, Voipio-Pulkki LM, Knuuti J, Toivonen L, Makela T, Makijarvi MA, Aronen HJ (2000) Multimodality MR imaging assessment of myocardial viability: combination of first-pass and late contrast enhancement to wall motion dynamics and comparison with FDG PET—initial experience. Radiology 217:729–736
Sandstede JJ, Lipke C, Beer M, Harre K, Pabst T, Kenn W, Neubauer S, Hahn D (2000) Analysis of first-pass and delayed contrast-enhancement patterns of dysfunctional myocardium on MR imaging: use in the prediction of myocardial viability. Am J Roentgenol 174:1737–1740
Baer FM, Voth E, Theissen P, Schicha H, Sechtem U (1994) Gradient-echo magnetic resonance imaging during incremental dobutamine infusion for the localization of coronary artery stenoses. Eur Heart J 15:218–225
Kuijpers D, van Dijkman PR, Janssen CH, Vliegenthart R, Zijlstra F, Oudkerk M (2004) Dobutamine stress MRI. Part II. Risk stratification with dobutamine cardiovascular magnetic resonance in patients suspected of myocardial ischemia. Eur Radiol 14:2046–2052
Kuijpers D, Janssen CH, van Dijkman PR, Oudkerk M (2004) Dobutamine stress MRI. Part I. Safety and feasibility of dobutamine cardiovascular magnetic resonance in patients suspected of myocardial ischemia. Eur Radiol 14:1823–1828
Dendale P, Franken PR, Block P, Pratikakis Y, De Roos A (1998) Contrast enhanced and functional magnetic resonance imaging for the detection of viable myocardium after infarction. Am Heart J 135:875–880
Baer FM, Voth E, LaRosee K, Schneider CA, Theissen P, Deutsch HJ, Schicha H, Erdmann E, Sechtem U (1996) Comparison of dobutamine transesophageal echocardiography and dobutamine magnetic resonance imaging for detection of residual myocardial viability. Am J Cardiol 78:415–419
Baer FM, Voth E, Schneider CA, Theissen P, Schicha H, Sechtem U (1995) Comparison of low-dose dobutamine-gradient-echo magnetic resonance imaging and positron emission tomography with [18F]fluorodeoxyglucose in patients with chronic coronary artery disease. A functional and morphological approach to the detection of residual myocardial viability. Circulation 91:1006–1015
Klein C, Nekolla SG, Bengel FM, Momose M, Sammer A, Haas F, Schnackenburg B, Delius W, Mudra H, Wolfram D, Schwaiger M (2002) Assessment of myocardial viability with contrast-enhanced magnetic resonance imaging: comparison with positron emission tomography. Circulation 105:162–167
Penzkofer H, Wintersperger BJ, Knez A, Weber J, Reiser M (1999) Assessment of myocardial perfusion using multisection first-pass MRI and color-coded parameter maps: a comparison to 99mTc Sesta MIBI SPECT and systolic myocardial wall thickening analysis. Magn Reson Imaging 17:161–1670
Nagueh SF, Mikati I, Weilbaecher D, Reardon MJ, Al-Zaghrini GJ, Cacela D, He ZX, Letsou G, Noon G, Howell JF, Espada R, Verani MS, Zoghbi WA (1999) Relation of the contractile reserve of hibernating myocardium to myocardial structure in humans. Circulation 100:490–496
Baer FM, Theissen P, Schneider CA, Voth E, Sechtem U, Schicha H, Erdmann E (1998) Dobutamine magnetic resonance imaging predicts contractile recovery of chronically dysfunctional myocardium after successful revascularization. J Am Coll Cardiol 31:1040–1048
Kim RJ, Wu E, Rafael A, Chen EL, Parker MA, Simonetti O, Klocke FJ, Bonow RO, Judd RM (2000) The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N Engl J Med 343:1445–1453
Knuuti MJ, Saraste M, Nuutila P, Harkonen R, Wegelius U, Haapanen A, Bergman J, Haaparanta M, Savunen T, Voipio-Pulkki LM (1994) Myocardial viability: fluorine-18-deoxyglucose positron emission tomography in prediction of wall motion recovery after revascularization. Am Heart J 127:785–796
Qureshi U, Nagueh SF, Afridi I, Vaduganathan P, Blaustein A, Verani MS, Winters WL Jr, Zoghbi WA (1997) Dobutamine echocardiography and quantitative rest-redistribution 201Tl tomography in myocardial hibernation. Relation of contractile reserve to 201Tl uptake and comparative prediction of recovery of function. Circulation 95:626–635
Ritchie JL, Bateman TM, Bonow RO, Crawford MH, Gibbons RJ, Hall RJ, O’Rourke RA, Parisi AF, Verani MS (1995) Guidelines for clinical use of cardiac radionuclide imaging. Report of the American College of Cardiology/American Heart Association Task Force on Assessment of Diagnostic and Therapeutic Cardiovascular Procedures (Committee on Radionuclide Imaging), developed in collaboration with the American Society of Nuclear Cardiology. J Am Coll Cardiol 25:521–547
Germano G, Kiat H, Kavanagh PB, Moriel M, Mazzanti M, Su HT, Van Train KF, Berman DS (1995) Automatic quantification of ejection fraction from gated myocardial perfusion SPECT. J Nucl Med 36:2138–2147
Faber TL, Cooke CD, Folks RD, Vansant JP, Nichols KJ, DePuey EG, Pettigrew RI, Garcia EV (1999) Left ventricular function and perfusion from gated SPECT perfusion images: an integrated method. J Nucl Med 40:650–659
Hausmann H, Meyer R, Siniawski H, Pregla R, Gutberlet M, Amthauer H, Felix R, Hetzer R (2004) Factors excersising an influence on recovery on hibernating myocardium after coronary artery bypass grafting. Eur J Cardiothorac Surg 26(1):89–95
Simonetti OP, Kim RJ, Fieno DS, Hillenbrand HB, Wu E, Bundy JM, Finn JP, Judd RM (2001) An improved MR imaging technique for the visualization of myocardial infarction. Radiology 218:215–223
Al-Saadi N, Nagel E, Gross M, Bornstedt A, Schnackenburg B, Klein C, Klimek W, Oswald H, Fleck E (2000) Noninvasive detection of myocardial ischemia from perfusion reserve based on cardiovascular magnetic resonance. Circulation 101:1379–1383
Nagel E, Lehmkuhl HB, Bocksch W, Klein C, Vogel U, Frantz E, Ellmer A, Dreysse S, Fleck E (1999) Noninvasive diagnosis of ischemia-induced wall motion abnormalities with the use of high-dose dobutamine stress MRI: comparison with dobutamine stress echocardiography. Circulation 99:763–770
Hunold P, Maderwald S, Eggebrecht H, Vogt FM, Barkhausen J (2004) Steady-state free precession sequences in myocardial first-pass perfusion MR imaging: comparison with TurboFLASH imaging. Eur Radiol 14:409–416
vom Dahl J, Schulz G, Koch KC (1998) Diagnosis of myocardial viability in chronic myocardial ischemia with nuclear medicine techniques. Z Kardiol 87 (suppl 2):92–99
Wagner A, Mahrholdt H, Holly TA, Elliott MD, Regenfus M, Parker M, Klocke FJ, Bonow RO, Kim RJ, Judd RM (2003) Contrast-enhanced MRI and routine single photon emission computed tomography (SPECT) perfusion imaging for detection of subendocardial myocardial infarcts: an imaging study. Lancet 361:374–379
Arnese M, Cornel JH, Salustri A, Maat A, Elhendy A, Reijs AE, Ten Cate FJ, Keane D, Balk AH, Roelandt JR et al (1995) Prediction of improvement of regional left ventricular function after surgical revascularization. A comparison of low-dose dobutamine echocardiography with 201Tl single-photon emission computed tomography. Circulation 91:2748–2752
Gallowitsch HJ, Unterweger O, Mikosch P, Kresnik E, Sykora J, Grimm G, Lind P (1999) Attenuation correction improves the detection of viable myocardium by thallium-201 cardiac tomography in patients with previous myocardial infarction and left ventricular dysfunction. Eur J Nucl Med 26:459–466
Heller EN, DeMan P, Liu YH, Dione DP, Zubal IG, Wackers FJ, Sinusas AJ (1997) Extracardiac activity complicates quantitative cardiac SPECT imaging using a simultaneous transmission–emission approach. J Nucl Med 38:1882–1890
Acknowledgements
The study was supported in part by grants from the Deutsche Forschungsgemeinschaft (BO-866/5-1). We thank Dr. Lutz Lüdemann and Dipl. math. Arne Janza for adapting the multimodality software Amira for our purposes. Furthermore, we thank the technicians, Mrs. Sylvia Foelz, Mrs. Virginia Ding-Reinelt, Mrs. Silvia Kurth, and Mrs. Catharina Oledtzki, and the medical editor, Ms. Anne Gale, for their assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gutberlet, M., Fröhlich, M., Mehl, S. et al. Myocardial viability assessment in patients with highly impaired left ventricular function: comparison of delayed enhancement, dobutamine stress MRI, end-diastolic wall thickness, and TI201-SPECT with functional recovery after revascularization. Eur Radiol 15, 872–880 (2005). https://doi.org/10.1007/s00330-005-2653-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-005-2653-9